Abstract
Background: Emerging evidence suggests that plant derived chemicals are promising sources for treatment of cancer, hundreds of which remain unexplored. Ferns are a group of vascular plants less investigated for their therapeutic potential. Ceratopteris thalictroides, a dietary aquatic fern investigated in the present study, is used as a traditional medicine for clotting blood from exposed cuts, healing wounds and inflammation, and as vegetable in raw or cooked form. Tribal populations in different parts of India, other Southeast Asian countries and Madagascar rely on these pteridophyte-based medicines for health benefits.
Aim of the study: The study involves investigation of the anticancer potential of shoot extract of Ceratopteris thalictroides.
Materials and Methods: The study involves extraction of bioactive from the plant material and evaluation of cytotoxicity in multiple cell lines, followed by flow cytometry and western blotting analyses post treatment. HPLC analysis of extract was performed to identify the major component responsible for anticancer activity. Tumor regression potential and toxicity of the extract in mice by body weight, histopathological examination and complete blood count, and western blotting of the mice tumor cells were also evaluated.
Results: The methanolic extract of C. thalictroides containing alkaloids or MECA induced cell death in multiple cell lines via apoptosis without causing cell cycle arrest. Although the extract was unable to generate reactive oxygen species (ROS) in the cells, it acted as a ROS quencher. HPLC analysis of methanolic extract revealed the presence of alkaloids as the major component. Elevated levels of active CASPASE 9, active CASPASE 3, CASPASE 8 along with PARP-1, PUMA, MDM2, Ku70 post treatment in leukemic cell line Nalm6 suggested the activation of intrinsic and extrinsic pathway of apoptosis. Further, oral administration of MECA led to tumor regression in mice bearing breast adenocarcinoma. Histopathological evaluation of internal organs of normal and tumor treated mice showed that the treated tissues resembled the normal tissues in architecture and integrity. Activation of CASPASE 9 and CASPASE 3 as well as cleavage of PARP-1 in the MECA treated mice along with downregulation of BCL-2 and elevated amounts of P53, BAK and BAX revealed activation of intrinsic pathway of apoptosis in treated mice tumor. Hematological parameters and survival of the treated mice were
comparable to that of normal mice, suggesting no significant toxic effects.
Conclusion: Results showed anticancer potential of C. thalictroides in cell lines and mice tumor model, without significant toxicity on normal cells.
Keywords
Anticancer, Apoptosis, Diet, Fern, Natural products, Toxicity, Cancer therapy
Abbreviations
ROS, Reactive oxygen species; MECA, methanolic extract containing alkaloids; HPLC, High performance liquid chromatography; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide; 2,4-DNP, 2,4-Dinitrophenol; H2DCFDA, 2,7 dichlorodihydrofluorescein diacetate; EAC, Ehrlich ascites carcinoma; CBC, Complete blood count; DPX, Dibutylphthalate polystyrene xylene; HE, Hematoxylin eosin.
Introduction
The global burden of cancer is constantly increasing. There were 18.1 million new cases and 9.6 million deaths due to cancer in 2018 and it is the second prominent cause of death globally, according to the World Health Organization. (https://www.who.int/cancer/PRGlobocanFinal.pdf). Many of the new research for cancer treatment focus on phytochemicals which also form the base for alternative cancer therapy along with diet modulation and exercise. Emerging evidence suggest that plant derived chemicals are a promising source for treatment of cancer and there are hundreds of them remain to be explored. Paclitaxel, vincristine, vinblastine, are some of the most common anticancer drugs of plant origin and used in the clinic. Numerous plants are still being investigated for their therapeutic effect against various diseases including cancer [1,2].
We have previously reported anticancer effects of the plant Vernonia condensata and a plant derived triterpenoid methyl angolensate [3,4]. We have also described tumor regression properties of the fruit’s sapodilla plum and strawberry in mice bearing breast adenocarcinoma [5,6].
Food derived phytochemicals are gaining significant attention because of their therapeutic abilities. It is now well established that diet which includes fruits and vegetables can reduce the risk of cancer. A recent clinical study following 56,048 participants on Danish diet for 23 years revealed that dietary intake of flavonoids is inversely related to cancer related deaths [7]. Another non-placebo, two arm study in women with breast cancer shows that walnut consumption increased the activation of genes involved in apoptosis and cell adhesion, at the same time inhibited genes involved in cell proliferation and migration [8]. These recent studies indicate the potential of dietary behaviors to improve human health and also the importance to focus on less studied vegetables and other plant materials for their medicinal properties.
Ferns are a less explored group of plants for their medicinal purposes. In the present study, we have investigated anticancer activity of the fern Ceratopteris thalictroides (L.) Brongn. (Figure 1a). This homosporous fern belongs to the family Pteridaceae, is an aquarium plant and commonly known as water sprite. Fronds of the plant are used as luxury vegetable in raw or cooked form in India, Bangladesh, China, Malaysia, Japan and Madagascar, and fresh fronds are used in salads or as substitute for asparagus [9]. Reportedly, this fern has abundant value as its rhizomes and fronds are used as medicine for fetal toxins and accumulation of phlegm.
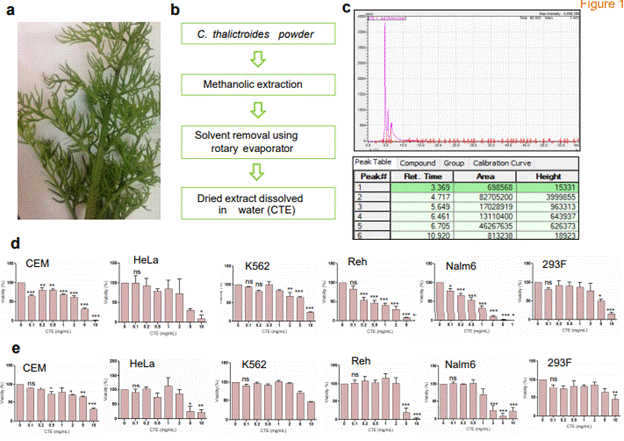
Figure 1. Cytotoxicity analysis of extract on cell lines. (a) Image showing the fern, Ceratopteris thalictroides used for the study. (b) Schematic showing preparation of C. thalictroides extracts used for the present study. (c) HPLC chromatogram of methanolic extract at 254 nm and table showing the retention time of major peaks, peak height and area. (d-e) CEM, HeLa, K562, Reh, Nalm6 and 293F were treated with various concentrations of the extract (0.1, 0.2, 0.5, 1, 2, 5, 10 mg/ml) and cytotoxicity was analyzed by trypan blue (d) and MTT (e) assays after 48 h (ns: not significant, *p< 0.05, **p< 0.005, ***p < 0.0001).
