Abstract
Objective: Reveal factors influencing sleep disorders in cancer patients receiving outpatient chemotherapy.
Method: The Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) self-report questionnaire was used to assess patients receiving outpatient chemotherapy for cancers of the blood and digestive organs. The PSQI-J comprises seven components (0~3 points) with total scores ranging from 0~21 points; a score of 6 points or more is deemed to indicate a sleep disorder. Influencing factors were analyzed using the Mann-Whitney U test. The result was judged to be statistically significant when p<0.05.PASW Statistics 22 was used for statistical analysis. Approval for the study was obtained from the Fukuoka University Institutional Review Board.
Results: Forty-three subjects participated in the study, of whom 62.8% were male and 86.0% were being treated for cancers of the digestive organs. The average age of the subjects was 56.1 years (±8.6). Average PSQI total score was 5.0 (±2.8), with sleep disorders indicated in 30.2%. Average scores for the components were as follows: subjective sleep quality 1.1 (±0.6), sleep latency 1.2 (±1.1), sleep disturbances 1.0 (±0.3), sleep duration 0.6 (±0.7), sleep efficiency 0.3 (±0.6), use of hypnotic agents 0.4 (±1.0), and daytime dysfunction 0.4 (±0.7). Participants with a sleep disorder had total PSQI score of 8.54(±2.03). Sleep disorders were significant in subjects with skin or taste disorders or use of hypnotic agents; in these subjects, sleep duration was 5.8(±1.5) hours, sleep latency was 46.2(±27.2) minutes, and their sleep efficiency was low. The ratio of using hypnotic agents was 13.9%, however, most of them had not improve sleep disorders.
Key words
outpatient chemotherapy, sleep disorders, side-effect symptom, PSQI-J
Introduction
Sleep plays an essential role in good health, both mentally and physically. However, cancer patients suffer from sleep disorders at the high frequency of thirty to fifty percent [1]. It has been reported that among patients receiving outpatient chemotherapy, 12.5%of patients have the symptoms of a sleep disorder and 25.8% of patients use hypnotic agents [2].
In recent years, the number of patients receiving outpatient chemotherapy has been increasing due to the development of anticancer agents, the improvement of supportive therapies and the additional fees for outpatient chemotherapy in the health care fee system. Outpatient chemotherapy is adopted by patients with side-effect symptoms up to grade two and with proper symptom management. However, chemotherapy requires a long-term treatment and patients receiving outpatient chemotherapy have troubles with pain and stress. Even a slight side-effect could turn into chronic pain and varied stresses and anxieties could arise, such as the financial burden of treatments, job problems and fear of cancer progression, which could induce sleep disorders. Patients could face a decline in Quality of Life.
It seems that patients receiving outpatient chemotherapy, in comparison with hospitalized patients, live at home maintaining their normal lives, and less sleep disorders are expected. However, not many investigations on outpatients sleep disorders have yet been reported, and evidence is not sufficient to build up a support system for outpatients. Thus, correlations between actual conditions and the related factors of sleep disorder must be clarified for urgent need to support patients who are undergoing outpatient chemotherapy treatment.
The objective of this study is to reveal the relations with patients undergoing outpatient chemotherapy between sleep disorders and the related side-effect symptoms.
Methods
Subjects
Subjects were patients who were receiving outpatient chemotherapy treatment in the Oncology Center of Fukuoka University Hospital. Participants fulfilled the following conditions:
ⅰ) to be diagnosed with hematological malignancy or solid carcinoma.
ⅱ) to be informed of the disease name.
ⅲ) to be treated mainly with chemotherapy and supportive therapy.
ⅳ) to be alert, conscious and capable of smooth communication.
ⅴ) to be adults in the age range of twenty to sixty-five.
Participants agreed to participate with informed consent documents after understanding the purpose of this study.
Methods of the survey
The survey was conducted, employing registered type self-report questionnaire by patients who received or waited for outpatient chemotherapy treatment. This survey was conducted with the consent of participants where they were informed verbally and in writing, of the purpose and methods of this study, ethical standard and then consented to participate in this research in writing with their signature. Participants were asked to fulfill questionnaires and drop them into a document collection box which was locked by a key, and was placed in the outpatient area of the Oncology Center of Fukuoka University Hospital.
Contents of the survey
Individual factors: Individual factors are age, gender, types of cancer, stage, treatment regimen, the presence or absence of prescription and its contents of a hypnotic agent, steroid and sedative, and side-effects, which were obtained through electronic health records. Fourteen side-effect symptoms were evaluated by Common Terminology Criteria for Adverse Events (CTCAE) v4.0 Japanese JCOG edition [3]. Performance Status (PS) was evaluated by the Eastern Cooperative Oncology Group (ECOG) [4].
Sleep disorder: “Sleep” in the survey was evaluated by the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) [5], which Doi et al. formed. PSQI-J consists of seven components and eighteen items: “sleep quality,” “sleep latency,” “sleep duration,” “sleep efficiency,” “sleep disturbance,” “use of hypnotic agent” and “daytime dysfunction due to sleepless”. Quantitative and qualitative information is involved and evaluated by Likert scale. Subscales yield a score from 0 to 3 and are calculated to yield a total score ranging from 0 to 21. The higher total score equates to a lower quality of sleep. With a total score of six or higher, the quality of sleep is assessed as sleep disorder. This scale has been proven reliable and valid and has been standardized.
