Abstract
Introduction: This study assessed the user performance and repeatability precision of the Contour Care blood glucose monitoring system in accordance with DIN EN ISO 15197:2015 criteria.
Methods: In the clinical trial (N=100), untrained subjects with diabetes self-tested capillary fingertip blood samples and completed an ease-of-use questionnaire. BGMS measurements of a single lot were compared to Cobas c111 results and assessed according to DIN EN ISO 15197:2015 and ADA guidelines.
Repeatability measurement precision was assessed using venous blood samples and three different test strip lots. As DIN EN ISO 15197:2015 does not specify acceptance criteria, standard deviations (SDs) and coefficients of variation (CV) for glucose concentrations < 100 mg/dL and ≥ 100 mg/dL, respectively, were calculated.
Results: In the clinical trial, the BGM demonstrated full compliance with all ISO 15197:2015 acceptance criteria: 100% of measurement results were within ± 15 mg/dL for blood glucose concentrations < 100 mg/dL and within ± 15% for concentrations ≥ 100 mg/dL. 100% of measurements fell within Zone A of the CEG. Due to a lack of hypoglycemic data, only the hyperglycemic range was evaluated in alignment with ADA guidelines and found to comply with all requirements. The majority of subjects found the BGMS easy to use, reflected in consistent user-friendliness and high acceptance ratings. Assessment of precision (SD and CV) also confirmed compliance with the predefined acceptance criteria.
Conclusion: The BGM exceeded minimum acceptance criteria and demonstrates exceptional usability. These data integrate into a consistent overall picture of the Contour® family's performance demonstrating that it represents a reliable solution for routine self-monitoring.
List of Abbreviations: ADA: American Diabetes Association; BGMS: Blood Glucose Monitoring System; SD: Standard Deviation; CV: Coefficient of Variation; MARD: Mean Absolute Relative Deviation
Keywords
performance evaluation by the user, repeatability measurement precision, system accuracy, ISO 15197, diabetes therapy, self-monitoring
Introduction
Within diabetes therapy, self-monitoring of blood glucose (SMBG) has long since become an integral part of the disease management, providing patients with the means for an active self-management of their condition and thus a generally a better glycemic control [1,2]. While advances in continuous glucose monitoring (CGM) enables people with diabetes to monitor their glycemic state in near real-time, this approach is not always feasible or indicated for various reasons [3-5], rendering conventional blood glucose monitoring systems (BGMS) a still relevant aspect of the treatment. This study evaluated the blood glucose monitoring system Contour Care (Ascensia Diabetes Care) in two clinical trial settings in accordance with DIN EN ISO 15197:2015, section 6.2.3. - Repeatability measurement precision and section 8. - Performance evaluation by the user as well as ADA guidelines [6,7]. The data integrates into a wider context of a post market follow up assessment of the device [8] and focuses on precision (i.e., random measurement errors within replicates), and accuracy as influenced by patient-related factors, including an evaluation of the ease of use for individuals with diabetes without prior experience with the device.
Materials and methods
Clinical trials
All tests were conducted across two separate clinical trials (clinicaltrials.gov IDs: user performance NCT06937736, repeatability precision NCT06037551) at the Institute of Diabetes Karlsburg facilities between September 2023 and August 2024. Ethical conduct, regulatory compliance, and scientific rigor were ensured throughout the study. Prior to subject recruitment, trial protocols, informed consent and other study forms were approved by the responsible ethics committee and exempted from approval by the competent authority. All participants were informed about the objective and procedures and potential risks. To participate, a signed informed consent was requested from the volunteer. Medications and/or supplements as well as relevant medical conditions were recorded.
Repeatability measurement precision
In accordance with ISO 15197:2015 requirements, evaluation of repeatability measurement precision was performed using fresh human venous blood of a single donor and completed within 8 h after sampling. Ten test meters and 3 reagent lots were used to analyze 5 sub-samples with glucose concentrations ranging between 30 and 400 mg/dL (see Table 1 in [6] and Table 1 of this report). For higher glucose concentrations, predefined amounts of a 20% glucose solution were added to the sample and incubated on a shaker for 30 minutes at room temperature. For hypoglycemic glucose ranges, samples were incubated in a water bath at a maximum of 37°C for 2–4 hours. Each meter/sample/lot-combination was measured 10 times under monitored environmental conditions using whole blood. Reference measurements were performed before and after test measurements using a Cobas c111 analyzer using plasma in duplicate to verify sample stability. All measurements were recorded in mg/dL. Hematocrit was measured electro-optically using a HemoCue Hb 801.
