Background: The exeresis surgery of the brain tumors especially low-grade gliomas is classically manifested by the occurrence of permanent or temporary postoperative neurological deficit. The emergence of new exploration techniques (fMRI, PET…) and surgical protocols; such as craniotomy exeresis in awake conditions; allowing to overcome considerably all deficits. The purpose of our study was to assess the impact of this usual technique in the life quality and survival of patients in our department and to highlight the optimization parameters of functional brain mapping by Direct Electrical Stimulation (DES).
Materials and patients: This is a prospective analytical study of 10 brain tumors cases of low-grade glioma type II, operated by common craniotomy in awake condition, in the department of neurosurgery UPR of IBN SINA Rabat University Hospital Center, accredited by the Word Federation of Neursurgical Societies as being WFNS Rabat Reference Center since 2002.
Results: Our study recorded a considerable improvement regarding the recovery of common postoperative neurological deficits during conventional tumor neurosurgery, particularly low grade gliomas. The Stimulation Threshold Intensity (STI) was 2,305±0.126 mA for sensory motor functions, against 1.335±0.197 mA for cognitive stimulation. The recovery time (RT) of the inhibited function from cessation of exeresis following the induced transient neurological deficit including partial convulsive crises. It varies from minimum RTmin=2,085 s [1,26–3,3] to maximum RTmax=11 s [4,3-11]. There is a statistically significant positive correlation between the peroperative neurological deficit and over-threshold stimulation.
Conclusion: In brain mapping, the optimization of stimulation threshold in DES represents a new standard whose effectiveness is proven scientifically. It has revolutionized the classic paradigm of the occurrence of transient and permanent neurological deficits due to the resection extent, which is done in an awake condition according to functional requirements and no longer according to onco-anatomical limits alone. Indeed, total functional recovery in postoperative care, even with rehabilitation, expresses the effectiveness and efficiency of this technique, for a better understanding of the brain function both in a plastic than connexionist context. Thus, the DES optimization without false negatives towards a sensitivity surrounding 100%, will free a new perspective toward a personalized and maximalist for functional surgical neuro-oncology without malignant transformation.
functional brain mapping, direct electrical stimulation, intraoperative neuroplasticity, awake surgery, low-grade gliomas
The exeresis surgery of the brain tumors especially low-grade gliomas is classically manifested by the occurrence of permanent or temporary postoperative neurological deficit. The emergence of new exploration techniques (fMRI, PET…) and surgical protocols; such as craniotomy exeresis in awake conditions; allowing to overcome considerably all deficits.
The use of craniotomy in awake mode is theoretically justified by maximalist exeresis in functional other than onco-anatomical limits, based on the functional brain mapping technique established by direct electrical sitmulation DES, exploiting the neurophysiological effect of a biphasic electrical current on the neuronal membrane potential of an electrical generator. Brain Mapping is an effective method of exploring functional brain during surgery, allowing an optimization of the total or supra- total tumors massively infiltrating subcortical connectivities in eloquent regions; historically not operable; with a significantly high functional recovery rate, simultaneously improving quality of life and survival medians.
The purpose of our study was to assess the impact of this usual technique in the life quality and survival of patients in our department and to highlight the optimization parameters of functional brain mapping by Direct Electrical Stimulation (DES).
Place of study
This is a prospective analytical study of a body of existing cases of the first 10 cases of low-grade glioma type II brain tumors, operated by common neurosurgical intervention in awaking mode, under ethical conditions and considerations, in the department of neurosurgery UPR of IBN SINA Rabat University Hospital Center, accredited by the Word Federation of Neursurgical Societies as being WFNS Rabat Reference Center since 2002.
The recruitment of patients is done with their consent after detailed explanations; according to the clinical indications objectified by biological, radiological (MRI, fMRI) and an appropriate orthophonic assessment.
Inclusion criteria
low-grade glioma.
Only consenting patients were included in this study after information illuminated, diagnosed carriers of primary brain tumors of intra-axial location and supra-tentorial in eloquent zones, objectified by the realization of an neuroimagery.
