Human herpes virus (HHV) 6 is a β-herpesvirus mostly known for causing the common childhood illness exanthema subitum. Nearly all children had contact with HHV 6 at the age of 2 [1] with a peak incidence between 9 and 21 months [2]. Primary infection typically leads to fever for 3 to 5 days with sudden onset of a maculopapular rash upon defervescence, [3] hence the name exanthema subitum. HHV 6 persists in monocytes and salivary glands and can be reactivated when immunity is impaired [4]. As a neurotropic herpes virus, HHV 6 also overcomes the blood-brain barrier and can cause multiple neurological symptoms: primary infection coincides with febrile seizures and accounts for one third of all febrile seizures inchildrenup to the age of two years [3,5]. It can also present as an acute encephalitis in immunocompetent populations [6,7] leading to headaches and seizures [8-11]. As HHV-6-associated encephalitis is described to be located in the limbic system, patients often show altered behavior, e.g. disorientation and confusion [9], drowsiness [8,12], aphasia [13-14], decreased appetite [10] and hallucinations [15]. Furthermore, HHV 6 is able to persist in the central nervous system [16] and reactivation has been associated with encephalitis and encephalopathy [17,18]. It has even been linked to neurodegenerative disorders such as multiple sclerosis [16].
Several cases of HHV 6 reactivation in patients after allogeneic stem cell transplantation (SCT) have been described leading to the so-called post-transplant acute limbic encephalitis (PALE) [11,21]. PALE presents similar to acute HHV 6 encephalitis with a broad spectrum of neurological symptoms including confusion, anterograde amnesia, emotional dysregulation, altered sleep/wake patterns and seizures [11,19-21]. Frequently, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and hyponatremia can be found [20]. PALE typically occurs about 2-6 weeks after transplant and comprises significant morbidity and mortality [21,22]. The diagnosis of PALE involves symptoms, analysis of blood and cerebrospinal fluid (CSF), MRI and EEG. In the CSF, there is often no or minimal pleocytosis, CSF glucose can be elevated or normal [11,23,24]. Laboratory abnormalities include hyponatremia due to SIADH, changes in leukocytes, thrombocytopenia [25,26] as well as elevated aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase [25-27]. Acute phase proteins as ferritin can be found elevated [25,26] while C-reactive protein (CRP) can be elevated or normal [9,28]. HHV 6 can be detected using PCR in plasma and CSF [8,29]. The EEG most commonly shows generalized slowing [13,14] or bilateral focal abnormalities over the temporal or frontotemporal leads with epileptiform activity [20]. However, the EEG can be without pathological findings, too [21]. In MRI, PALE characteristically presents with unilateral or bilateral signal abnormalities in the limbic system, particularly in the amygdaloid and hippocampal areas [22,30,31]. Neurologic sequelae such as ataxia, epilepsy and developmental disabilities, for example loss of verbal and social skills and memory loss have been described following acute HHV-6-associated encephalitis [8,22,24,32] as well as PALE [21,23,33-38].
In addition to neurological symptoms, HHV 6 causes acute myocarditis and inflammatory cardiomyopathy in immunocompetent adults as well as pediatric patients with unexplained sudden heart failure [39-43]. It leads to restriction in left ventricular ejection fraction (LVEF) [42,44] as well as pericardial effusion [44,45]. In patients after allo-SCT, only one case of acute myocarditis following HHV 6 reactivation has been described in adults [46] and in children, [44] respectively.
Therapeutic strategies in HHV 6 reactivation include antiviral therapy with ganciclovir [44] or foscarnet [21]. There have even been studies using foscarnet prophylaxis to prevent HHV 6 reactivation with some [46] or limited success [47]. There are also contradictory findings regarding steroid therapy as it has been found to induce either higher viral load [48] or lower viral load [49], one study showed that pulse steroid therapy is associated with lower risk of neurological sequelae [9].