Performance evaluation by the user
A total of 101 people with type 1 and type 2 diabetes (ICD-10: E10, 11) aged ≥ 18 years were enrolled for the performance evaluation by the user. Exclusion criteria were implemented, including prior experience with the test device or underlying medical conditions that might impose a risk towards study personnel or the patient himself. Participants were also excluded if they reported using medications or supplements listed in Appendix A of [6] and their measurements were found to be deviant, potentially indicating an interference with the test system.
The participants were provided with the test device of their preferred measurement unit and all accompanying material as provided in the standard commercial packaging. After sufficient time to familiarize with the device and manual or quicks start guide, subjects obtained capillary blood by a fingertip puncture using a disposable lancet. Measurements were repeated in case of an error message. Following self-measurement using the test meter, a sample of capillary blood was taken by trial staff for the determination of the packed cell volume and glucose within 5 minutes of user testing. Hematocrit was measured electro-optically using a HemoCue Hb 801, glucose was measured in plasma using a Cobas c111 Analyzer (Hexokinase, Roche Diagnostics). Following the user test of the device, subjects were asked to complete a thirteen-item ease-of-use questionnaire to evaluate the user experience comprising factors such as ease of operation, clarity of instructions, the device’s usability, and maintenance. All responses were based on a 5-point Likert scale (very positive / strongly agree, positive / agree, neutral, negative / disagree, very negative / strongly disagree), no weights were applied. All measurements with the reference device were recorded in mg/dL. Test meter measurements were recorded in mg/dL and mmol/L.
Data analysis
Repeatability precision: For each sample (i.e., glucose concentration according to Table 1), the mean result, the standard deviation (SD) and the coefficient of variation (CV) were calculated for each test strip lot separately. DIN EN ISO 15197:2015 does not stipulate minimum requirements for the repeatability measurement precision. Acceptance criteria were SD < 5 mg/dL for means < 100 mg/dL and CV < 5% for means ≥ 100 mg/dL. No data were excluded.
Performance evaluation: All analyses and calculations were performed in mg/dL. Measurements recorded in mmol/L were converted using the formula:
Frm. 1 Gluc(mg/dL) =Gluc(mmol/L) × 18.01802
Self-measurement results were compared with laboratory reference results using the trial staff-obtained blood sample. Analytical accuracy was assessed based on ISO 15197:2015 guidelines requiring that ≥ 95% of measurements lie within ± 15 mg/dL /% of mean reference results, and ≥ 99% of measurements lying in zones A and B of the consensus error grid. Analysis included regression analysis, construction of modified Bland-Altman plots, Consensus-Error Grid for Diabetes Type I analysis [9,10], as well as an assessment of established performance metrics (i.e. MARD, BIAS and an ad-hoc confidence metric, i.e. the narrowest error margin comprising at least 95% of meter inaccuracies [8]).
Results
Repeatability measurement precision
In the glucose range between 93.7 mg/dL - 307.4 mg/dL evaluation of the repeatability measurement precision resulted in a mean SD of 1.3 mg/dL for samples with blood glucose concentrations < 100 mg/dL and in a mean CV of 2.0% for glucose concentrations ≥ 100 mg/dL (Table 2). The system fully complies with the acceptance criteria.
|
Within specified error limits |
Performance metrics |
ISO 15197 |
± 5 mg/dL /± 5% |
± 10 mg/dL / ± 10% |
± 15 mg/dL /
± 15% |
BIAS |
MARD |
ad-hoc |
< 100 mg/dL (n=16) |
79.1 – 100
mg/dL |
15/16
(93.8%) |
15/16
(93.8%) |
16/16
100%) |
100/100 (100%) |
1.7 |
-0.9 |
3.2 |
3.5 |
6.6 mg/dL |
8.4
mg/dL /% |
≥ 100 mg/dL (n=84) |
100 – 348.7
mg/dL |
67/84
(79.8%) |
83/84
(98.8%) |
84/84
(100%) |
-1.4 |
3.6 |
8.4% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Table 2. Summary of blood glucose monitoring system results from assessment of analytical accuracy according to DIN EN ISO 15197:2015 and additional performance metrics
Analytical accuracy
After exclusion of 1 participant due to intake of substance(s) and/or underlying medical conditions that may interfere with blood glucose measurements as outlined in Appendix A of [6], a total of 100 evaluable measurements and questionnaire user-feedbacks were obtained. Key demographic characteristics of the study cohort are summarized in Figure 1. Assessment of analytical accuracy in self-application demonstrated that the system fully complies with the DIN EN ISO 15197:2015 acceptance criteria. In the glucose concentration range of 79.1 – 348.7 mg/dL, 100% of measurement results were within ± 15 mg/dL or 15% of the reference (Figure 2, Table 3). While no hypoglycemic samples < 70 mg/dL were available for analysis, evaluation of the normoglycemic and hyperglycemic range (i.e., 70 - 180 mg/dL and > 180 mg/dL, respectively) attest full compliance with the standard showing that 100% of measurements were within 15 mg/dL / % of the reference. The ad-hoc performance indicator was calculated at 8.4 mg/dL / % and 8.1%, and the BIAS shifting from -0.4 mg/dL towards -2.3%, respectively (compare Figure 2A).