Traditionally it is used as medicine in Asia. The leaves and root parts are used as a poultice against skin complaints, such as cuts, wounds, inflammation, in India, China, Malaysia and Philippines [9-13]. (http://flora.huh.harvard.edu/china/). Apart from this, it has been also used as a green manure in paddy fields [9]. Traditional uses of the Pteridophyte have been studied in tribal populations in different parts of India, including Chandraprabha Wildlife Sanctuary (Chandauli, Uttar Pradesh), Simlipal Biosphere Reserve (Orissa), Sirumalai hills of Eastern Ghats (Tamil Nadu), Shivalik mountain and south east of Corbett national Park (Terai region, Uttarakhand), as medicine and food [9,13-16]. The known metabolites of this fern are pterosins, flavonoids and phenolics [17].
Here, for the first time, we report the anticancer potential of C. thalictroides in various cell lines and in mouse model of breast adenocarcinoma. HPLC analysis of methanolic extract indicates the presence of alkaloids as major component, and hence referred to as methanolic extract containing alkaloids (MECA). The methanolic extract of C. thalictroides (Figure 1b) induced apoptosis in cell lines. Treatment of leukemic cell line Nalm6 with methanolic extract activates the extrinsic and intrinsic pathway of apoptosis. Interestingly, it also exhibited reactive oxygen species (ROS) scavenging property. Importantly, administration of MECA resulted in significant reduction in the tumor volume in a mice model without causing toxic effect in normal cells. Western blot analyses of the mice tumor cells showed activation of intrinsic pathway of apoptosis in vivo.
Materials and methods
Cell lines and culture
Human B cell leukemia cell lines, Nalm6 and Reh; human embryonic kidney cell line, HEK293F; human chronic myelogenous leukemia cell line, K562; human cervix adenocarcinoma cell line, HeLa; T cell leukemic cell line CEM were cultured in RPMI 1640 or DMEM with 10% FBS and 100 μg/ml of Penicillin G and Streptomycin, as described before [18,19].
Animals
Swiss albino mice (6-8 weeks) were purchased from the Central Animal Facility, Indian Institute of Science (IISc) and maintained in polypropylene cages. Animals were provided standard pellet diet (Agro Corporation Pvt. Ltd, Bangalore, India) and water ad libitum. Animals were kept under controlled conditions of temperature and humidity with 12 h dark/light cycles. The experimental design and methods followed institutional guidelines and were approved by Institutional Animal Ethics Committee (CAF/Ethics/248/2011), IISc, Bangalore.
Chemicals and reagents
All the chemicals used in this study were procured from Sigma Aldrich, USA and SRL, India. Antibodies were obtained from Santa Cruz Biotechnology, USA; Cell Signaling Technologies, USA; Abcam, UK and BD Biosciences, USA.
Preparation of extract
C. thalictroides was collected from Kerala, India and identified by the botanist M S Kiranraj, Department of Botany, Sree Narayana College, Kollam, India (Accession number: SNCH-2804). The plant material (shoot) was shade dried and powdered. 10 g of the plant powder was extracted with 200 ml hexane for 6 h at room temperature with continuous shaking. The plant material was filtered using Whatman No.1 filter paper and extracted twice with 200 mlmethanol for 6 h at room temperature with constant shaking. The filtrate was concentrated to dried form in a rotary evaporator (Buchi, Switzerland) and vacuum concentrator (Concentrator
plus, Eppendorf, Germany). The dried plant material was mixed in autoclaved double distilled water; vortexed intermittently for 1 h, centrifuged at 9600×g for 10 min and supernatant was collected. This extract was then used for all the studies.
HPLC analysis
The HPLC analysis was performed using C18 analytical column in the Shimadzu HPLC system (Shimadzu, Japan). Gradient of acetonitrile and water was used as mobile phase and peaks were detected at 254 nm. The injection volume was 20 μl, and the flow rate was 0.8 ml/min. Samples were prepared by adding acetonitrile to the extract followed by filtration with 0.22 μm filter.
Trypan blue dye exclusion assay
The cytotoxic potential of extract was checked in the cancer cell lines Nalm6, Reh, CEM, K562, HeLa and HEK293F at various concentrations (0.1, 0.2, 0.5, 1, 2, 5 and 10 mg/ml) using trypan blue assay as described before (4). Briefly, 0.75×105 cells/ml was seeded and the respective concentrations of extract were added. The assay was performed after 48 h incubation by either using hemocytometer (Carl Zeiss AxioVision, Germany) or using an automated cell counter (LUNA-II, Logos Biosystems, South Korea). Cells along with media served as control.
MTT assay
MTT assay was performed to test the effect of MECA (0.1, 0.2, 0.5, 1, 2, 5 and 10 mg/ml) on cell proliferation as explained before [20]. In short, 100 µl of the uniform cell suspension after 48 h incubation with MECA was treated with 5 mg/ml MTT reagent and change in the color intensity was measured at 570 nm in microplate reader (Bio-Rad, USA).
Flow cytometry analyses
Trypan blue dye exclusion assay
Cells were treated with 0.5, 1 and 2 mg/ml of MECA and 1.5×105 cells were harvested after 48 h of incubation for all flow cytometry (CytoFLEX, Beckman Coulter, USA) analyses. Cells without MECA served as control.
Apoptosis detection by Annexin V/PI double staining
Annexin V/PI double staining was performed post treatment with the extract for quantification of early and late apoptotic cells as well as necrotic cell population in Nalm6 cells as described previously [5]. Cells were stained with Annexin V- FITC (0.2 mg/ml) and PI (0.05 mg/ml) for acquisition in CytoFLEX (Beckman Coulter, USA). Quantification of percentage of cells in early, late and necrotic populations was performed using GraphPad Prism.
