Abstract
It is a physical phenomenon for biological systems that the cartilage surface is hydrophilic and negatively charged. However, there is a lack of knowledge about some parameters possess by cartilage surface, e.g., interfacial surface energy, amphoteric, wettability, negatively charged bilayers, and lamellar slippage. Some of these parameters will be taken from literature to support and correct Hills [1] hydrophobic lubrication model which is hydrophilic and negatively charged.
Keywords
hydrophilic and negatively charged surface, amphoteric articular cartilage, interfacial energy, friction coefficient, model of hydrophilic lubrication
Introduction
The mechanism of surface articular cartilage lubrication has been a frequently controversial subject in the past two decades. For a description of this model, it is necessary to recognize a function of parameters depending on the presence of phospholipid bilayers on the surface. To these parameters belong interfacial surface energy, pH, and wettability, number of PLs bilayers. The lipid bilayers on the surface of the normal joint cartilage contain mainly phosphatidyl-choline (41%), phosphatidylethanolamine (27%), and sphingomyelin (32%) of total phospholipids [2]. Liposomes and lamellar phases are composite structures made of phospholipids in synovial fluid and bilayers on cartilage surface (see Figure 1).
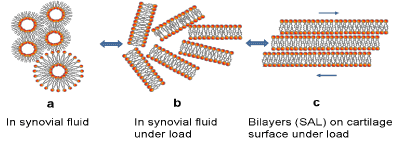
Figure 1. Phospholipids in synovial fluid (SF) (a) liposome and hexagonal phases, (b) lamellar phases in SF under load and (c) phospholipid bilayers on the surface of cartilage under load (3).
Phospholipids as lubricant are highly self-organized biomolecules in aqueous media, and their structure lets them form spontaneously vesicles, lamellar phases, and membrane (see Fig. 1). The multilamellar structure of phospholipids, namely the surface amorphous layer (SAL), covers the natural surface of articular cartilage. A very high porosity (75 to 80 %) is concluded to be a critical factor in providing excellent hydration lubrication properties of articular cartilage [3].
We have shown, in agreement with published literature that the main driving force for the interaction between hydrophilic and negatively charged surface with a phosphatidylcholine membrane is the van der Waals attraction. In a zwitterion of phosphatidylethanolamine and sphingomyelin, the positive charge of the nitrogen atom of the head group is slightly closer to the surface membrane than the phosphorous atom.
In this study, we have considered cartilage model with PLs functional group (-NH2, -PO4-), to provide a range of surface wettability and charge. Currently, the relative importance of surface wettability and surface charge in influencing how PLs adhere to a surface [4]. Verification of hydrophobic or hydrophilic model, the following studies have been undertaken: interfacial energy, friction (cartilage/cartilage) pair, and wettability cartilage surface (values measured for the air-dry surfaces).
Experimental
The cattle knees (aged 15-20 months) were used to take AC specimens. Two kinds of osteochondral plugs: 5 and 10 mm in diameter were cut out of lateral and medial femoral condyles by a circular stainless steel cutter. The AC sample discs with underlying bone were formed into 3-mm plugs and stored at 253 K in a 0.155 M NaCl (pH = 6.9) solution. Before the experiments, the samples were fully defrosted. Next, the AC specimens were glued to the disc and pinned to stainless steel surfaces, and the friction measurements were carried out in the universal buffer solution.
Tensiometer
The contact angle between a droplet of a 0.15 M saline solution and a sample of the air-dry surface of AC was measured with the use of a KSV CAM100 tensiometer. The specimen of cartilage was normal, partial and completely depleted. On each sample, five experiments were performed.
Friction test
A sliding pin-on-disc tribotester T-11, manufactured by the NIST Research from Radom, Poland, was used to perform the friction measurements. Before the friction measurements, the lubricants were prepared on the base of the universal Britton-Robinson buffer solution [5], where pH ranged from 2.0 to 9.5. Because of the assumption of preserving conditions of the physiological state of the lubricant [6], the measurements of AC/AC tribopairs friction coefficients were completed at room temperature, in the time of 5 and 10 minutes, when the velocity was 1 mm/s and load 15N.
