Abstract
De-escalation of surgery for axillary lymph nodes in breast cancer treatment has advanced following the development of the sentinel lymph node biopsy procedure for clinical axillary lymph node metastasis-negative breast cancer. Sentinel lymph node biopsy procedures following downstaging by neoadjuvant chemotherapy (NAC) in clinical axillary lymph node metastasis-positive cases provide such advancements. However, sentinel lymph node biopsies performed after NAC have a high false negative rate (12.6-14.6%) and are unable to provide a definitive basis for assessing axillary lymph node metastases. Targeted axillary dissection (TAD) is a new method designed to overcome these disadvantages by allowing for more accurate assessment of axillary lymph node metastases following NAC through the placement of clips at lymph nodes with metastasis prior to NAC, and such procedures have been attracting research attention. I125seed labeling and wire localization were frequently performed in prior studies as methods for extracting indwelled clips from lymph nodes, but these methods were considered to be invasive, and their adoption in Japan was not achieved. For this study, we designed a variant of the TAD method involving labeling indwelled lymph node clips using pyoktanin dye (crystal violet), and we believe this method could potentially be adopted in Japan.
Keyword
breast cancer, neoadjuvant chemotherapy, sentinel lymph node biopsy, targeted axillary dissection
The current axillary surgery for breast cancer
Primary breast cancer therapies include localized treatments, such as surgery and radiation therapy, and multidisciplinary approaches, such as a combination of systemic therapies that include hormone therapy, chemotherapy, and molecular-targeted therapy. Localized treatment has gradually decreased, due to advances in systemic therapy. The NSABP B-32 trial demonstrated that in cases that are negative for clinical axillary lymph node metastasis, additional axillary dissection does not affect prognosis if the patient is negative for sentinel lymph node metastasis[1]. Owing to this, the new standard procedure for surgery is not to perform axillary dissection in cases that are negative for clinical axillary lymph node metastasis if the patient is negative for sentinel lymph node metastasis. Additionally, the Z0011 trial demonstrated that axillary dissection can be avoided under the following condition: if there are two or fewer macro-metastases to the sentinel lymph nodes, then breast-conserving surgery is performed when the tumor T stage is T2 or below, and appropriate systemic therapy is introduced [2].
Downstaging by neoadjuvant chemotherapy for clinical node-positive breast cancer
In cases that are negative for clinical axillary lymph node metastasis, de-escalation of axillary treatment is progressing. However, axillary dissection is still the standard axillary treatment in cases where axillary lymph node metastasis has been observed clinically.
For some time, downstaging through neoadjuvant chemotherapy(NAC) has been attempted as a means to eliminate axillary dissection in cases that are positive for axillary lymph node metastasis. The original goal of NAC was downstaging, in order to perform radical surgery for treating inoperable advanced breast cancer. NAC applications are now expanding to operable early-stage breast cancer as well.
The following are two major overseas clinical trials on sentinel lymph node biopsy following NAC. The SENTINA trial studied sentinel lymph node biopsy in cases where neoadjuvant chemotherapy was performed [3].Within the study, Arm C was a group of patients who were positive for clinical axillary lymph node metastasis and underwent sentinel lymph node biopsy after NAC was performed (N=592). In this group, the rate of false negatives for sentinel lymph node biopsies was 14.2% (32/226). However, the rate of false negatives was 8.6% (6/70) in cases where three or more sentinel lymph nodes were successfully excised intraoperatively.The ACOSOG Z1071 trial studied sentinel lymph node biopsy following NAC in cases that were positive for clinical axillary lymph node metastasis, as in Arm C of the SENTINA trial [4].Among the 701 cases studied, the rate of false negatives was 12.6% (39/310). Additionally, in this trial, the rate of false negatives was 9.1% in cases where three or more sentinel lymph nodes were successfully excised.Within Japan, Enokido et al. conducted a study of 143 cases at eight facilities that analyzed sentinel lymph node biopsy following NAC in cases that were positive for clinical axillary lymph node metastasis [5]. The rate of false negatives for all cases was 16.0% (13/81), a higher value than the two studies mentioned previously. Although the rate of false negatives was not stated for each possible number of sentinel lymph nodes excised, the overall rate was 9.1% (13/143) in cases where three or more sentinel lymph nodes were successfully excised, and this is presumed to be the cause of the high false negative rate.