Exclusion criteria
This study excludes all patients who have not met the criteria for to be a candidate for awake surgery, namely patients with carriers:
- Tumors in the brainstem.
- Metastases or meningeal tumors.
- Patients operated under awake conditions for malformations were also excluded arteriovenous or as part of epilepsy.
Methods and materials and data collection in order to identify all aspects related to our research theme, our study consisted, initially, of the recruitment of low-grade glioma to their admission and in a second phase the standard brain awake craniotomy.
Functional brain mapping by DES
It is done by direct electrical stimulation (DES) exploiting the neurophysiological effect of a bipolar electrode on the neuronal membrane potential, marked by a "negative" response of the patient following a temporary functional altering of the cortex, located between the two extremities of the bipolar electrode (5 mm spacing probe) [1].
The initial condition
The biphasic electrical current must check the following parameters: Rectangular pulses of 1 ms, Frequency 60 Hz, Intensities of 1 to 6 mA (local anaesthesia), and 4 to 18 mA (general anaesthesia), variable in steps of 0.5 mA, Stimulation time: 1 second (sensorimotor), 4 seconds (cognitive functions)
The boundary conditions
Searching for the Right Stimulation Threshold Intensity (mA) to overcome this limit condition.
The stimulation threshold intensities established for the mapping are between 4 and 18 mA
- Over-threshold stimulation: causes partial and total seizures
- Sub-threshold stimulation: False negative results
Statistical analysis
Statistical analysis was performed by using R software version R-3.6.1; Quantitative variables were expressed in median [quartiles] or on mean±standard deviation (ds).
The Student T-Test and the Pearson product-moment correlation coefficient(PPMCC) were used for the univariate comparison of quantitative variables with a threshold of significance < 0.05.
Ethical considerations
The study was submitted to the Ethics Committee of the Faculty of Medicine and Pharmacy of Rabat for approval. It is done under ethical conditions and considerations, in the department of neurosurgery UPR of IBN SINA Rabat University Hospital Center, accredited by the Word Federation of Neursurgical Societies as being WFNS Rabat Reference Center since 2002.
The DES for each patient reveals that the mean respectively ( median) of Stimulation Threshold Intensity (STI) was 2,305±0.126 mA; (2,325 mA [2 - 2,45]) for sensory motor functions, against 1.335±0.197; (1,325 mA [1-1,65]) for cognitive stimulation, for a mean intensity difference of (0,97±0,07 mA) and 1 mA on median. Thus the correction factor was 1.726 for having the cognitive threshold from the sensory motor threshold value, of the areas that require more intensity to cause a transient deficit subject of positive functional brain mapping. (Figure 1, Table 1).
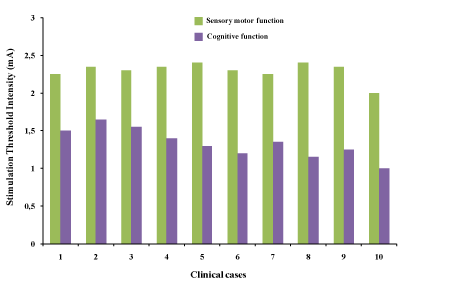
Figure 1. Stimulation Threshold Intensity (STI) distribution during functional brain mapping in awake surgery of our 10 low-grade gliomas according to the brain sensoy motor and cognitive functions
The recovery time (RT) of the inhibited function from cessation of exeresis following the induced transient neurological deficit including partial convulsive crises. It varies from minimum RTmin=2,085 s [1,26–3,3] to maximum RTmax=11 s [4,3-11] on median and 2.107±0.678 to 6.748±2.241 on mean. however in tukey box representation, it is noted as one outlier value for the patient number 10 which shows an aberrant value concerning stimulation threshold intensity in sensory motor as in cognitive functions, justified by the child age of this clinical case (Figure 2).