Analysis of mitochondrial membrane potential variation
JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide) staining assay was followed to check any variation in the mitochondrial membrane potential of CEM cells with MECA treatment as described before [21]. Cells were stained with 0.5 µM JC-1 dye. The change in fluorescence from red to green was checked in all the samples. 2, 4-Dinitrophenol-treated cells (2,4-DNP) were used as positive control. Quantification of percentage of cells with high and low membrane potential was performed using GraphPad Prism.
Analysis of intracellular ROS generation
Generation of intracellular ROS with 2 mg/ml extract was tested in CEM cell line at various time intervals (30 min, 1, 2, 4 and 6 h). The cells were harvested and treated with 2,7 dichlorodihydrofluorescein diacetate (H2DCFDA) as explained before [20] and acquired using CytoFLEX. H2O2 was used as positive control.
Analysis of ROS scavenging potential of MECA
For the analysis of ROS scavenging activity of MECA, ROS was generated by adding H2O2 and then treated with different concentrations (0.5, 1 and 2 mg/ml) of MECA for 5 min. The analysis was performed in CEM cells pretreated with H2DCFDA. The amount of ROS remaining after treatment was analyzed as described before [5,21]. Quantification of percentage of cells was performed using GraphPad Prism.
Immunoblotting analysis after MECA treatment in Nalm6 cells
Immunoblotting was performed with ~30 μg of Nalm6 cell lysates. Briefly, Nalm6 cells were treated with 0.5, 1 and 2 mg/kg of extract for 48 h. Cells were harvested and lysate was prepared [22,23]. Lysates were resolved on 8-12% SDS PAGE gels and transferred to PVDF membrane (Millipore, USA) and probed with respective antibodies. We have used the primary antibodies; MCL-1, PARP-1, CASPASE 3, CASPASE 8 and CASPASE 9, PUMA, MDM2, FAS, XRCC4 and Ku70. The blots were developed using chemiluminescent substrate (Immobilon western, Millipore) and images were captured using gel documentation system (LAS 3000, FUJI, JAPAN).
Anticancer potential of the extract in breast adenocarcinoma mice model
Tumor was induced by injecting 1×106 EAC cells into the left thigh region of Swiss albino mice and the treatment started once the tumor size was approximately 0.15 cm3 which took 7-9 days after injection of tumor cells as described previously [24]. Control was the group of mice without treatment. The initial study was performed with 3, 9 and 27 mg/kg of MECA (data not shown) from which we have selected 27 mg/kg as the concentration for in vivo tumor studies.
Preliminary experiment consisted of seven continuous doses orally administered to Swiss albino female mice (control-4, MECA group-6 mice each). Subsequent to this, we increased the doses to 10 continuous doses and the effect was investigated in both male (control-6, MECA group-10) and female mice (control-12, MECA group-19). The animals were monitored and the tumor volume was measured at time intervals. Tumor was measured using Vernier calipers and volume was calculated using the formula: V=0.5×a×b2, where ‘a’ and ‘b’ indicate major and minor tumor diameters respectively [23]. Mice were sacrificed on the 25th day, and the internal organs were collected.
Immunoblotting analysis of tumor cells
Immunoblotting was performed for tumor cells collected from control and MECA treated mice as described before with modification [22,23]. Swiss albino female mice were categorized to three groups; control, 27 and 54 mg/kg treatment groups. Tumor was induced as explained in above section. Ten continuous doses of above concentrations of the extract were given orally and after 24 h of the last dose, the animals were sacrificed, tumor cells were collected from the thigh muscle and snap frozen in liquid nitrogen. The cells were later stored at -80ºC. For western blot analysis, tumor cells were thawed washed twice in 1X PBS and cell lysates were prepared in RIPA buffer. Cell lysates were resolved on 10-12% SDS-PAGE, transferred to PVDF membrane (Millipore, USA) and probed with respective antibodies. We have used the primary antibodies; P53, BCL-2, MCL-1, PARP-1, BAX, BAK, SMAC/DIABLO, cleaved CASPASE 3 and CASPASE 9. ACTIN acted as the loading control. The blots were developed using chemiluminescent substrate (Immobilon western, Millipore) and images were captured using gel documentation system (LAS 3000, FUJI, JAPAN).
Histological analysis
On the 25th day of oral administration, mice from both control and treated group (from the tumor study) were dissected. Kidney, liver and thigh muscle were collected, fixed in 4% paraformaldehyde, and processed as described before [25]. A small piece of each tissue was embedded in paraffin wax blocks and sectioned to 5 µm slices using rotary microtome (Leica Biosystems, USA). Paraffin was removed from the sections and stained with hematoxylin and eosin (HE) as explained elsewhere [25]. The sections were mounted in DPX and images
were taken using bright field microscope (Carl Zeiss AxioVision, Germany).
Toxicity analysis and survival study
In order to check if MECA can cause toxicity in mice, we performed hematological analysis, complete blood count (CBC) and also survival of the animals after treatment as described before [20,24,26]. Two groups of mice were orally fed with 10 continuous doses of 27 and 54 mg/kg MECA along with the control mice without treatment. Each group contained eight animals. On 28th day of treatment, blood was collected from three mice of each group and CBC was checked. Another set of experiment was executed to test the survival as well as any variations in the appearance, behavior, and food and water intake of the MECA treated mice from the normal mice. Here also we used 10 continuous doses of 27 and 54 mg/kg of MECA and the animals were monitored for a period of six months.
Statistical analysis
All the experiments were performed minimum three times independently and values in the figures are expressed as mean ± SEM. Statistical analyses were conducted by student’s t test and one way ANOVA using GraphPad Prism 5. Values were considered significant when p< 0.05 (ns: not significant, *p< 0.05, **p< 0.005, ***p < 0.0001).