The friction coefficient measurements of cartilage/cartilage tribopair were carried out over the pH range between 2.5 and 9.5. The samples were equilibrated with each buffer solution under a load 15N for 5 minutes with previously measured pH. During the experiment, AC specimens were immersed in the lubricant to ensure its presence at their contact interface. A total number of 5 tests, where fresh sample were used, were performed. During each test, at least four repetitions per the specimen pair were carried out. Finally, the mean and standard deviation were calculated, and the graph of the friction coefficient of the pH solution was prepared.
The interfacial energy measurements method
In the literature, the effect of pH on the interfacial energy ( ) of spherical lipid bilayers formed from phospholipid (PLs) has already been described in details [3,7-9], phosphatidylserine, and sphingomyelin) is around pH 4, and the model phospholipidic membrane the interfacial energy “bell-shaped curve” vs. pH based on literature data was made.
Results and discussion
Hydrophilic cartilage model with negatively charged phosphate ions of surface
The strong adsorption of PLs molecules by their quaternary ammonium positive ion (Me3N+-) to hydrophilic cartilage surface (a proteoglycan) is [10] hydrophobic model of the cartilage surface. Strong cohesion between phosphate ions and calcium (II): (-PO4—Ca++-PO4--) making the close-packed hydrophobic solid layer (Figure 2a). However, considering pH 7.4 condition and properties of phospholipids (PLs) of being highly self-organized, bilayer is formed and the surface is negatively charged (Figure 2b). The multilamellar structure of phospholipids, namely the surface amorphous layer (SAL), covers the natural surface of articular cartilage. We conclude that a very high porosity (75%) is a critical factor in providing excellent hydration lubrication properties of articular cartilage.
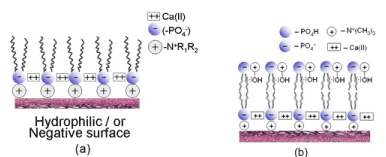
Figure 2. The hydrophobic (a) Hills (2002) model for boundary lubrication of cartilage surface and (b) hydrophilic cartilage surface.
Changes in interfacial energy of spherical lipid bilayers and cartilage friction coefficient
The effect of pH on the interfacial energy ( ) of spherical lipid bilayers formed from phospholipids (PLs) all four (phosphatidylcholine, phosphatidylethanolamine, phosphatidylse-rine, and sphingomyelin) has been described previously in details [3,7-9]. The isoelectric point of all four phospholipids is around pH 4, and the model phospholipidic membrane the interfacial energy vs. pH based on literature “bell-shaped curve” was created (Figure 3).
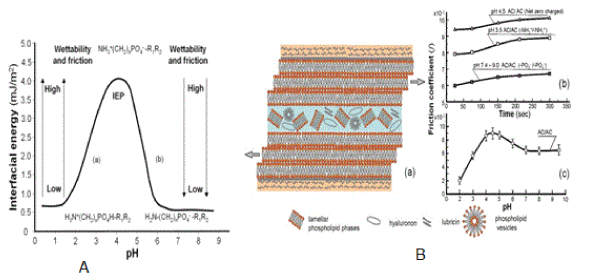
Figure 3. The interfacial energy curve (A) of spherical phospholipid bilayers vs. pH “bell-shape curve” formed from phospholipid (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine and sphingomyelin), (a) (-NH3+→ -NH2) and (b) (-PO4H → -PO4-). (B) The friction coefficient of the bovine (cartilage/cartilage) tribological pair surfaces. (a) Lamellar slippage of the bilayers lubrication mechanism, (b) friction with positively charged surface (+/+) at pH 3.5, with negatively charged surface (-/-) at pH 7.4 - 9.0 and at the isoelectric point, IEP, (±/±) at a pH 4.3 and (c) the friction coefficient curve the bovine (cartilage/cartilage) surfaces against pH 2.0 to 9.5 of buffer solutions under a 15N load and 1 mm/s sliding velocity during 10 min.