A 7 year-old boy was admitted to our hospital for his second allogeneic SCT. Five years ago, he suffered from a Burkitt’s lymphoma, after relapsing he underwent his first allo-SCT 3 years ago and at that time was diagnosed with Li-Fraumeni’s syndrome. A second relapse led to CAR-T-cell therapy, after which he developed secondary myelodysplastic syndrome progressing to acute myeloid leukemia (AML M6) setting the indication for a second allo-SCT. Chemotherapy-based conditioning was performed using busulfan (reduced dose to minimize toxicity, 3x 3.6mg/kg, target AUC 60ng*h/l), cyclophosphamide (2 x 60mg/kg), melphalan (1 x 140 mg/m2) and ATG (3 x 20 mg/kg). For Graft versus Host disease (GvHD) prophylaxis our patient received short-term methotrexate on day +1 and +3 (2 x 10 mg/m2) and cyclosporine A (starting dose 3mg/kg, then adjusted to drug level). After conditioning, he received a bone marrow transplant of a matched unrelated donor, HLA 9/10 (4.2 x 10e6 CD34+ per kg bodyweight, 43.3 x 10e6 CD3+ per kg bodyweight). Our patient showed a quick and satisfactory hematopoetic regeneration with a take of leukocytes (>1000/µl) on day +12.
During engraftment, our patient developed severe sepsis with increased CRP to a maximum of 199 g/l on day +13 leading to acute renal failure and cardiorespiratory insufficiency requiring non-invasive ventilation and catecholamine support. We escalated the empiric antimicrobial therapy to broad-spectrum and our patient improved rapidly.
On day +19, our patient developed hypotension and tachycardia, the following transthoracic echocardiogram revealed a global systolic dysfunction with a LVEF of 48 % as well as pericardial effusion with a diameter of ca. 1 cm (Figure 1). High sensitivity Troponin T peaked at 66.4 ng/l (normal ≤ 14 ng/l) and NT-pro-BNP peaked at 33905 pg/ml (normal ≤ 145 pg/ml). The cardiac failure was treated with noradrenaline. In the following days, heart function stabilized, Troponin T and NT-pro-BNP rapidly decreased. Follow-up ultrasound on day +23 showed a normalized left ventricular function (LVEF 63,8%); therefore we abstained from a biopsy of the myocardium to identify the cause of this episode of acute heart failure.
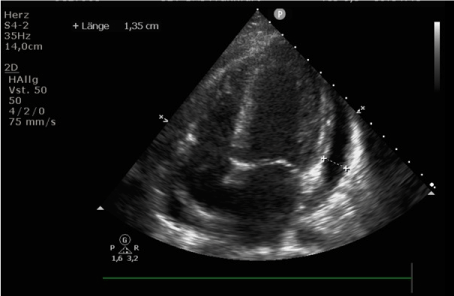
Figure 1. Transthoracic echocardiogram performed on day +19 after allogeneic SCT due to novel hypotension and tachycardia showed pericardial effusion with a diameter of ca. 1 cm
However, on day +26 he developed progressive drowsiness, slurred speech and a general slowing-down in verbal and motor reaction. He showed signs of anterograde and retrograde amnesia, insomnia and hallucinations. Moreover, he exhibited an impressive alteration in mood and affect as he was very neutral, quiet and lethargic in contrast to his usually very distinctive expression of emotion. The EEG showed generalized slowing without epileptiform activities. With posterior reversible encephalopathy syndrome (PRES) as one possible differential diagnosis in mind, we stopped the administration of CsA and steroids and started our patient on mycofenolat-mofetil (2 x 15 mg/kg) and levetiracetam (2 x 15mg / kg) to prevent seizures. We conducted a cranial MRI, which showed novel symmetric laminar restrictions in diffusion on both hippocampi with hyperintense T2/Flair correlates (Figure 2). Additionally, our patient showed hyponatremia (130 mmol/l) as a sign of SIADH. In the CSF as well as in the blood, we could detect HHV6 confirming PALE as the cause of these symptoms. We promptly started intravenous therapy with forscarnet (3 x 60 mg/kg). After one week of antiviral therapy, HHV 6 could not be detected in the blood anymore. Over the following weeks the neurological status improved slowly but steadily. The follow-up MRI after 4 weeks of antiviral treatment showed a full recovery of the PALE-associated changes, therefore we stopped antiviral therapy. Five months after PALE, our patient presents fully recovered without any neurological sequelae.