In all three studies, the rate of false negatives was high, and it was considered safe to omit axillary dissection in over 10% of cases. However, the results of the ACOSOG Z1071 and SENTINA trials showed that the rate of false negatives decreased in cases where three or more sentinel lymph nodes were successfully excised [3,4]. Based on this, we believe that, in cases that are positive for clinical axillary lymph node metastasis, it is better to excise as many sentinel lymph nodes (preferably three or more) as possible in order to achieve highly accurate axillary lymph node analysis following NAC. However, due to blockage caused by lymph duct cancer cells or scarring in lymph nodes that had already metastasized, it is often difficult to evaluate sentinel lymph node biopsies following NAC in cases that were positive for clinical axillary lymph node metastasis, and the rate of identification is about 87.8% to 92.7%[3-5]. Furthermore, in cases where three or more sentinel lymph nodes were successfully excised, the rates were 34% (201/592) in the SENTINA trial, 56.3% (388/689) in the Z1071 trial, and 9.1% in the Enokido study. Based on this observation, it is believed that even when downstaging with NAC is performed for cases that are positive for clinical axillary lymph node metastases, axillary dissection can only be omitted in a limited number of cases.
Targeted axillary dissection
In a clinical trial by Caudle et al. for patients who were positive for clinical axillary lymph node metastasis, NAC was performed after attaching clips to axillary lymph nodes with metastasis, and the clipped lymph nodes were excised while performing a sentinel lymph node biopsy using the regular dual tracer method after NAC [6].Although the rate of false negatives was 10.1% (7/69) when only sentinel lymph nodes were evaluated, the rate of false negatives changed to 1.4% (1/74) when evaluation of the clipped lymph nodes was added. This demonstrated a major improvement.
Additionally, for the 170 cases in the previously mentioned Z1071 trial, the rate of false negatives improved to 7.2% when clips were attached to lymph nodes in which metastasis was observed before NAC, and the evaluation of clipped lymph nodes was performed during surgery.As described here, the methods in which axillary lymph nodes with prior metastasis are excised after NAC, and axillary lymph node metastases are evaluated, is referred to as targeted axillary dissection (TAD). Because the National Comprehensive Cancer Network guidelines also recommend the omission of axillary dissection after NAC in cases that are positive for clinical axillary lymph node metastases, we can expect this to become a standard treatment in Japan as well.However, the clips attached to lymph nodes are usually under 5 mm in size and are often difficult to visually identify during surgery. In the previously mentioned Z1071 trial, out of 170 cases with clip placement, 107 sentinel lymph nodes were identified as clipped lymph nodes during surgery[7]. Among the remaining cases, clips were not found in the 34 lymph nodes that remained after axillary dissection and the 29 lymph nodes that were removed during sentinel lymph node or axillary dissections. The method used by Abigail involves inserting I125 seed into lymph nodes clipped preoperatively and locating the clips under the guide of a navigator. This method produces an intraoperative identification rate for clipped lymph nodes of 97.5%, extremely high rate. Similarly, Donker et al. performed TAD using I125 seeds and produced positive results, demonstrating an identification rate of 97% (97/100)[8].The approach used in these studies is referred to as the MARI (Marking Axillary Lymph Node with Radioactive Iodine Seed) procedure.
The results of TAD for each clinical trial are shown in table 1.Taking into account the studies by Abigail and Donker, marking tumors with I125 seeds is essential for performing TAD[6,7]. However, in Japan, I125 seed is only used for therapeutic purposes such as radiation therapy for prostate cancer, and exposes patients to radiation. As such, it will likely be difficult for I125 seed methodologies to be accepted in Japan. Plecha et al. have reported results regarding TAD that use wire localization on clipped lymph nodes [9].In cases where lymph nodes clipped before NAC were preoperatively localized with wires, the TAD success rate was 97.4% (71/73), compared to 79.4% (27/34) in cases where this was not performed. Eun et al. also published a report on the results of TAD using wire localization. Their study included 20 subjects, and a total of 24 clips were placed, with two clips placed in four of the subjects. Twenty-three clips were identified during surgery, and the TAD success rate, as a result of wire localization use, was 95.8% (23/24). In addition to confirming the feasibility of TAD, the use of wire localization allows for multiple lymph node TAD operations in a single case.Table 1 shows the results of each study concerning TAD. As a result of the use of I125seed and wire localization, all TAD success rates were greater than90% [10].
Table 1: Clipped note detection and false negative rates in major studies related to TAD.