Results
HPLC analysis of extract
Methanolic extract of the shade-dried powder from the shoot of Ceratopteris was used for characterization and evaluation of biological properties (Figure 1a-1c). For identification of major component present in the extract, HPLC analysis was performed. A broad range of 190-600 nm was analyzed to detect the major component. We observed a major peak between 3-11 min at 234 nm wavelength (Figure 1c). Based on previous literature, we identified that major compounds in the extract are alkaloids, prominent being magnoflorine [27-30]. Hence, based on this result, the methanolic extracts were thereafter renamed as Methanolic extract containing alkaloids
(MECA).
MECA induces cytotoxicity in multiple cancer cell lines
Cytotoxicity analysis showed significant reduction in cell viability in the case of leukemic cell lines, Nalm6 and Reh, when incubated with increasing concentrations of MECA (Figure 1d-1e). IC50 was determined to be 1.042 mg/ml and 2.528 mg/ml, respectively after trypan blue and MTT assays (Figure 1d-1e, Table 1). Although B cell derived leukemia cell lines were maximally sensitive to MECA, its cytotoxic effect was limited in K562 (Figure 1d-1e, Table 1). A variation in the IC50 was observed between trypan blue and MTT assay methods. This might be due to the difference in the mechanism of detection of cytotoxicity by both methods, as trypan blue detects cell death while MTT assesses the cell metabolic activity. Hence IC50 was calculated taking average of both methods. IC50 of MECA in each cell line is showed in Table 1.
Table 1. IC50 values of MECA in various cell lines. IC50 was determined by taking average of trypan blue and MTT assay.
Cell line |
IC50 (mg/ml) |
CEM |
3.497 |
HeLa |
3.74 |
K562 |
8.501 |
Reh |
2.528 |
Nalm6 |
1.042 |
293F |
6.01 |
MECA induces cell death via apoptosis without causing cell cycle arrest
Following the investigation of cytotoxic potential of MECA, we investigated the mechanism of cell death in Nalm6. Cell cycle analysis did not show any cell cycle arrest post treatment (data not shown). Annexin V-FITC/PI double-staining revealed that the apoptotic cell death was directly proportional to the concentration of extract used (Figure 2a, b). There was 10.07, 2.16 and 0.47% of cells in the early apoptotic, late apoptotic and necrotic phases, respectively after 48 h treatment with 1 mg/ml MECA. There was considerable increase in early
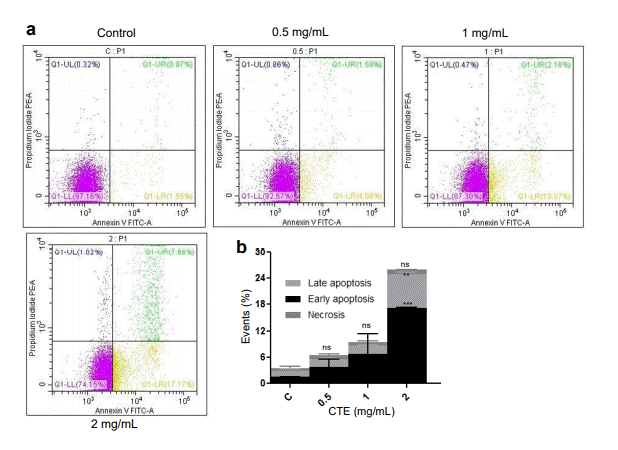
Figure 2. Evaluation of cell death mechanisms following MECA treatment after Annexin V-FITC and PI staining. (a) Analysis of the apoptotic and necrotic populations 48 h after treatment of Nalm6 cells with increasing concentrations of MECA (0.5, 1 and 2 mg/ml). (b) Bar diagram represents the % of necrotic, late and early apoptotic cells following treatment with the extract (ns: not significant, *p< 0.05, **p< 0.005, ***p < 0.0001).
(17.17 %) and late apoptotic (7.66%) populations when the concentration was increased to 2 mg/mL (Figure 2a, b). It was interesting to note that the cell population was shifting to early apoptotic stage rather than necrotic with increasing concentrations of MECA.
Mitochondrial membrane potential (MMP) variation was measured as a shift from red to green fluorescence since the mitochondrion accumulating dye moves to the cytoplasm. Flow cytometry analysis in CEM cells and comparison with 2,4-DNP showed an increase in the green fluorescence with increasing concentrations of MECA (Figure 3a, b). This data confirms that the extract causes membrane depolarization, which in turn leads to opening of mitochondrial permeability transition pore and induction of apoptosis.
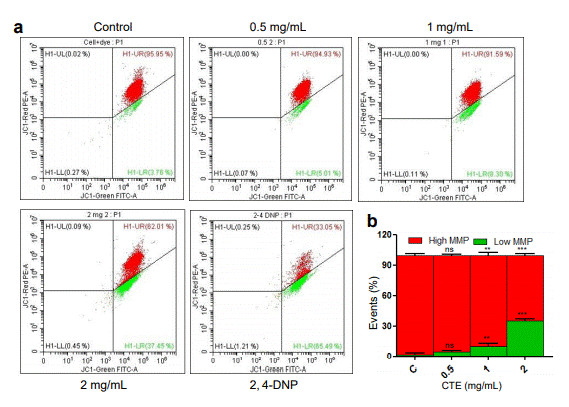
Figure 3. Effect of MECA on mitochondrial membrane integrity of CEM cells. (a) Variation in mitochondrial membrane potential after treatment (48 h) with increasing concentrations (0.5, 1 and 2 mg/ml) of the extract lead to a shift from red to green after JC-1 staining. (b) Bar diagram illustrates the quantitation of shift from red to green after JC-1 staining following MECA treatment (ns: not significant, *p< 0.05, **p< 0.005, ***p < 0.0001).
MECA is a ROS scavenger, but does not induce ROS generation
Several plant extracts are known to possess antioxidant potential. Therefore, we were interested in testing ability of MECA as a ROS quencher. MECA showed ROS scavenging capacity with the lowest amount tested i.e., 0.5 mg/ml and the ROS quenching was more efficient with higher concentrations of extract (Figure 4a, b).