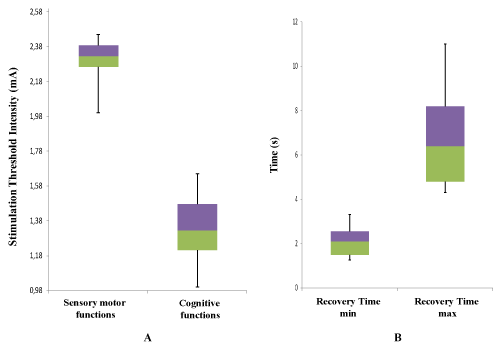
Figure 2. Dispersion of Stimulation Threshold Intensity (STI) and Recovery Time (RT) in Tukey box [median, quartiles, minimum, maximum or deciles] A. Stimulation Threshold Intensity (STI) of positive functional brain mapping for cognitive and sensory motor areas. B. RT extremum (RTmin, RTmax) after intraoperative transient neurological deficit in awake surgery: Recovery time of the inhibited function from cessation of exeresis following the induced transient neurological deficit including partial convulsive crises
The statistical analysis by Student T-test reveals that in 95% confidence interval with significative smaller p-value (6.803 10-13), the best stimulation threshold intensity was into the interval of confidence: [2.215065, 2.394935] with 2.305±0.126 (Mean±sd) in sensory motor mapping, against [1.193879, 1.476121] with 1.335±0.197 (Mean±sd) in cognitive mapping (Table 1).
Table 1. Distribution of our data statistical series by quantitative variables of Student T-Test
|
|
|
Student T-Test
|
|
Type of variability
|
Mean±SD
|
95 % confidence interval
Alternative hypothesis: true mean is not equal to 0
|
t-value
|
df
|
p-value
|
|
Upper
|
Lower
|
|
STI sm, I
|
2.305±0.126
|
2.394935
|
2.215065
|
57.978
|
9
|
6.803.10-13
|
|
STI c, I
|
1.335±0.197
|
1.476121
|
1.193879
|
21.4
|
9
|
4.995.10-9
|
|
RTmin, t
|
2.107±0.678
|
2.592109
|
1.621891
|
9.8254
|
9
|
4.143.10-6
|
|
RTmax, t
|
6.748±2.241
|
8.350768
|
5.145232
|
9.5242
|
9
|
5.361.10-6
|
STIsm: Stimulation threshold intensity of sensory motor function; STIc: Stimulation threshold intensity of cognitive function; I: Intensity (mA); t: Time (s); t-value; df: Degree of freedom; p- value: The mean difference is statistically significant at p<0.05
The analysis of Table 2 revealed a statistically significant positive correlation (P=0.087) between the peroperative neurological deficit and the STI. The best correlation is between the STI sm and the RTmax with a correlation coefficient (CC) of 0.567 versus 0.398 for STIc.
Table 2. Data correlation study of quantitative variables by Student T-Test.
|
Type of variability
|
RT max
|
RT min
|
|
STI sm
|
CC=0.567
p-value=0.087
t-value=1.944
|
CC=- 0.328
p-value=0.354
t-value=- 0.982
|
|
STI c
|
CC=0.398
p-value=0.254
t-value=1.229
|
CC=- 0.162
p-value=0.655
t-value=- 0.462
|
STIsm: Stimulation threshold intensity of sensory motor function; STIc: Stimulation threshold intensity of cognitive function; RT min: Minimum of recovery time; RT max: Maximum of recovery time t-value; CC: Correlation coefficient
The correlation analysis by the Pearson product-moment correlation coefficient (PPMCC) allowed that correlation analysis revealed that there is a correlation between elevation of stimulation threshold intensity and intraoperative neurological deficit.
According to Table 3, for significative p-value (p-value= 0.087) we have a significant proof when a null hypothesis that the slope is null in favor of an alternative different to zero. Thus, the coefficient of determination (Multiple R-squared=0.3209) shows that in intraoperative care, 32.09% of transient neurological deficits incidences are explained by high pacing intensities in sensory motor areas.
Table 3. STIsm - RTmax correlation by Pearson coefficient
|
(STIsm,RTmax)
Correlation
|
Residual standard error
|
Multiple
R-squared
|
Adjusted R-squared
|
F-statistic
|
p-value
|
|
1.958 on 8 degrees of freedom
|
0.3209
|
0.236
|
3.78 on 1 and 8 df
|
0.08776
|
In postoperative care, all functions were totally preserved (2 cases without complications) even with transient deficits in peroperative (10 cases) and postoperative neurological deficits after 10 weeks of reeducation (8 cases). In peroperative care, total convulsive crises (2 cases) were expressed towards the end of exresis surgery in deep eloquent zones marking the functional limit of the tumor (Figure 3).