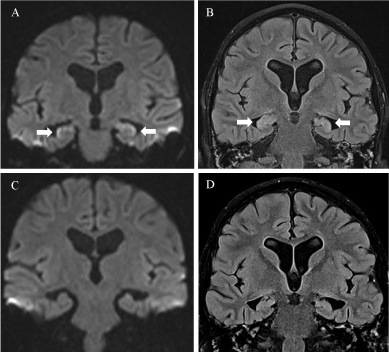
Figure 2. Acute limbic encephalitis after allogeneic stem cell transplantation. A and B: DWI (A) and FLAIR (B) at presentation (day +28): Limbic encephalitis-like manifestations on MRI images. (A) Coronal DWI-MRI image shows bilateral diffusion restriction of the hippocampus with hypersignal and swelling on FLAIR (B). C and D: DWI (C) and FLAIR (D) after 5 weeks (day +62) show complete imaging resolution
As our patient developed a maculopapular rash and pruritus typical for acute GvHD of the skin, we started therapy with methylprednisolone (2 x 0,5 mg/kg) on day +33 and resumed the therapy with CsA.
HHV 6 is a widely spread DNA virus that over 90% of the population have had contact with [1]. Persisting in monocytes and salivary glands, it can be reactivated when the immune system is compromised – e.g. after allo-SCT. HHV 6 reactivation typically occurs in the first 2-4 weeks after transplantation [4,49] and can account for a wide variety of symptoms including myocarditis [45,46] and encephalitis [19,21,23,33,34].
Our patient developed acute heart failure accompanied by pericardial effusion which was self-limiting. At this moment, HHV 6 reactivation was not yet in scope of our differential diagnoses. Then, he developed a general slowing, slurriness of speech, short-term memory impairment and hallucinations. When hyperintense signals on both hippocampal regions in T2-weighted MRI were found, we performed a lumbar punction and could verify the HHV 6 reactivation in the CSF leading to our diagnosis of a HHV-6-associated PALE. Our patient did not show any electroencephalographic changes. We immediately started antiviral therapy with foscarnet leading to a slow but steady improvement in the neurological symptoms of our patient. Whether the self-limiting episode of acute myocarditis was HHV 6-related remains unclear but as HHV 6 has been described to cause acute myocarditis in immunocompetent as well as in patients after allo-SCT, it is a reasonable possibility.
This case study highlights the wide spectrum of potential organ involvement due to HHV 6 reactivation after allo-SCT in a pediatric patient. Furthermore, our data confirm the tolerability and efficacy of treatment with foscarnet in pediatric patients with PALE. We hope our case report helps to improve recognition of organ damage patterns that are associated with HHV 6 reactivation in pediatric patients after allo-SCT as they are an inflammatory complication that can potentially be treated with antiviral therapy.
Informed written consent was obtained from the patient and his parents; we thank our patient and his family for giving us the opportunity to share our experience. We thank the Department of Pediatric Neuroradiology, University Hospital Frankfurt for providing the images for our publication. Furthermore, we thank all team members of the Division for Stem Cell Transplantation, Department for Children and Adolescents, University Hospital Frankfurt, for their competent patient care as well as all other colleagues involved in patient care.
The authors report no conflict of interest.
- Okuno T, Takahashi K, Balachandra K, Shiraki K Yamanishi K, et al. (1989) Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol 27: 651-653. [Crossref]
- Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang M, et al. (2005) A population-based study of primary human herpesvirus 6 infection. N Engl J Med 352: 768-776. [Crossref]
- Asano Y, Yoshikawa T, Suga S, Kobayashi I, Nakashima T, et al. (1994) Clinical Features of Infants With Primary Human Herpesvirus 6 Infection (Exanthem Subitum, Roseola Infantum). Pediatrics 93: 104-108. [Crossref]
- Jeulin H, Agrinier N, Guery M, Salmon A, Clément L, et al. (2013) Human herpesvirus 6 infection after allogeneic stem cell transplantation: incidence, outcome, and factors associated with HHV-6 reactivation. Transplantation 95: 1292-1298 [Crossref]
- Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, et al. (1994) Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med 331: 432-438. [Crossref]
- Yavarian J, Gavvami N, Mamishi S (2014) Detection of human herpesvirus 6 in cerebrospinal fluid of children with possible encephalitis. Jundishapur J Microbiol 7: e11821. [Crossref]
- Yamamoto S, Takahashi S, Tanaka R (2015) Human herpesvirus-6 infection-associated acute encephalopathy without skin rash. Brain Dev 37: 829-332.