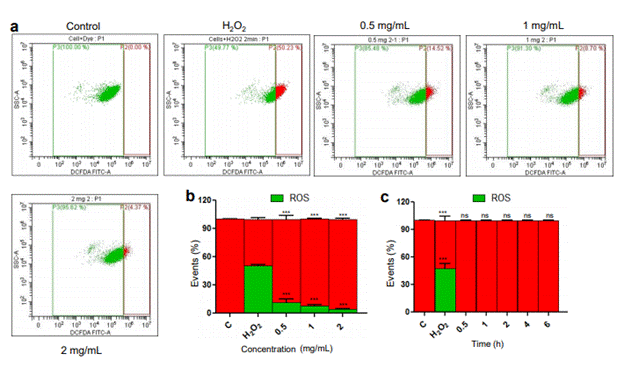
Figure 4. Effect of MECA on reactive oxygen species (ROS) in CEM cells. (a) Removal of ROS generated by H2O2 by different concentrations of MECA (0.5, 1 and 2 mg/ml). (b) Bar diagram showing the quenching of ROS following addition of increasing concentrations of MECA (p value < 0.0001). (c) Bar diagram depicting the generation of ROS at various time points following treatment with MECA (2 mg/ml) (ns: not significant, *p< 0.05, **p< 0.005, ***p < 0.0001).
Many plant metabolites and other bioactives can cause generation of intracellular ROS which lead to cell death. Our investigation for ROS induction based on fluorescence of DCFDA turned out to be negative as there was no detectable ROS with 2 mg/ml MECA at any of the time points tested (Figure 4c).
MECA induces cell death in cancer cells by activation of both intrinsic and extrinsic pathway of apoptosis
Dose dependent increase in cleaved CASPASE 3 and cleaved CASPASE 9 and spliced MCL-1 indicates the activation of intrinsic pathway of apoptosis ex vivo (Figure 5). We also observed the increase in CASPASE 8, PUMA, MDM2 and FAS upon treatment which suggests the activation of extrinsic pathway of apoptosis (Figure 5). Cleavage of PARP-1, a DNA damage repair protein by CASPASE 3 was observed in treated samples but not in the control (Figure 5). Interestingly, levels of NHEJ protein KU70 showed a significant reduction suggesting altered levels of double-strand break repair (Figure 5). Altogether, our results suggest that MECA can induce both intrinsic and extrinsic pathway of apoptosis and lead to the cell death.
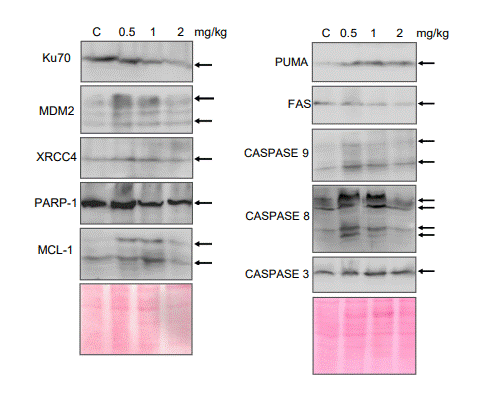
Figure 5. Analysis of apoptotic markers in Nalm6 cells after treatment with the extract. Western blot analysis for the apoptotic markers from the lysate prepared from Nalm6 cells after 48 h of MECA treatment (0.5,1 and 2 mg/ml). The blots are derived from multiple gels and ponceau image is showing as loading control. Band of interest are marked with an arrow.
MECA treatment leads to tumor regression
As a preliminary study, we administered seven continuous oral doses of MECA (27 mg/kg) and found a significant reduction in the tumor size of treated (1.7 cm3) animals comparing to the tumor control (2.8 cm3) group (Figure 6a). We further increased the number of doses to 10 continuous days without changing the amount of extract. The study was conducted in both males and females (Figure 6b, c). Interestingly, the tumor size in female mice group was reduced to 0.98 cm3, whereas the tumor size of control female mice group was 2.09 cm3 after the study period (Figure 6b). MECA treatment caused remarkable tumor regression in the female group. However, the male group of mice did not show much variation in tumor size compared to the control group. The control group had an average tumor size of 2.6 cm3 and the treated group had tumor volume of 2.18 cm3 at the end of the study period (Figure 6c).
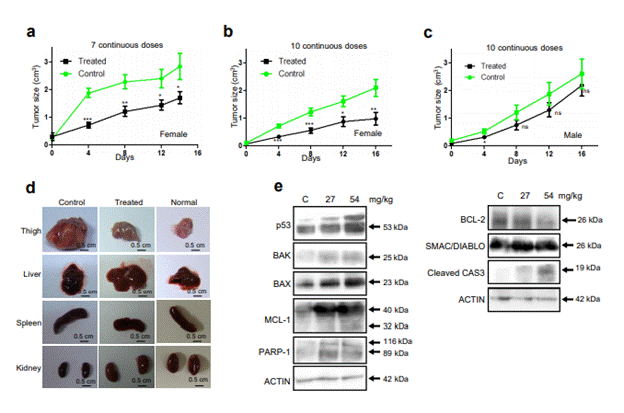
Figure 6. Effect of the extract on progression of solid EAC tumor in Swiss albino mice. (a) Effect of 7 continuous doses of MECA (27 mg/kg) on tumor progression in female mice. (b, c) Effect of 10 continuous doses of MECA (27 mg/kg) on tumor progression in female (b) and male mice (c). Control denotes tumor control. (d) Morphological evaluation of different organs from tumor control, MECA treated and normal mice (ns: not significant, *p< 0.05, **p< 0.005, ***p < 0.0001). (e) Western blot analysis of expression of apoptotic proteins in mice where extracts prepared from tumor control and MECA treated (27 and 54 mg/kg) mice were used. Actin served as loading control. The blots shown are derived from multiple gels; membrane was cut based on molecular weight. Band of interest is indicated with an arrow.
Apart from tumor, we compared the size, morphology and appearance of internal organs and found variation only in the size of spleen of control animal (Figure 6d) while the size was normal in the treated mice.