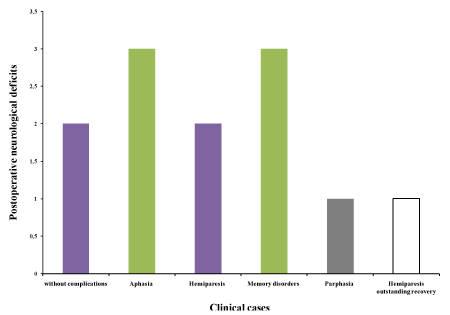
Figure 3. Occurrence of postoperative neurological deficits recovered by reeducation and rehabilitation programs
Obtaining good functional brain mapping is a witness to the success of the DES practice by optimum threshold intensity, it requires a controlled optimization of the DES parameters. Thus, compliance with DES optimization standards has made it possible to have less fluctuating optimal thresholds for both brain functions (Figure 1, Table 1), explaining the occurrence minimum of partial convulsive crises frequency and total convulsive crises number in intraoperative care (2 cases), depending on the stimulation threshold intensity, location and tumor infiltration [2]. So in our study the mean of threshold intensities (Figure 2A) reflects the good results objectified by total recovery of all neurological deficits of which 8 cases with reeducation program, allowing the survival against ever deeper tumors in eloquent areas, despite this, the report of neurological sequelae has largely changed without recidivism in postoperative care but still regularly monitored.
Brain recovers the inhibited function even in partial convulsive crises in a few seconds by momentary cessation of exeresis (Figure 2B), giving the time to reorganize itself in the ipsilesional, contralesional hemispheres and areas homologous or reactivate other neurofunctional pathways within perilesional areas of their previous functions (intraoperative neuroplasticity). The end of exeresis according to the onco-functional limit was marked sometimes by the occurrence of total convulsive crise [3-6].
E. Mandonnet et al, has concludued a short period of 3 s precludes observing any of the aforementioned phenomena whose occurrence is time dependent, including: a delayed onset of deficit, a fast recovery by dynamical short-term plasticity and a late recovery by biological long-term plasticity [7]. In correlation with results of Figure 2B, the functional recovery time varies from 2,085 s [1,26–3,3] observed in cortical and subcortical, to 11s [4,3-11] observed mainly during excision in deeper eloquent areas. This recovery marking intraoperative neuroplasticity is mainly done first by synaptic remodelling of all functional reactivation and then pruning of the branches consolidating the new neurofunctional interconnectivity as part of functional reorganization. This process almost lasts some milliseconds to a few minutes will be made by approximation or removal of existing synapses, appearance of new synapses or strengthening of two synapses interaction [8].
The requirement in functional brain mapping of threshold stimulation outside the boundary conditions, leads us towards an analytical statistical verification (Tables 1 and 2) in order to best ensure correlations between technical parametrs optimization and the post-surgical results obtained. so, there is a significant positive correlation CC=0.567 with (P=0.087) between the peroperative neurological deficit and the STIsm. More to that in intraoperative care, the coefficient of determination (Multiple R-squared=0.3209) indicated that 32.09% of transient neurological deficits incidences mostly occurring by partial or total convulsive crises are caused by high pacing intensities in sensory motor areas, especially cognitive functions which will be stimulated by an intensity almost doubled because increase by a coefficient of 1.76 (Table 3).
Although in a recent comparative correlation, Anthony L. Ritaccio et al, reported terms of satisfaction concerning of DES characteristics with ohter different functional brain mapping techniques, in which has shown reliability, efficiency and lower cost, but which requires better optimization to be able to avoid false functional mapping in order to improve the sensitivity of this technique (Table 4) [9]. However, the DES without false-negative according to strict standards allows to certainly obtain improving sensitivity and therefore to operate with a very high functional safety [10].