Analysis methods: The evaluations of individual factors and side-effects were shown by frequency distribution. The qualitative evaluations of sleep were shown by descriptive statistics values from the data; calculations of subscale scores and total scores of PSQI-J. The quantitative evaluations of sleep were shown by descriptive statistics value from the written data; calculation of sleep latency and sleep duration. Data of side-effects were classified into two groups, the no side-effect group of Grade zero and the side-effect group of Grade one-three. Comparisons of Quantitative sleep evaluations were shown in individual factors and in the presence or absence of a sleep disorder. Comparisons of qualitative sleep evaluations were shown in individual factors, in the presence or absence of side-effects and in the presence or absence of sleep disorder.
Data were statistically analyzed using the Mann-Whitney U test. The result was judged to be statistically significant when p<0.05. PASW Statistics ver. 22 for Windows was employed as statistic analysis software.
Ethical considerations: Approval for the study was obtained from the Fukuoka University Institutional Review Board (registration number: 16-0-05). This researcher informed subjects, verbally and in writing, using an explanation paper and questionnaire. The following were explained to subjects; what the purpose and methods of this study were, participation was based on voluntary consent to cooperate in the survey, no demerit would occur due to refusal of participation, personal information was kept confidential, how data were stored and secured, what the possible discomforts or risks were and how they were dealt with, what benefits or interests were, how survey results would be publicized and what the conflicts of interests was.
Results
Outline of participants
In this study, fifty patients consented to participate in this survey. Questionnaires of forty-seven participants were returned (94 % of recovery rate). Subjects for analysis were forty-three, after eliminating four data sets due to omission of recording. Age range was from thirty-six to sixty-five: the mean value, 56.1(±8.6). The proportion of male was 62.8 % (twenty-seven participants) and the proportion of female was 37.2 % (sixteen participants). As for types of cancer, there were seven participants with blood cancer, thirty-five with digestive organs cancer and one with breast cancer. In stage of cancer, seven participants (16.3%) were in the second stage, nine participants (20.9%) in the third stage, twenty-four participants (55.8%) in the fourth stage and one participant was unknown. Further, eleven participants (25.6%) were with Performance Status (PS) of 1 and thirty-two participants (74.4%) were with PS of 1. It was recognized that six participants (13.9%) took hypnotic agents, twenty-nine (67.4%) took steroids and twenty-one (48.8%) took analgesic; these three were administered orally (Table 1).
|
|
Variable
|
n
|
%
|
|
|
Age
|
30~39
|
4
|
9.3%
|
mean(SD) 56.1(±8.6)
|
|
40~49
|
5
|
11.6%
|
|
50~59
|
15
|
34.9%
|
|
60~69
|
19
|
44.2%
|
|
Gender
|
Male
|
27
|
62.8%
|
|
|
Female
|
16
|
37.2%
|
|
Types of Cancer
|
Digestive organs
|
35
|
81.4%
|
colorectal 21
|
|
pancreatic 6
|
|
gastric 5
|
|
duodenum 3
|
|
Breast
|
1
|
2.3%
|
|
|
Blood
|
7
|
16.3%
|
|
|
Stage
|
Ⅱ
|
7
|
16.3%
|
|
|
Ⅲ
|
9
|
20.9%
|
|
Ⅳ
|
24
|
55.8%
|
|
unkown
|
1
|
2.3%
|
|
PS
|
0
|
11
|
25.6%
|
|
|
1
|
32
|
74.4%
|
|
2
|
0
|
0.0%
|
Table 1. Outline of the patients
Evaluation of side-effect symptoms due to chemotherapy
All of the side-effects were recognized in Grade one. Four of the fourteen items are shown in descending order as follows: peripheral neuropathy of twenty participants (46.5%), anorexia of nineteen (44.2%), skin disorder of seventeen (39.5%) and constipation of fifteen (34.9%). Grade two had seven items: peripheral neuropathy of three participants (7%) and anorexia of three (7%), which were fewer than in Grade one. In Grade three, only one item, leukopenia of one participant (2.3%) was recognized (Table 2).