In order to understand the mechanism of cell death induced by MECA, we have studied the expression of apoptotic proteins in the tumor cells collected from control and treated female mice (Figure 6e). There was a concentration dependent increase in the expression of P53 in the treated cells compared to control which is the first indication that MECA can induce tumor regression in vivo (Figure 6e). We have checked the expression levels of BAK and BAX, the principal regulators of intrinsic pathway of apoptosis in the BCL2 family that lead to permeabilization of mitochondrial membrane [31]. Interestingly, we found remarkable dose dependent buildup of BAX and BAK in the treatment groups which suggests the activation of intrinsic pathway of apoptosis. Consistent to this, downregulation of anti-apoptotic protein, BCL2 was also observed in the treated groups compared to control. This was further substantiated by the increase in the levels of SMAC/DIABLO; a pro-apoptotic activator of CASPASE in the treated groups (Figure 6e). Splicing of MCL-1 protein resulting in its pro-apoptotic form (32 kDa) was noticed as a faint band in the highest treatment group. We extended our investigation to check the levels of activated CASPASES which are other members associated with the pathway (Figure 6e). An increase in the amount of cleaved CASPASE 3 in the 54 mg/kg group ascertained our hypothesis that MECA can lead to apoptosis in mice. Cleavage of PARP-1, a DNA damage repair protein by CASPASE 3 was evident in the treated samples, but not in the control (Figure 6e). Altogether, our results suggest that MECA can induce intrinsic pathway of apoptosis and lead to tumor regression.
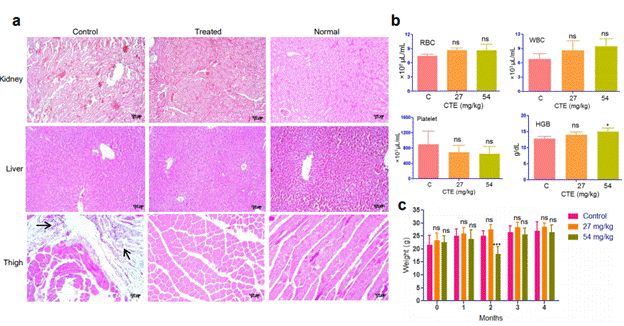
Figure 7. Toxicity analyses of MECA in mice. (a) Haematoxylin-Eosin-stained sections of kidney, liver and thigh tissues from tumor control, MECA treated and normal mice. Tumor cells are marked with arrows. (b) Various blood parameters including RBC, WBC, platelet and HGB after treatment with the extract (C- control, 27 and 54 – 27 and 54 mg/kg respectively). (c) Survival of mice treated with the extract (27 and 54 mg/kg) up to five months. ‘0” denotes the body weight before the start of experiment (ns: not significant, *p< 0.05, ***p < 0.0001).
Histopathological analysis
HE staining of organs was performed to examine the microscopic anatomy for any histopathological variations (Figure 7a). The appearance of liver sections showed similar architecture and integrity in control and treated mice. Histological features of the kidney tissue from control varied from the treated with expanded spaces among cells. Thigh muscle of control animal showed infiltrated tumor cells and the morphology was significantly different from the treated group (Figure 7a). The thigh muscles of control mouse showed irregularity in the size and shape with much interstitial spaces among them (Figure 7a). On the other hand, normal morphology was restored in the treated mouse. Histopathological analyses revealed that MECA treatment had not caused toxicity or damage in the internal organs of mice.

Figure 7. Toxicity analyses of MECA in mice. (a) Haematoxylin-Eosin-stained sections of kidney, liver and thigh tissues from tumor control, MECA treated and normal mice. Tumor cells are marked with arrows. (b) Various blood parameters including RBC, WBC, platelet and HGB after treatment with the extract (C- control, 27 and 54 – 27 and 54 mg/kg respectively). (c) Survival of mice treated with the extract (27 and 54 mg/kg) up to five months. ‘0” denotes the body weight before the start of experiment (ns: not significant, *p< 0.05, ***p < 0.0001).
Hematological analysis for toxicity
Complete blood count after MECA treatment with two different doses (27 and 54 mg/kg) did not show much variation from normal (Figure 7b). An increase in the number of white blood cells and decrease in platelet count was observed for the treatment groups but they were within the reported normal range [20,32]. We found in our study that the variations in blood counts were not significant with consumption of MECA. Survival and weight of the mice were recorded for a period of four months (Figure 7c). There was no change in appearance, behavior, and food and water intake for the treated animals from the normal mice group. Although there was a reduction in body weight in treated animals during the second month, the overall body weight increased in the consecutive months with no significant difference compared to control. The toxicity analyses results underscore the safe use of C. thalictroides as a normal food ingredient.
Discussion
Latest research suggests that diet can influence the course of cancer where the various dietary components can intensify or reduce the risk of cancer. In a recent study, researchers discovered that indole-3-carbinol (I3C), a small molecule found in broccoli and other cruciferous vegetables can activate expression of the tumor suppressor PTEN leading to reduced tumor burden in mice [33]. Alternatively, another study [34] uncovered that excess dietary intake of asparagine can lead to metastasis of triple-negative breast cancer in mice models, and limiting asparagine can prevent the spread. These studies suggest that modulation of diet might impact on the patient’s response to treatment. Moreover, traditional medicines which make use of herbal formulations for cancer treatment are gaining remarkable attention. Studies are underway where researchers are trying to understand whether the herbal formulations alone or in combination with chemotherapy and radiation can improve the life of cancer patients.
The current study focuses on the anti-tumor potential of a poorly studied aquatic fern, C. thalictroides which is used as a vegetable and also against skin ailments in different parts of the world. There is no literature available on the therapeutic potentials of this plant and for the first time we have investigated the anti-tumor and antioxidant potential of the extract along with the analyses of any toxicity caused by this plant in mice. The initial proof of the in vitro anticancer activity came from the cytotoxicity analyses in multiple cell lines. Flow cytometry analysis after Annexin V-FITC/PI staining revealed that the cytotoxicity induced by MECA was due to apoptosis. This result was further endorsed by the observation of mitochondrial membrane depolarization after JC-1 staining of MECA treated CEM cells, which also confirmed the involvement of mitochondria in commencement of apoptosis. Interestingly, the extract did not generate ROS even at the highest concentration used, but on the other hand, removed the ROS generated by H2O2. Thus, our results suggest that generation of ROS is not the cause of apoptosis induced by MECA. The contribution of antioxidant activity by MECA towards the anticancer potential has to be investigated further. Further, HPLC analysis reveals that the major component which is responsible for anticancer activity is alkaloids. Taken together, these suggest that the common aquarium plant under study is a good source of antioxidants and alkaloids, and thus might provide health benefits when included in the diet.