Table 4. Characteristics of different functional mapping techniques
|
Technique
|
Cost
|
Expertise
|
Active/Passive
|
Availability
|
|
ESM (DES)
|
+
|
+
|
A
|
+++
|
|
fMRI
Passive ECoG
|
+++
|
+++
|
P
|
++
|
|
++
|
++
|
P
|
++
|
|
TMS
|
++
|
+++
|
A
|
++
|
|
MEG
|
+++
|
+++
|
P
|
+
|
+Minimal; ++Average; +++Maximal; A: Active; ECoG: Electrocarticography; ESM: Electrical stimulation mapping; fMRI: Functional magnetic resonance imaging; MEG: Magnetoencephalography; P: Passive; TMS: Transcranial magetic stimulation
It happens that the DES of an area did not cause an intraoperative deficit (negative mapping), while its surgical excision caused a permanent neurological deficit for the patient "false negative"; due to a lower stimulation intensity than the patient's threshold [11]. As sometimes stimulation causes a neurological deficit (positive mapping) whereas the excision of this region would not have caused a permanent deficit for the patient "false positive" by Triggering a disorganizing electrical post-discharge of the cortex at a distance from the stimulated region and patient tirdness [12,13].
In brain mapping, the optimization of stimulation threshold in DES represents a new standard whose effectiveness is proven scientifically. It has revolutionized the classic paradigm of the occurrence of transient and permanent neurological deficits due to the resection extent, which is done in an awake condition according to functional requirements and no longer according to onco-anatomical limits alone. Indeed, total functional recovery in postoperative care, even with rehabilitation, expresses the effectiveness and efficiency of this technique, for a better understanding of the brain function both in a plastic than connexionist context. Thus, the DES optimization without false negatives towards a sensitivity surrounding 100%, will free a new perspective toward a personalized and maximalist for functional surgical neuro-oncology without malignant transformation.
The data [Exploitation sheet, Clinical informations] used to support the results of this study are available upon request from the corresponding author.
The data used to support the findings of this study are included within the article.
There are no conflicts of interest regarding the publication of this paper.
The research and publication of this article has not received any specific funding and is done by myself.
- Duffau H (2004) Peroperative functional mapping using direct electrical stimulations. Methodological considerations. Neurochirurgie 50: 474-483.
- Purves D, Augustine GJ, Fitzpatrick D, et al. (2001) Editors. Neuroscience. Sunderland (ma).
- Grafman J (2000) Conceptualizing functional neuroplasticity. J Commun Disord 33: 345-356.
- Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor functioning chronic stroke. Ann Neurol 55: 400-409.
- Anglade C, Thiel A, Ansaldo AI (2014) The complementary role of thecerebral hemispheres in recovery from aphasia after stroke: A critical review of literature. Brain Inj 28: 138-145.
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, et al. (2007) Functional MRI language in aphasia: A review of the literature and the methodological challenges. Neuropsychol Rev 17: 157-177.
- Mandonnet E, Duffau H (2011) Intraoperative electrical mapping: advances, limitations and perspectives, brain mapping from neural basis of cognitionto surgical applications. Springer-Verlag/Wien 105: 101 -108.
- Castren E, Hen R (2013) Neuronal plasticity and antidepressantactions. Trends Neurosci 36: 259-267.
- Ritaccio AL, Brunner P (2018) Gerwin Schalk Electrical stimulation mapping of the brain: Basic principles and emerging alternatives. Journal of Clinical Neurophysiology 35.
- Mandonnet E, Winkler PA, Duffau H (2010) Direct electrical stimulation as an input gate into brain functional networks : principles, advantages and limitations. Acta Neurochir (Wien) 152: 185-193.
- Jayakar P (1993) Physiological principles of electrical stimulation. Adv Neurol 63: 17-27.
- Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, et al. (2003) Usefulness of intraoperative electrical subcortical mapping during surgery forlow-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 98: 764-778.
- Ishitobi M, Nakasato N, Suzuki K, Nagamatsu K, Shamoto H, et al. (2000) Remote discharges in the posterior language area during basal temporal stimulation. Neuroreport 11: 2997-3000.