Table 2. Evaluation of the side-effect symptom by a chemotherapy
|
Grade
|
Leukopenia
|
Thrombocytopenia
|
Anemia
|
Fatigue
|
Pain
|
Nausea · vomiting
|
Oral mucosa disorder
|
Peripheral neuropathy
|
Skin disorder
|
Taste disorder
|
Constipation
|
Diarrhea
|
Anorexia
|
Dapilation
|
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
|
0
|
38
|
88.4
|
30
|
69.8
|
28
|
65.1
|
27
|
67.4
|
31
|
72.1
|
33
|
76.7
|
36
|
83.7
|
20
|
46.5
|
26
|
60.5
|
36
|
83.7
|
28
|
65.1
|
34
|
79.1
|
21
|
48.8
|
39
|
90.7
|
|
1
|
2
|
4.7
|
12
|
27.9
|
13
|
30.2
|
15
|
30.2
|
11
|
25.6
|
8
|
18.6
|
7
|
16.3
|
20
|
46.5
|
17
|
39.5
|
7
|
16.3
|
15
|
34.9
|
9
|
20.9
|
19
|
44.2
|
4
|
9.3
|
|
2
|
2
|
4.7
|
1
|
2.3
|
1
|
2.3
|
1
|
2.3
|
1
|
2.3
|
2
|
4.7
|
0
|
0.0
|
3
|
7.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
3
|
7.0
|
0
|
0.0
|
|
3
|
1
|
2.3
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
0
|
0.0
|
Quantitative sleep evaluation of the Pittsburgh Sleep Quality Index-J, PSQI-J
Quantitative sleep evaluation of PSQI-J indicated that the mean value of sleep duration was 6.7 (±1.1) hours: eighteen participants (41.8%) had sleep duration from three hours to less than seven hours, fifteen (34.8%) had from seven hours to less than eight hours, ten (23.2%) had from eight hours to less than nine hours. The mean value of sleep latency was 25.2 (±22.2) minutes: twenty-four participants (56%) had sleep latency less than thirty minutes, twelve (27.9%) had from thirty minutes to less than sixty minutes, and seven (16.3%) had over sixty minutes. The mean value of sleep efficiency was 93.8% (±17.8): twenty-nine participants (67%) had sleep efficiency over 90%, five (11.6%) had from 80% to less than 90%, six (14%) had from 70% to less than 80% and three (6.9%) had from 60% to less than 70% (Figure 1,2).
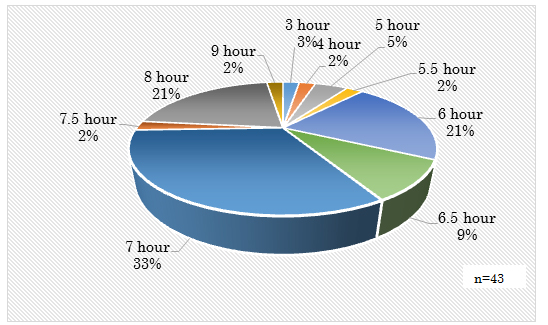
Figure 1: Objective evaluation of duration sleep
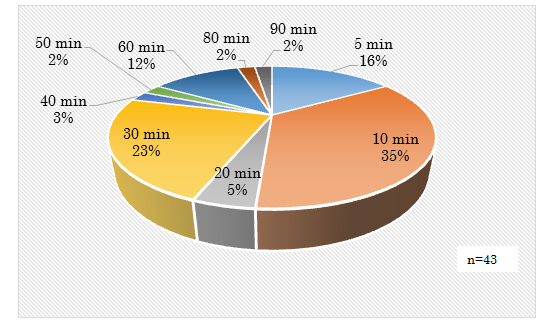
Figure 2: Objective evaluation of Sleep latency
Qualitative sleep evaluation and sleep disorders evaluation of PSQI-J
The mean value of total scores of PSQI-J was 5.0 (±2.8). In mean values of subscales, sleep quality was 1.1(±0.6), sleep latency was 1.2(±1.1), sleep disturbance was 1.0(±0.3), sleep duration was 0.6 (±0.7), sleep efficiency was 0.3 (±0.6), use of hypnotic agent was 0.4 (±1.0), and daytime dysfunction due to sleepless was 0.4(±0.7). Thirteen participants (30.2%) were recognized as having a sleep disorder, which had a six or higher total PSQI score.
Participants with a sleep disorder had total PSQI score of 8.54(±2.03), sleep quality of 1.54(±0.66), sleep latency of 2.15 (±1.28), sleep duration of 1.23 (±1.01) and sleep efficiency of 0.77(±0.83). On the other hand, participants who did not have a sleep disorder had a total PSQI score of 3.47 (±1.41), sleep quality of 0.93(±0.45), sleep latency of 0.73(±0.74), sleep duration of 0.30(±0.47) and sleep efficiency of 0.17 (±0.46). The mean values of participants with a sleep disorder were significantly higher than that of participants without sleep disorder in sleep quality, sleep latency, sleep duration and sleep efficiency (Table 3).