Further in vivo experiments in breast adenocarcinoma mouse model revealed the anti-tumor potential of C. thalictroides. The syngeneic mouse model developed by injecting EAC tumor cells served as an excellent model system to study the tumor reducing potential of MECA. Reduction in tumor volume was achieved with 27 mg/kg and the in vivo studies revealed that MECA contains bioactives that hold the potential to prevent tumor progression. Though there are studies indicating the presence of phenolics and pterosins from C. thalictroides, a thorough analysis of the metabolome is necessary to unravel the molecules involved in its therapeutic potential. Besides, more investigation is required to understand the cause for observed less antitumor activity by MECA in male mice compared to females. A study on sex differences on subcutaneous inoculation of EAC tumor cells demonstrated that the tumors in male mice were larger than in female mice [35]. There are previous reports suggesting that it is easier to induce tumor immunity in female mice and they also possess greater natural immunity to EAC than male mice [36]. However, the factors behind this occurrence remain to be elucidated.
In vivo toxicity analysis by comparison of CBC between MECA treated and normal mice revealed that all the blood parameters tested were within range [26,37]. Blood parameters are essential markers of diseases in humans as well as animals. Hence toxicology analysis based on CBC can indicate any harmful side effect upon consumption of food and medicines. Observation of the animals over a four-month period did not show any indication of toxicity and they remained healthy throughout the study. Toxicity analyses denote the safe use of C. thalictroides as a dietary ingredient. Histopathological analysis of internal organs from the treated mice showed no cellular level damage following MECA treatment which is in line with the hematology and mice survival data that underline that MECA administration is non-toxic.
Our attempts to elucidate the mechanism behind cell death caused by MECA revealed the activation of intrinsic pathway of apoptosis in vivo and activation of intrinsic and extrinsic pathway of apoptosis ex vivo. Using western blot analyses, we demonstrated the overexpression and activation of multiple apoptotic marker proteins, starting from the pro-apoptotic BAX and BAK to the executioner CASPASE 3 following MECA treatment in mice. The loss of mitochondrial membrane potential in treated samples was evident from the JC-1 staining assay. We have found up-regulation of multiple proteins involved in the intrinsic pathway of apoptosis which proved that the tumor regression in mice was accomplished through programmed cell death. Downregulation in the expression of Ku70 and PARP1 followed by XRCC4 and FAS. Also increased expression of PUMA and MDM2 suggests the activation of extrinsic and intrinsic pathways after 48 hours of treatment in Nalm6 cells.
Conclusion
Current study establishes that ingestion of C. thalictroides can slow down cancer cell proliferation in mice without causing any detrimental side effects. Apart from the anticancer effect, the work also demonstrates antioxidant potential of this less explored vegetable. A detailed analysis is required to identify various compounds present in the plant and also to delineate the mechanisms and pathways involved in various biological activities.
Acknowledgements
We thank Dr Namrata Nilavar and other members of SCR laboratory for critical reading and comments on the manuscript. We thank Dr. Supriya Vartak and Dr. Vidya Gopalakrishnan for help in mice handling and cell culture. We thank FACS and Central Animal Facilities of IISc for their help. This work was supported by grants from DBT-COE (BT/PR/3458/COE/34/33/2015), Glue grant (BT/PR23078/MED/29/1253/2017) and IISc-DBT partnership programme [DBT/BF/PR/INS/2011-12/IISc] to SCR. KKV is supported by SERB-NPDF (PDF/2017/000785) from DST, India.
Author Contributions
S.C.R., A.S. and K.K.V. conceived and designed the experiments; K.K.V., A.S., U.R., S.S. and A.K. performed the experiments. S.C.R., K.K.V., U.R. and S.S. analyzed the data and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Availability of data and materials
The datasets used in the current study are available from the corresponding author on reasonable request.
References
- Mann J (2002) Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2: 143-148. [Crossref]
- Drew DA and Chan AT (2018) Towards a cancer-chemopreventive diet. Nat Biomed Eng 2: 6-7. [Crossref]
- Thomas E, Gopalakrishnan V, Somasagara RR, Choudhary B, Raghavan SC (2016) Extract of Vernonia condensata, Inhibits Tumor Progression and Improves Survival of Tumorallograft Bearing Mouse. Sci Rep 6: 23255.
- Chiruvella KK, Kari V, Choudhary B, Nambiar M, Ghanta RG, Raghavan SC (2008) Methyl angolensate, a natural tetranortriterpenoid induces intrinsic apoptotic pathway in leukemic cells. FEBS Lett 582: 4066-4076. [Crossref]
- Srivastava M, Hegde M, Chiruvella KK, Koroth J, Bhattacharya S, et al. (2014) Sapodilla Plum (Achras sapota) Induces Apoptosis in Cancer Cell Lines and Inhibits Tumor Progression in Mice. Sci Rep 4: 6147. [Crossref]
- Somasagara RR, Hegde M, Chiruvella KK, Musini A, Choudhary B, et al. (2012) Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One 7: e47021. [Crossref]
- Bondonno NP, Dalgaard F, Kyrà C, Murray K, Bondonno CP, et al. (2019) Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun 10: 3651.
- Hardman WE, Primerano DA, Legenza MT, Morgan J, Fan J, et al. (2019) Dietary walnut altered gene expressions related to tumor growth, survival, and metastasis in breast cancer patients: a pilot clinical trial. Nutr Res 66: 82-94. [Crossref]
- Singh SK, Rajkumar SD (2017) Biodiversity and Indigenous Use of Medicinal Ferns in Chandraprabha Wildlife Sanctuary, Chandauli, Uttar Pradesh. Int J Res Stud Biosci 5: 19-25.
- Sen A, Ghosh PD (2011) A note on the ethnobotanical studies of some pteridophytes in Assam. Ind J Trad Know 10: 292-295.