In Figure 3 (A) the isoelectric point, IEP, with the pH at which a phospholipid molecule carries no net electrical charge,
IEP ; H2N (CH2)n PO4H-R1R2 D H3N+(CH2)n PO4--R1R2
pH 0.2 to 4.0 (-NH3+→ -NH2) (pH 4.0 to 6.5 ) (-PO4H → -PO4-)
(a) (Left-hand side of the curve) (b) (Right-hand side of the curve)
The isoelectric point, IEP of all four phospholipids is around pH 4.0 ± 0.2 an interfacial energy (mJ/m2) is summarized as follows: phosphatidylcholine (3.53), phosphatidylethanolamine (4.06), phosphatidylserine (2.93) and sphingomyelin (4.42) (values taken from Table 1 in [10] and [3]. Changes in interfacial energy correspond well to the amino (-NH3+→ -NH2) transition at a low pH; after IEP pH 4.0, and the phosphate (-PO4H → -PO4-) transition at a higher pH range of 4.0 – 6.5
Friction tests were conducted to investigate the role of the phospholipid bilayers charged positively (+/+), negatively (-/-) and at an isoelectric point, IEP, (±/±). Cartilage/cartilage friction tests were conducted on fresh, healthy cartilage.
Figure 3 summarizes the interfacial energy, the wettability and coefficient of friction (f) obtained for PLs terminated with a spherical bilayer (Figure 3A) and (cartilage/cartilage) friction (Figure 3B) of either PLs of functional groups (-NH3+/ -NH2) and (-PO4H / -PO4-) over the buffer solution pH range of 0.2-9.0. The interpretation of the results requires consideration of the charge density of the functional groups.
At low pH buffer solution, PLs bilayer the amino groups almost all in the (-NH3+) form, but as the pH of the buffer is increased, the amino groups begin to lose their charge (-NH3+→ -NH2) leaving the bilayer with a more hydrophobic character, which causes an increase in the contact angle. After IEP, in the case of (-PO4H) groups of PLs bilayer, the contact angle decreases with increasing buffer pH because the (-PO4H) groups begin to gain their charge (-PO4H→ -PO4-) (Figures 3A and 3B). In contrast, at pH values above pH 6.5 – 9.0, the cartilage surfaces are both negatively charged, with the charge density (repulsive interactions) and low friction with increasing pH. Dependence of the surface friction on the solution pH was previously observed for (poly (L-lysine)/ hyaluronic acid and SiO2 surface) [11,12].
Conclusion
- The bilayers on the surface of articular cartilage at pH 7.4 of synovial fluid are hydrophilic and negatively charged.
- Model of boundary lubrication of natural surfaces supported by lamellar slippage of the bilayers lubrication mechanism.
- Hills hydrophobic surface model of AC has no support in all experimental facts presented in this paper and current literature which actually supports the concept that AC is amphoteric, hydrophilic with the negatively charged surface (-PO4-), Figure 4 [3].

Figure 4. Cover book page.
References
- Hills BA, Butler BD (1984) Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann Rheum Dis 43: 641-648.
- Sarma JAV, Powell GL, LaBerge M (1991) Phospholipid composition of articular cartilage boundary lubricant. J OrthopResearch 19: 671-676.
- https://wwwamazoncom/dp/B07B42P1JY
- Leckband D, Chen YL, Israelachvili J, Wickman HH, Fletcher M, et al. (1993) Measurements of conformational changes during adhesion of lipid and protein (polylysine and S-layer) surface. Biotechnol Bioeng 42: 167-177.
- Britton HTK, Robinson RA (1931) Universal buffer solutions and the dissociation constant of veronal. J Chem Soc 1456-1462.
- Hills BA (2002) Surface-active phospholipid: a Pandora's box of clinical applications Part II Barrier and lubricating properties. Int Med Journ 32: 242-251.
- Petelska AD, Figaszewski ZA (2000) Effect of pH on the interfacial tension of bilayer lipid membrane. Biophysical J 78: 812-817.
- Petelska AD, Figaszewski ZA, (2002a) Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylcholine or phosphatidylserine. Biochim Biophys Acta 1561: 135-146.
- Petelska AD, Figaszewski ZA (2002b) Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylethanolamine. Biochim Biophys Acta 1567: 79-86.
- Petelska AD, Figaszewski ZA (2009) pH effect of the sphingomyelin membrane interfacial tension. J Membrane Biol 230: 11-19.
- Burke SE, Barrett CJ (2003) pH-Responsive Properties of Multilayered Poly(L-lysine)/ Hyaluronic Acid Surfaces. Biomacromolecules 41773-1783.
- Marti A, Hahner G, Spencer ND (1995) Sensitivity of frictional forces to pH on a nanometer scale: A lateral force microscopy study. Langmuir 11: 4632-4635.