Table 3. Qualitative evaluation and Sleep disorders by PSQI-J
|
n
|
Total
|
Sleep disorders
|
|
No
|
Yes
|
|
|
43
|
|
30
|
13
|
|
|
|
mean
|
SD
|
mean
|
SD
|
mean
|
SD
|
p
|
|
Sleep quality
|
1.12
|
0.59
|
0.93
|
0.45
|
1.54
|
0.66
|
0.006**
|
|
Sleep latency
|
1.16
|
1.13
|
0.73
|
0.74
|
2.15
|
1.28
|
0.001***
|
|
Sleep duration
|
0.58
|
0.79
|
0.30
|
0.47
|
1.23
|
1.01
|
0.003**
|
|
Sleep efficiency
|
0.35
|
0.65
|
0.17
|
0.46
|
0.77
|
0.83
|
0.03*
|
|
Sleep disturbance
|
0.98
|
0.27
|
0.93
|
0.25
|
1.08
|
0.28
|
0.488
|
|
Use of hypnotic agent
|
0.37
|
0.98
|
0.03
|
0.18
|
1.15
|
1.52
|
0.062
|
|
Daytime dysfunction
|
0.44
|
0.70
|
0.37
|
0.56
|
0.62
|
0.96
|
0.648
|
|
PSQI total score
|
5.00
|
2.85
|
3.47
|
1.41
|
8.54
|
2.03
|
0***
|
Mann-Whiyney U-test
*p<0.05 **p<0.01 ***p<0.001
Comparison of quantitative sleep evaluations based on individual factors and sleep disorder
A significant difference was recognized in the sleep latency between 46.2 (±27.2) minutes of participants with sleep disorder and 16.2 (±11.2) minutes of participants without sleep disorder. There was no significant difference in sleep latency due to hypnotic agent, while participants taking a hypnotic agent had 42.0 (±21.7) minutes longer than participants not taking a hypnotic agent.
A significant difference was recognized in the sleep duration between 5.8 (±1.5) hours of patients with sleep disorder and 7.1 (±0.7) hours of patients without sleep disorder. Sleep efficiency showed a significant difference between 83.9% (±13.5) and 98.1% (±17.8) respectively. Participants who had sleep disorder symptoms showed longer sleep latency and shorter sleep duration. It was confirmed that participants with a sleep disorder had low quantity and efficiency of sleep (Table 4).
Table 4. Comparison of quantitative evaluation of the sleep by Individual Factors
|
|
|
total
|
Sleep disorder
|
|
Sex
|
|
Types of Cancer
|
|
Hypnotic agent
|
|
|
|
|
No
|
Yes
|
|
Male
|
Female
|
|
Blood
|
Solid
|
|
No
|
Yes
|
|
|
|
n
|
43
|
30
|
13
|
|
27
|
16
|
|
7
|
36
|
|
38
|
5
|
|
|
|
unit
|
mean
|
SD
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
|
sleep latency
|
minute
|
25.2
|
22.2
|
16.2
|
11.2
|
46.2
|
27.2
|
0.000 ***
|
22.0
|
21.4
|
30.6
|
23.2
|
0.136
|
30.0
|
32.1
|
24.3
|
20.2
|
0.711
|
23.0
|
21.6
|
42.0
|
21.7
|
0.065
|
|
duration sleep
|
hour
|
6.7
|
1.1
|
7.1
|
0.7
|
5.8
|
1.5
|
0.000 ***
|
6.7
|
1.2
|
6.8
|
1.0
|
0.918
|
6.3
|
0.8
|
6.8
|
1.2
|
0.156
|
6.7
|
1.1
|
7.0
|
1.2
|
0.898
|
|
sleep efficiency
|
%
|
93.8
|
17.8
|
98.1
|
17.8
|
83.9
|
13.5
|
0.007 ***
|
92.1
|
13.2
|
96.7
|
23.8
|
0.938
|
86.1
|
10.7
|
95.3
|
18.6
|
0.112
|
93.8
|
18.7
|
94.1
|
8.9
|
0.898
|
Mann-Whitney U-test
***p<0.001 **p<0.01
Comparison of qualitative sleep evaluations based on individual factors and side-effects
Based on the types of cancer, there was no significant difference in sleep quality of PSQI-J subscales between patients with blood cancer and patients with solid cancer, while the sleep quality of participants with blood cancer showed 1.57 (±0.53) lower than that of participants with solid cancer 1.03(±0.56). Contrarily, in comparing the total PSQI scores, participants using a hypnotic agent had a significantly higher score 8.60 (±0.89) than participants not taking hypnotic agent 4.53 (±2.67). Patients who receive outpatient chemotherapy and use a hypnotic agent showed a higher score and had sleep disorder symptoms (Table 5).