- Mannonen L, Herrgard E, Valmari P (2007) Primary human herpesvirus-6 infection in the central nervous system can cause severe disease. Pediatr Neurol 37: 186-191.
- Olli-Lahdesmaki T, Haataja L, Parkkola R, Waris M, Bleyzac N, et al. (2010) Highdose ganciclovir in HHV-6 encephalitis of an immunocompetent child. Pediatr Neurol 43: 53-56 [Crossref]
- Jones CM, Dunn HG, Thomas EE, Cone RW, Weber JM (1994) Acute encephalopathy and status epilepticus associated with human herpes virus 6 infection. Dev Med Child Neurol 36: 646-50 [Crossref]
- Eliassen E, Hemond CC, Santoro JD (2020) HHV-6-Associated Neurological Disease in Children: Epidemiologic, Clinical, Diagnostic, and Treatment Considerations. Pediatr Neurol 105: 10-20. [Crossref]
- Takaya J, Araki A, Mori K, Kaneko K (2007) Usefulness of diffusion-weighted MRI in human herpesvirus-6 encephalitis. Acta Paediatr 96: 137-138. [Crossref]
- Bozzola E, Krzysztofiak A, Bozzola M, Calcaterra V, Quondamcarlo A, et al. (2012) HHV6 meningoencephalitis sequelae in previously healthy children. Infection 40: 563-566. [Crossref]
- Pulickal AS, Ramachandran S, Rizek P, Narula P, Schubert R (2013) Chorea and developmental regression associated with human herpes virus-6 encephalitis. Pediatr Neurol 48: 249-251. [Crossref]
- Kawamura Y, Ohashi M, Asahito H, Takahashi Y, Kojima S, et al. (2012) Posterior reversible encephalopathy syndrome in a child with post-transplant HHV-6B encephalitis. Bone Marrow Transplant 47: 1381-1382. [Crossref]
- Hogestyn JM, Mock DJ, Mayer-Proschel M (2018) Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regen Res 13: 211-221 [Crossref]
- Poppe M, Bruck W, Hahn G, Heubner G, Goebe HH, et al. (2001) Fulminant course in a case of diffuse myelinoclastic encephalitis-- a case report. Neuropediatrics 32: 41-44 [Crossref]
- Berry SJ, Smith R (2010) 10-year-old male with fever and headache. Clin Pediatr (Phila) 49: 707-709. [Crossref]
- Gewurz BE, Marty FM, Baden LR, Katz JT (2008) Human herpesvirus 6 encephalitis. Curr Infect Dis Rep 10: 292-299. [Crossref]
- Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, et al. (2007) Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology 69: 156-165 [Crossref]
- Santoro JD, Hemond CC (2019) Human herpesvirus 6 associated post-transplant acute limbic encephalitis: Clinical observations of biomarkers for risk of seizure in a pediatric population. Transpl Infect Dis 21: e13003 [Crossref]
- Ogata M, Fukuda T, Teshima T (2015) Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant 50: 1030-1036 [Crossref]
- Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, et al. (2009) Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev 31: 731-738. [Crossref]
- Yoshikawa T, Ohashi M, Miyake F, Fujita A, Usui C, et al. (2009) Exanthem subitum-associated encephalitis: nationwide survey in Japan. Pediatr Neurol 41: 353-358. [Crossref]
- Tadokoro R, Okumura A, Nakazawa T, Hara S, Yamakawa Y, et al. (2010) Acute encephalopathy with biphasicseizures and late reduced diffusion associated with hemophagocytic syndrome. Brain Dev 32: 477-81. [Crossref]
- Matsumoto H, Hatanaka D, Ogura Y, Chida A, Nakamura Y, et al. (2011) Severe human herpesvirus 6-associated encephalopathy in three children: analysis of cytokine profiles and the carnitine palmitoyltransferase 2 gene. Pediatr Infect Dis J 30: 999-1001. [Crossref]
- Akasaka M, Sasaki M, Ehara S, Kamei A, Chida S (2005) Transient decrease in cerebral whitematter diffusivity on MR imaging in human herpes virus-6 encephalopathy. Brain Dev 27: 30-33. [Crossref]
- Ahtiluoto S, Mannonen L, Paetau A, A Vaheri, M Koskiniemi, et al. (2000) In situ hybridization detection of human herpesvirus 6 in brain tissue from fatal encephalitis. Pediatrics 105: 431-433. [Crossref]