- Sarker SK (2009) Pteridophytes of greater Mymensingh district of Bangladesh used as vegetables and medicines. Bangl J Plant Taxon 16: 47-56.
- Grubben GJH, Denton OA (2004) Plant Resources of Tropical Africa 2. Vegetables, Backhuys Publishers, Wageningen, Netherlands.
- Thirupathi M, Karthikeyan S (2015) Distribution and ethnomedicinal uses of petridophytes in Sirumalai hills, eastern ghats of Tamilnadu, India. Int J Curr Res 7: 17224- 17227.
- Joshi BT, Srivastava A, Mishra RK (2019) Ecology and Ethnobotany of Ceratopteris thalictroides (L.) Brongn. Horticult Ecol stud Managt 140-146.
- Rout SD, Panda T, Mishra N (2009) Ethnomedicinal studies on some pteridophytes of Similipal Biosphere Reserve, Orissa, India. Int J Med Medical Sci 1: 192- 197.
- Baskaran XR, Geo Vigila AV, Zhang SZ, Feng SX, Liao WB (2018) A review of the use of pteridophytes for treating human ailments. J Zhejiang Univ Sci B 19: 85-119. [Crossref]
- Chen CY, Chiu FY, Lin Y, Huang WJ, Hsieh PS, et al. (2015) Chemical constituents analysis and antidiabetic activity validation of four fern species from Taiwan. Int J Mol Sci 16: 2497-2516. [Crossref]
- Thomas E, Gopalakrishnan V, Hegde M, Kumar S, Karki SS, et al. (2016) A Novel Resveratrol Based Tubulin Inhibitor Induces Mitotic Arrest and Activates Apoptosis in Cancer Cells. Sci Rep 6: 34653.
- Gopalakrishnan V, Dahal S, Radha G, Sharma S, Raghavan SC, et al. (2019) Characterization of DNA double-strand break repair pathways in diffuse large B cell lymphoma. Mol Carcinog 58: 219-233. [Crossref]
- Vartak SV, Hegde M, Iyer D, Gaikwad S, Gopalakrishnan V, et al. (2016) A novel inhibitor of BCL2, Disarib abrogates tumor growth while sparing platelets, by activating intrinsic pathway of apoptosis. Biochem Pharmacol 122: 10-22. [Crossref]
- Kavitha CV, Nambiar M, Narayanaswamy PB, Thomas E, Rathore U, et al. (2013) Propyl-2-(8-(3,4-difluorobenzyl)- 2',5'-dioxo-8-azaspiro[bicyclo[3.2.1] octane-3,4'-imidazolidine]-1'-yl) acetate induces apoptosis in human leukemia cells through mitochondrial pathway following cell cycle arrest. PLoS One 8: e69103. [Crossref]
- Sharma S, Choudhary B, Raghavan SC (2011) Efficiency of nonhomologous DNA end joining varies among somatic tissues, despite similarity in mechanism. Cell Mol Life Sci 68: 661-676. [Crossref]
- Iyer D, Vartak SV, Mishra A, Goldsmith G, Kumar S, et al. (2016) Identification of a novel BCL2-specific inhibitor that binds predominantly to the BH1 domain. FEBS J 283: 3408-3437. [Crossref]
- Srivastava M, Nambiar M, Sharma S, Karki SS, Goldsmith G, et al. (2012) An Inhibitor of Nonhomologous End-Joining Abrogates DoubleStrand Break Repair and Impedes Cancer Progression. Cell 151: 1474-1487. [Crossref]
- Sebastian R, Raghavan SC (2016) Induction of DNA damage and erroneous repair can explain genomic instability caused by endosulfan. Carcinogenesis 37: 929-940. [Crossref]
- Sharma S, Varsha KK, Kumari S, Gopalakrishnan V, Jose AE, et al. (2020) Acute toxicity analysis of Disarib, an inhibitor of BCL2. Sci Rep 10: 15188. [Crossref]
- Woldemariam TZ, Betz JM, Houghton PJ (1997) Analysis of aporphine and quinolizidine alkaloids from Caulophyllum thalictroides by densitometry and HPLC. J Pharm Biomed Anal 15: 839-843. [Crossref]
- Smitha V, Vadivel V (2019) Phytochemical screening for active compounds in Ceratopteris thalictroides (L.) Brogn. J Pharmacognosy Phytochem 8: 3556-3559.
- Mithraja MJ, Antonisamy JM, Mahesh M, Paul ZM, Jeeva S (2012) Chemical diversity analysis on some selected medicinally important pteridophytes of Western Ghats, India. Asian Pacific J Trop Biomed 2: S34-S39.
- Chen CY, Chiu FY, Lin YS, Huang WJ, Hsieh PS, et al. (2015) Chemical Constituents Analysis and Antidiabetic Activity Validation of Four Fern Species from Taiwan. Int J Mol Sci 16: 2497-2516. [Crossref]
- Pena-Blanco A, Garcia-Saez AJ (2018) Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J 285: 416-431. [Crossref]
- Restell TI, Porfirio LC, Souza ASd, Silva IS (2014) Hematology of Swiss mice (Mus musculus) of both genders and different ages. Acta Cirurgica Brasileira 29: 306-312.
- Lee Y-R, Chen M, Lee JD, Zhang J, Lin S-Y, et al. (2019) Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 364: eaau0159. [Crossref]
- Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, et al. (2018) Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554: 378-381. [Crossref]
- Thunold S (1967) Sex differences in tumour growth and lymphoid reactions in mice with Ehrlich’s Ascites Carcinoma. Acta Pathol Microbiol Scand 69: 521-533. [Crossref]
- Hartveit F (1962) Further Observations on the Results of Combining Freund's Adjuvant with Living Ehrlich Ascites Carcinoma. Br J Cancer 16: 331–339. [Crossref]
- Srivastava M, Nambiar M, Sharma S, Karki SS, Goldsmith G, et al. (2012) An inhibitor of nonhomologous end-joining abrogates double-strand break repair and impedes cancer progression. Cell 151: 1474-1487. [Crossref]