Table 5. Comparison of qualitative evaluation of sleep by individual factors
|
|
Types of cancer
|
Sex
|
PS
|
Hypnotic agent
|
|
|
Blood
|
Solid
|
|
Male
|
Femal
|
|
0
|
1
|
|
No
|
Yes
|
|
|
n
|
7
|
36
|
|
27
|
16
|
|
11
|
32
|
|
38
|
5
|
|
|
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
|
Sleep quality
|
1.57
|
0.53
|
1.03
|
0.56
|
0.05
|
1.07
|
0.62
|
1.19
|
0.54
|
0.561
|
0.91
|
0.54
|
1.19
|
0.59
|
0.252
|
1.05
|
0.57
|
1.60
|
0.55
|
0.092
|
|
Sleep latency
|
1.29
|
1.38
|
1.14
|
1.10
|
0.89
|
1.00
|
1.18
|
1.44
|
1.03
|
0.149
|
1.45
|
1.21
|
1.06
|
1.11
|
0.356
|
1.03
|
1.05
|
2.20
|
1.30
|
0.065
|
|
Sleep duration
|
0.86
|
0.69
|
0.53
|
0.81
|
0.2
|
0.56
|
0.75
|
0.63
|
0.89
|
0.921
|
0.27
|
0.47
|
0.69
|
0.86
|
0.209
|
0.61
|
0.82
|
0.40
|
0.55
|
0.755
|
|
Sleep efficiency
|
0.43
|
0.53
|
0.33
|
0.68
|
0.53
|
0.33
|
0.68
|
0.38
|
0.62
|
0.622
|
0.36
|
0.67
|
0.34
|
0.65
|
0.924
|
0.39
|
0.68
|
0.00
|
0.00
|
0.308
|
|
Sleep disturbance
|
1.00
|
0.58
|
0.97
|
0.17
|
0.94
|
0.96
|
0.34
|
1.00
|
0.00
|
0.649
|
0.91
|
0.30
|
1.00
|
0.25
|
0.671
|
0.97
|
0.28
|
1.00
|
0.00
|
0.927
|
|
Use of hypnotic agent
|
0.43
|
1.13
|
0.36
|
0.96
|
0.99
|
0.37
|
0.97
|
0.38
|
1.02
|
0.867
|
0.55
|
1.21
|
0.31
|
0.90
|
0.773
|
0.03
|
0.16
|
3.00
|
0.00
|
0.000***
|
|
Daytime dysfunction
|
0.57
|
1.13
|
0.42
|
0.60
|
0.91
|
0.44
|
0.80
|
0.44
|
0.51
|
0.549
|
0.09
|
0.30
|
0.56
|
0.76
|
0.082
|
0.45
|
0.72
|
0.40
|
0.55
|
0.927
|
|
PSQI total score
|
6.14
|
3.89
|
4.78
|
2.61
|
0.34
|
4.74
|
3.02
|
5.44
|
2.56
|
0.298
|
4.55
|
2.58
|
5.16
|
2.95
|
0.732
|
4.53
|
2.67
|
8.60
|
0.89
|
0.002**
|
Mann-Whiyney U-test
*p<0.05 **p<0.01 ***p<0.001
In examining relations between PSQI score totals and side-effects due to chemotherapy, the presence or absence of skin and taste disorders significantly affected the total score. The total score mean value of participants with a skin disorder 6.24 (±2.93) was significantly higher than that of participants without a skin disorder 4.19 (±2.53). Furthermore, participants who had a taste disorder side-effect showed a significantly higher mean value of total score 7.14 (±3.39) than participants who did not 4.58 (±2.58). Participants with both a skin and taste disorder had higher total PSQI scores and had sleep disorder symptoms (Table 6).
Table 6. Comparison of subjective sleep due to symptoms
|
|
Skin disorder
|
Oral mucosa disorder
|
Taste disorder
|
Constipation
|
Diarrhea
|
|
|
No
|
Yes
|
|
No
|
Yes
|
|
No
|
Yes
|
|
No
|
Yes
|
|
No
|
Yes
|
|
|
n
|
26
|
17
|
|
36
|
7
|
|
36
|
7
|
|
28
|
15
|
|
34
|
9
|
|
|
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
|
Sleep quality
|
1.12
|
0.52
|
1.12
|
0.70
|
0.93
|
1.08
|
0.60
|
1.29
|
0.49
|
0.508
|
1.06
|
0.58
|
1.43
|
0.53
|
0.198
|
1.00
|
0.61
|
1.33
|
0.49
|
0.082
|
1.15
|
0.56
|
1.00
|
0.71
|
0.607
|
|
Sleep latency
|
0.92
|
0.98
|
1.53
|
1.28
|
0.123
|
1.14
|
1.15
|
1.29
|
1.11
|
0.711
|
1.08
|
1.05
|
1.57
|
1.51
|
0.488
|
1.07
|
1.18
|
1.33
|
1.05
|
0.345
|
1.15
|
1.13
|
1.22
|
1.20
|
0.872
|
|
Sleep duration
|
0.46
|
0.71
|
0.76
|
0.90
|
0.244
|
0.53
|
0.84
|
0.86
|
0.38
|
0.097
|
0.47
|
0.70
|
1.14
|
1.07
|
0.104
|
0.64
|
0.78
|
0.47
|
0.83
|
0.321
|
0.59
|
0.86
|
0.56
|
0.53
|
0.736
|
|
Sleep efficiency
|
0.23
|
0.51
|
0.53
|
0.80
|
0.193
|
0.36
|
0.68
|
0.29
|
0.49
|
1
|
0.31
|
0.62
|
0.57
|
0.79
|
0.41