- Winestone LE, Punn R, Tamaresis JS, Buckingham J, Pinsky BA, et al. (2018) High human herpesvirus 6 viral load in pediatric allogeneic hematopoietic stem cell transplant patients is associated with detection in end organs and high mortality. Pediatr Transplant 22: 10. [Crossref]
- Provenzale JM, vanLandingham KE, Lewis DV, Mukundan S Jr, White LE (2008) Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology 249: 955-963. [Crossref]
- Sadighi Z, Sabin ND, Hayden R, Stewart E, Pillai A (2015) Diagnostic Clues to Human Herpesvirus 6 Encephalitis and Wernicke Encephalopathy After Pediatric Hematopoietic Cell Transplantation. J Child Neurol 30: 1307-1314. [Crossref]
- Oki J, Yoshida H, Tokumitsu A, Takahashi S, Miyamoto A, et al. (1995) Serial neuroimages of acute necrotizing encephalopathy associated with human herpesvirus 6 infection. Brain Dev 17: 356-359. [Crossref]
- Raspall-Chaure M, Armangue T, Elorza I, Sanchez-Montanez A, Vicente-Rasoamalala M, et al. (2012) Epileptic encephalopathy after HHV6 post-transplant acute limbic encephalitis in children: confirmation of a new epilepsy syndrome. Epilepsy Res 105: 419-422. [Crossref]
- Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, et al. (2012) Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant 18: 1638-1648. [Crossref]
- Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, et al. (2007) Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 39: 705-709. [Crossref]
- Nakamura Y, Matsumoto H, Zaha K (2017) A case of Dravet syndrome complicated by human herpesvirus-6 infection-associated acute encephalopathy and choreoathetosis. No To Hattatsu 49: 32-36. [Crossref]
- Kawamura Y, Yamazaki Y, Ohashi M, Ihira M, Yoshikawa T (2013) Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B-associated acute encephalopathy with biphasic seizures and late reduced diffusion. J Med Virol 86: 512-518. [Crossref]
- Tanuma N, Miyata R, Nakajima K, Okumura A, Kubota M, et al. (2014) Changes in cerebrospinal fluid biomarkers in human herpesvirus-6-associated acute encephalopathy/febrile seizures. Mediators Inflamm 2014: 564091. [Crossref]
- Reddy S, Eliassen E, Krueger GR, Das BB (2017) Human herpesvirus 6-induced inflammatory cardiomyopathy in immunocompetent children. Ann Pediatr Card 10: 259-268 [Crossref]
- Bigalke B, Klingel K, May AE, Kandolf R, Gawaz MG (2007) Human herpesvirus 6 subtype A-associated myocarditis with 'apical ballooning'. Can J Cardiol 23: 393-395. [Crossref]
- Yoshikawa T, Ihira M, Suzuki K, Suga S, Kito H, et al. (2001) Fatal acute myocarditis in an infant with human herpesvirus 6 infection. J Clin Pathol 54: 792-795. [Crossref]
- Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, et al. (2006) Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114: 1581-1590. [Crossref]
- Kühl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, et al. (2005) Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112: 1965-1970. [Crossref]
- Stefanski HE, Thibert KA, Pritchett J, Prusty BK, Wagner JE, et al. (2016) Fatal Myocarditis Associated With HHV-6 Following Immunosuppression in Two Children. Pediatrics 137: 2015-1352. [Crossref]
- Brennan Y, Gottlieb DJ, Baewer D, Blyth E (2015) A fatal case of acute HHV-6 myocarditis following allogeneic haemopoietic stem cell transplantation. J Clin Virol 72: 82-84. [Crossref]
- Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, et al. (2011) Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant 46: 863-869. [Crossref]
- Ogata M, Satou T, Inoue Y, Takano K, Ikebe T, et al. (2013) Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transplant 48: 257-264. [Crossref]
- Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, et al. (2006) Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis 193: 68-79. [Crossref]
- Ishida T, Kano Y, Mizukawa Y, Shiohara T (2014) The dynamics of herpesvirus reactivations during and after severe drug eruptions: their relation to the clinical phenotype and therapeutic outcome. Allergy 69: 798-805. [Crossref]