Source | No. of case | Method of Detection clipped node | False negative rate with TAD | Detection rate of clipped node |
Boughey et al. [4] | 140 | Not mentioned | 6.80% | 62.90% |
Donker et al. [8] | 100 | Insert l125Seed | 7% | 97.00% |
Caudle et al. [6] | 208 | Insert l125Seed | 4.20% | 97.50% |
Plecha et al. [9] | 107 | Wire localiation | Not mentioned | 97.30% |
Kim et al. [10] | 20 | Wire localiation | Not mentioned | 95.80% |
Total 24 clip inserted
As described, although research on TAD and the excision of clipped lymph nodes has been actively undertaken overseas, TAD is not yet widespread in Japan, and TAD techniques for Japanese individuals have not yet been established. Additionally, because wire localization and I125 seed placement are very invasive, it will likely be difficult for such procedures to be accepted in Japan.
Our study related to TAD
The clinical study we are conducting is a Phase 1 clinical trial that adopts pyoktanin dye (crystal violet) marking as a method for excising clipped lymph nodes, in order to conduct TAD in Japan. The patient group is composed of patients with breast cancer, aged 18 years and above, with tumors having T stages of T1-T3 and N stage of N1, without distant metastasis. Selected patientswere planning to have standard neoadjuvant chemotherapy using sequential dosing of anthracyclines and taxanes. Patients who are [regnant or breastfeeding, and patients with bilateral breast cancer (whether at the same time or not),were excluded from the study.
Inclusion and exclusion criteria, as well as an overview of the study, are provided in figure1. The first step of the study is to clip axillary lymph nodes in which metastasis were observed. Histological diagnosis using pathological cytology is required to diagnose axillary lymph node metastasis.The second step is to perform what is currently considered the standard treatment: neoadjuvant chemotherapy using sequential dosing of anthracyclines and taxanes. In HER2-type cases, we addedtrastuzumab. Patients were excluded if the primary lesion or lymph nodes enlarged when neoadjuvant chemotherapy was performed.
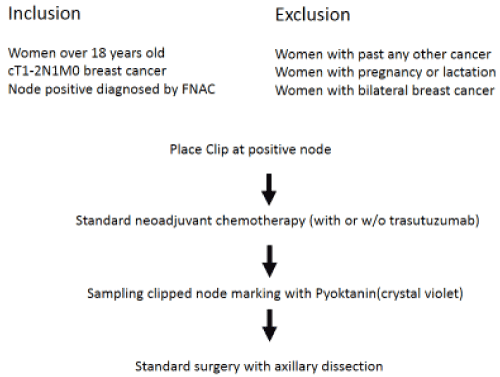
Figure 1: Study design
Following neoadjuvant chemotherapy, standard surgeries, including axillary dissection,were performed. Sampling was conducted during surgery by identifying clipped lymph nodes through ultrasound, then making lymph nodes visible through pyoktanin marking. The specimens sampled during surgery underwent X-ray analysis, and TAD was considered a success if a clip was confirmed.The outcome was TAD success rate in cases where TAD was attempted. The planned number of cases was 15, and an overall success rate of 95% or above was expected.Once the target number of subjects was reached and the TAD success rate was deemed sufficient, the next step was to eliminate axillary dissection using TAD.
Authorship and contributorship
Writing of manuscript: Masaru Takemae
Study design:Masaru Takemae, Jiro Ando, Michiko Harao, Nobuo Hoshi
Audit of study:Masahiro Wada
Approval of study:Takao Inada
Acknowledgement
Our study funded by Tochigi Cancer Center.
References
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, et al. (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11:927-933. [Crossref]
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, et al. (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569-575. [Crossref]
- Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, et al. (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14: 609-618. [Crossref]
- Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, et al. (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 310: 1455-1461. [Crossref]
- Enokido K, Watanabe C, Nakamura S, Ogiya A, Osako T, et al. (2016) Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy in Patients With an Initial Diagnosis of Cytology-Proven Lymph Node-Positive Breast Cancer. Clin Breast Cancer 16: 299-304.[Crossref]
- Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, et al. (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J ClinOncol 34: 1072-1078. [Crossref]
- Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, et al. (2015) Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: Results from ACOSOG Z1071 (Alliance). Ann Surg263:802-7.8) [Crossref]
- Donker M, Straver ME, Wesseling J, Loo CE, Schot M, et al. (2015) Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 261: 378-382. [Crossref]
- Plecha D, Bai S, Patterson H, Thompson C, Shenk R (2015) Improving the accuracy of axillary lymph node surgery in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann SurgOncol 22: 4241-4246. [Crossref]
- Kim EY, Byon WS, Lee KH, Yun JS, Park YL, et al. (2018) Feasibility of Preoperative Axillary Lymph Node Marking with a Clip in Breast Cancer Patient Before Neoadjuvant Chemotherapy: A Preliminary Study. World J Surg 42:582-589. [Crossref]