|
0.50
|
0.75
|
0.07
|
0.26
|
0.036*
|
0.38
|
0.65
|
0.22
|
0.67
|
0.471
|
|
Sleep disturbance
|
0.92
|
0.27
|
1.06
|
0.24
|
0.103
|
0.97
|
0.29
|
1.00
|
0.00
|
0.91
|
0.94
|
0.23
|
1.14
|
0.38
|
0.448
|
1.00
|
0.27
|
0.93
|
0.26
|
0.432
|
0.97
|
0.30
|
1.00
|
0.00
|
0.895
|
|
use of hypnotic agent
|
0.27
|
0.83
|
0.53
|
1.18
|
0.535
|
0.33
|
0.96
|
0.57
|
1.13
|
0.529
|
0.36
|
0.96
|
0.43
|
1.13
|
1
|
0.25
|
0.80
|
0.60
|
1.24
|
0.373
|
0.38
|
0.99
|
0.33
|
1.00
|
0.895
|
|
daytime dysfunction
|
0.27
|
0.45
|
0.71
|
0.92
|
0.103
|
0.42
|
0.69
|
0.57
|
0.79
|
0.64
|
0.36
|
0.54
|
0.86
|
1.21
|
0.468
|
0.43
|
0.79
|
0.47
|
0.52
|
0.403
|
0.38
|
0.70
|
0.67
|
0.71
|
0.243
|
|
PSQI total score
|
4.19
|
2.53
|
6.24
|
2.93
|
0.018*
|
4.83
|
2.98
|
5.86
|
1.95
|
0.156
|
4.58
|
2.58
|
7.14
|
3.39
|
0.049*
|
4.89
|
2.87
|
5.20
|
2.88
|
0.969
|
5.00
|
2.98
|
5.00
|
2.40
|
0.895
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pain
|
Peripheral neuropathy
|
Nausea · vomiting
|
Anorexia
|
Fatigue
|
|
|
No
|
Yes
|
|
No
|
Yes
|
|
No
|
Yes
|
|
No
|
Yes
|
|
No
|
Yes
|
|
|
n
|
31
|
12
|
|
20
|
23
|
|
33
|
10
|
|
21
|
22
|
|
27
|
16
|
|
|
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
mean
|
SD
|
mean
|
SD
|
p
|
|
Sleep quality
|
1.03
|
0.60
|
1.33
|
0.49
|
0.22
|
1.10
|
0.64
|
1.13
|
0.55
|
0.897
|
1.03
|
0.59
|
1.40
|
0.52
|
0.149
|
1.05
|
0.67
|
1.18
|
0.50
|
0.498
|
1.15
|
0.66
|
1.06
|
0.44
|
0.581
|
|
Sleep latency
|
1.06
|
1.12
|
1.42
|
1.16
|
0.37
|
1.00
|
1.08
|
1.30
|
1.18
|
0.380
|
1.12
|
1.11
|
1.30
|
1.25
|
0.702
|
1.05
|
1.12
|
1.27
|
1.16
|
0.501
|
1.04
|
1.13
|
1.38
|
1.15
|
0.306
|
|
Sleep duration
|
0.52
|
0.72
|
0.75
|
0.97
|
0.57
|
0.45
|
0.51
|
0.70
|
0.97
|
0.690
|
0.48
|
0.67
|
0.90
|
1.10
|
0.402
|
0.52
|
0.75
|
0.64
|
0.85
|
0.711
|
0.74
|
0.90
|
0.31
|
0.48
|
0.122
|
|
Sleep efficiency
|
0.29
|
0.64
|
0.50
|
0.67
|
0.33
|
0.25
|
0.55
|
0.43
|
0.73
|
0.398
|
0.27
|
0.57
|
0.60
|
0.84
|
0.341
|
0.33
|
0.66
|
0.36
|
0.66
|
0.824
|
0.37
|
0.69
|
0.31
|
0.60
|
0.882
|
|
Sleep disturbance
|
0.97
|
0.18
|
1.00
|
0.43
|
0.88
|
0.95
|
0.39
|
1.00
|
0.00
|
0.526
|
0.97
|
0.30
|
1.00
|
0.00
|
0.899
|
1.00
|
0.32
|
0.95
|
0.21
|
0.582
|
0.93
|
0.27
|
1.06
|
0.25
|
0.105
|
|
use of hypnotic agent
|
0.39
|
1.02
|
0.33
|
0.89
|
0.90
|
0.35
|
0.93
|
0.39
|
1.03
|
0.903
|
0.39
|
1.00
|
0.30
|
0.95
|
0.832
|
0.14
|
0.65
|
0.59
|
1.18
|
0.098
|
0.26
|
0.81
|
0.56
|
1.21
|
0.452
|
|
daytime dysfunction
|
0.39
|
0.62
|
0.58
|
0.90
|
0.62
|
0.40
|
0.75
|
0.48
|
0.67
|
0.532
|
0.45
|
0.75
|
0.40
|
0.52
|
0.899
|
0.52
|
0.81
|
0.36
|
0.58
|
0.602
|
0.41
|
0.64
|
0.50
|
0.82
|
0.799
|
|
PSQI total score
|
4.65
|
2.63
|
5.92
|
3.29
|
0.33
|
4.50
|
2.86
|
5.43
|
2.83
|
0.238
|
4.73
|
2.78
|
5.90
|
3.03
|
0.299
|
4.62
|
2.97
|
5.36
|
2.74
|
0.32
|
4.89
|
2.81
|
5.19
|
2.99
|
0.751
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
2021 Copyright OAT. All rights reserv
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mann-Whiyney U-test
*p<0.05 **p<0.01 ***p<0.001
Discussion
Sleep disorder of cancer patients receiving outpatient chemotherapy
This study clarified sleep conditions of cancer patients receiving outpatient chemotherapy that the mean value of sleep duration showed6.7 (±1.1) hours, sleep disorders were present in 30.2% of participants, and the use of hypnotic agents was 13.9%.
Fukui et al. reported that in blood cancer patients, who received inpatient chemotherapy, sleep disorder symptoms occurred at the proportion of 63.5% [6]. The sleep disorder conditions of outpatients were apparently improved. Outpatient chemotherapy treatment contributed to the improvement of sleep environments where outpatients tended to live a normal life, in comparison with inpatients, maintaining their pace in daily life and having advantages in sleeping and eating habits [7]. Outpatient chemotherapy is applied only for patients with side-effect Grade 0-2, which induced a decrease in sleep disorder along with performance status and side-effect management.
However, patients with a sleep disorder had low sleep efficiency, as the mean value of sleep duration was 5.8 (±1.5) hours and sleep latency was 46.2 (±27.2) minutes. Sufficient sleep is different from person to person, as some people require only five hours for good sleep and some need as long as ten hours of sleep. Therefore, sufficient sleep could mean that people feel comfortable waking up and do not have extreme drowsiness during the daytime. Medical treatment is required for disease-induced sleep disorder with a lack of sleep at night and extreme drowsiness during the daytime [8]. The sleep disorder symptoms examined in this research were not related to the difficulty in staying awake during the day. Thus, drowsiness during the daytime does not affect daily life activities. However, a decline of sleep efficiency and sleep quality were shown in this survey. It was indicated that sleep latency was long, which resulted in short sleep duration and long waking duration which was spent worrying in bed. Therefore, it is a serious concern on the influences in health that sleep deprivation hinders the body and brain from relaxing. There is an urgent need to construct support systems for patients receiving outpatient chemotherapy who need assistance in falling asleep without difficulties and to secure sufficient sleep duration.
Related factors of sleep disorder
This study revealed that sleep disorder symptoms occurred more seriously in the cases using a hypnotic agent, or that have a skin or taste disorder.
Firstly, there would be a possibility to not prescribe appropriate medication according to the symptoms of the sleep disorder, as the effects of hypnotic agents are categorized in a diversified manner, into long-short-term, short-term, intermediate-term and long-term [9]. Assessment and evaluation of sleep must be fully performed. In addition, it must be considered that some patients would feel anxiety about medication for sleep disorder symptoms. Only half of patients with a sleep disorder took a hypnotic agent. In comparison with other side-effects of chemotherapy, care and support for a sleep disorder might not be satisfactory. Patients with chemotherapy are likely to be prescribed varied medications for supportive care and some patients feel it is a burden to use a hypnotic agent. Therefore, it is essential to assess the usages and effects of hypnotic agents according to symptoms of sleep disorders and to support patients with a deep understanding of psychological reactions.
Secondarily, a skin disorder is not an immediate threat to life but could advance in severity with pain, which could even require changes in treatment scheme. This study showed that participants who had skin disorder symptoms accounted for 39.5% with complaints of dry skin, rashes on limbs, physical disorder feelings and tingling sensations and of persistent slight symptoms on fingers, toes and face. Skin disorder symptoms do not involve severe pain or itching. It might be recognized even by medical professionals that symptoms are under control and are being managed. However, this study revealed that skin disorders affected sleep disorders. While among the side-effects of chemotherapy, nausea/vomiting, fatigue and anorexia are limited in the period of occurrence and these syndromes subside after the peak, skin disorder symptoms could accumulate and strengthen as medication treatment is repeated [10]. Slight but persistent skin disorder was a burden for patients, and anxiety and irritation affected patients because the syndromes did not improve or even became worse. When they went to bed and tried to be relaxed, they became sensitive in a quiet environment and overly conscious of physical discomfort, which could hinder them from falling asleep. Medical professionals must keep this situation in mind and not underestimate even a slight syndrome, but to deeply understand their sleep conditions and to support patients.
Thirdly, a taste disorder means conditions of changes in feeling taste or texture of food. It is recognized that diversified factors could induce a taste disorder, such as dysfunction of gustatory cell, decline in the ability to transport taste substance due to decreased salivary secretion, dysfunction of nervous conduction and decreased zinc [11].
One of indicators for taste disorder is anorexia. Chemotherapy for cancer in digestive organs and blood cancer requires concomitant medication and nausea/vomiting would occur for several days after administration. These syndromes are relatively weak and disappear in approximately one week, because antiemetic is prescribed to prevent and control the symptoms. However, the syndromes of nausea/vomiting do not completely disappear and this induces unpleasant sensations and anorexia. Decreased food intake tends to cause decreased salivary secretion and decreased self-maintenance of oral hygiene. Other than anorexia, alimentary deficiency could be triggered along with the decline of self-care ability due to increased fatigue.
Zinc deficiency, which is an inducer of taste disorder, induces hyposecretion of gonadotropin-releasing hormone and growth hormone [12]. Sleep disorder induces hyposecretion of growth hormone, because growth hormone as well as gonadotropin-releasing hormone is secreted during sleep at night [13]. Matuno et al. reported that patients with a serious growth hormone deficiency have low quality of life with low quality of sleep in comparison with adults in general. The report also indicated that hormone replacement therapy improved sleep disorder from 48.6 ± 26.0 to 55.4 ± 22.7 [14] of sleep. However, the mechanism for how growth hormones affect sleep disorders has not been clarified.
It seems that taste disorders would not directly impact sleep conditions. However, it is possible that low growth hormone, due to zinc deficiency, could be related to sleep disorders. It is a future task to examine correlations between sleep disorders and data of food intake and zinc.
This study found that in the side-effects data for chemotherapy, pain, fatigue and nausea/vomiting did not indicate an influence on sleep disorders. It was assumed that the management of these side-effects would be performed as appropriate support therapy. However, further surveys are necessary for long-term and continuous evaluation of side-effects. This survey was conducted on the day of chemotherapy administration to evaluate side-effect conditions of the day on the survey and PSQI-J evaluated sleep conditions for the previous month. However, chemotherapy side-effect symptoms are diverse and changeover time from the day of the administration of anticancer agent: some side-effects disappear after the peak and some are strengthened as administration is conducted repeatedly. Therefore, observations of a fixed point would not fully cover all the side-effects of outpatient chemotherapy. The further examination of this study is to evaluate side-effects in a long-term and continuous survey.
Conclusion
This study was conducted to examine the sleep of cancer patients receiving outpatient chemotherapy and clarified the following:
1. It was recognized that the mean value of sleep duration was 6.7 (±1.1) hours, the ratio of sleep disorder was 30.2% and the ratio of using hypnotic agents was 13.9%.
2. The participants with sleep disorder had short sleep duration 5.8 (±1.5) hours and long sleep latency 46.2 (±27.2) minutes, and their sleep efficiency was low.
3. The participants who were using hypnotic agents with skin and taste disorders had more sleep disorders.
4. Although few chemotherapy outpatients experience difficulties in their daily lives due to sleep disorders, degraded sleep quality due to difficulties falling or remaining asleep are a concern.
5. A review of sleeping medications and intervention in the case of patients with skin or taste disorders are necessary.
Acknowledgment
This study was supported by a Grant-in-Aid (26463363) for Scientific Research from the Japanese Government.
A partial of the summary of this article was presented at the third International Society of Caring and Peace Conference (March, 2017).
References
- Humpel N, Iverson DC (2010) Sleep quality, fatigue and physical activity following a cancer diagnosis. Eur J Cancer Care (Engl) 19: 761-768. [Crossref]
- Theobald DE (2004) Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin Cornerstone 6: S15-21. [Crossref]
- CTCAE v 4.0. [http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40]. [Accessed 2016-10-10].
- ECOG Performance Status. Common Toxicity Criteria, Version 2.0. [http://www.jcog.jp/] [Accessed 2016-10-3].
- Doi Y, Minowa M, Uchiyama M, Okawa M. (1998)Development of the Pittsburgh Sleep Quality Index Japanease version. Jpn J Psychiat Treatment 13: 755-763.
- Fukui A, Kamei H, Hirano S, Hanya M, et al. (2010) Survey on Isomnia in Patients with Hematological Malignancies. Jpn J Pharm Health Care and Sci 36: 580-586.
- Syouji M, Ikeda H, Aoki M, Mori A, et al. (2015) Perceptions and realities of outpatients’ lif under long-term cancer chemotherapy. J K W U Acad Nurs 41: 86-96.
- Uchiyama M (2009) Response of sleep disorders and treatment guidelines. Tokyo: Jiho Inc (in Japanease).
- Takihara T, Sawada Y (2015) Pocket medicine collection. Fukuoka: HAKUBUNSHA Co (in Japanease).
- Ooji T, Nishimura Y, Fukuda H, Tsuji A (2016) Adverse-drug-reaction program: The first cancer-chemotherapy nursing: 46-97, Osaka: MEDICUS SHUPPAN, Publishers Co (in Japanease).
- Kobayashi Y, Nakanishi H (2013) Management of eating disorders by nausea, vomiting, dysgeusia with cancer chemotherapy. J Jpn Society for Parenteral and Enteral Nutrition 28: 627-634.
- Nishi Y (1996) Zinc and growth. J Am Coll Nutr 15: 340-344. [Crossref]
- Ito E, Murata A, Yamamoto K, Kudo S (2003) [Relationship between sleep disordered breathing and body weight loss in patients with chronic obstructive pulmonary disease]. Nihon Kokyuki Gakkai Zasshi 41: 268-275. [Crossref]
- Matuno A, Takano K, Tahara S (2016) Study on the treatment outcome of patients with severe adult growth hormone secretory insufficiency. Annual Research Report The Foundation for Growth Science 39: 25-31.