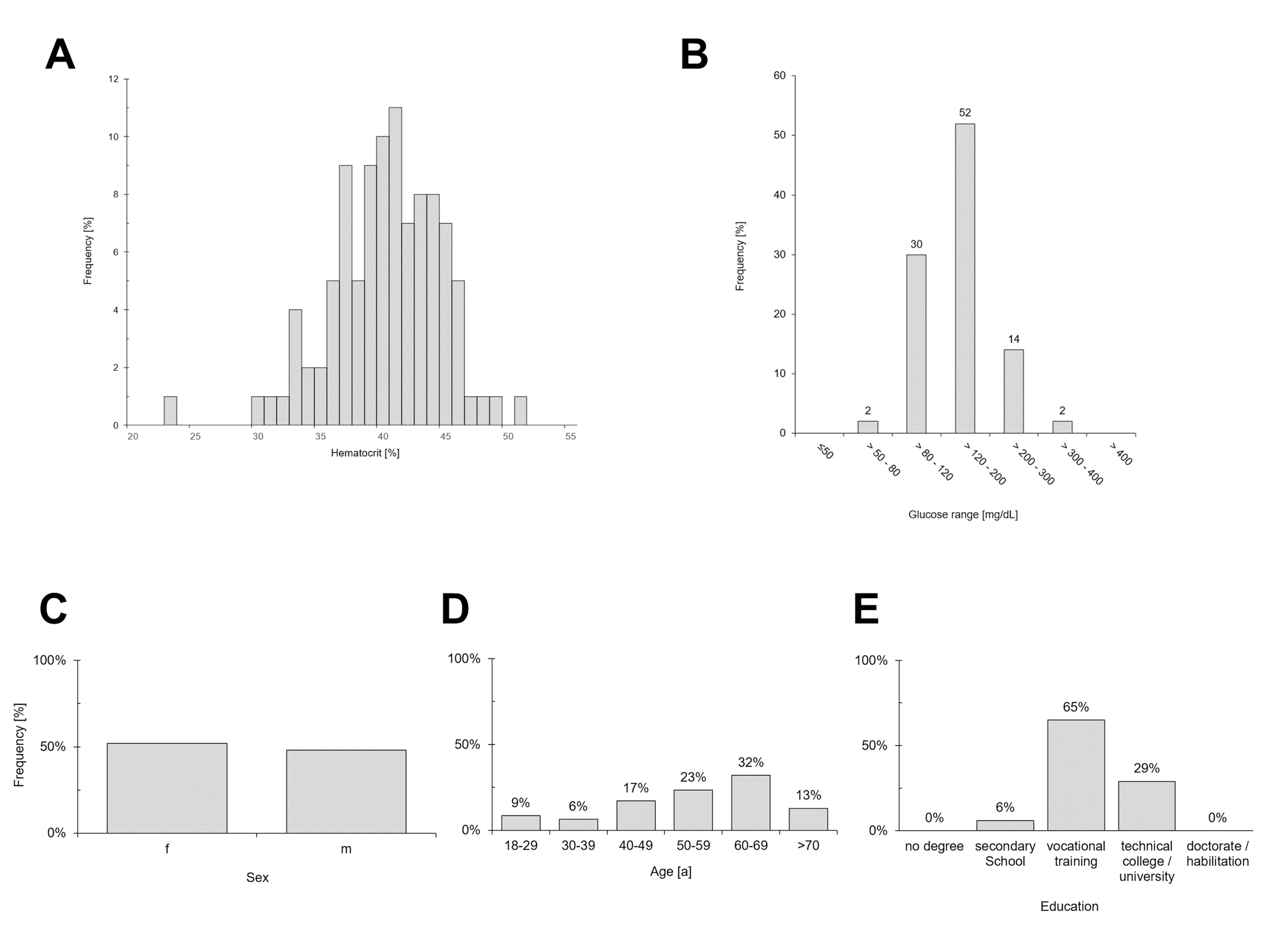
Figure 1. Overview of demographic characteristics for the performance evaluation by the user. (A) Sampled hematocrit values following a normal distribution, (B) sampled glucose ranges, (C) gender distribution, (D) age distribution, (E) educational levels
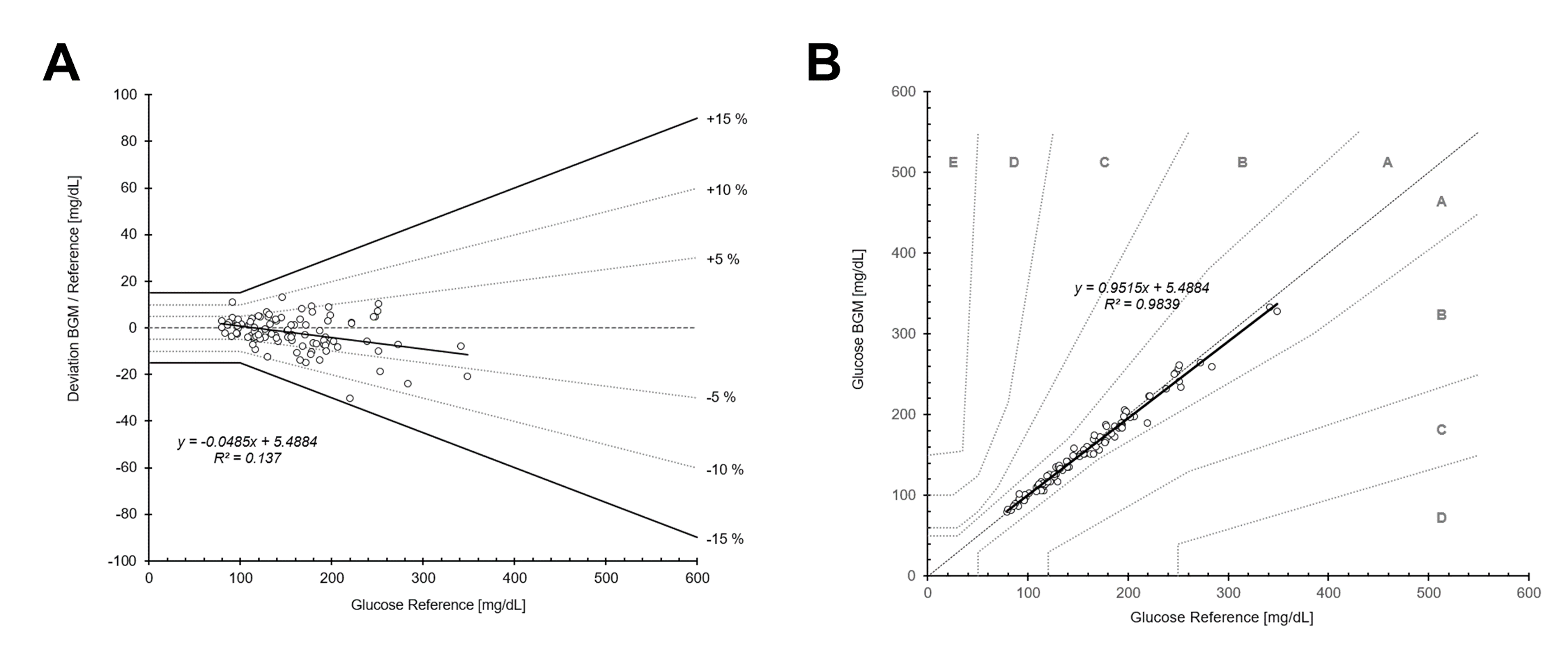
Figure 2. Accuracy assessment of the performance evaluation by the User. Bland-Altman-plot showing deviation from blood glucose monitor glucose to reference measurements (A) and Consensus-Error-Grid for Diabetes Type I (B) including regression analysis
|
Within specified error limits |
Performance metrics |
ADA SMCD |
± 5 mg/dL /± 5% |
± 10 mg/dL / ± 10% |
± 15 mg/dL /
± 15% |
BIAS |
MARD |
ad-hoc |
hypo |
< 70
mg/dL
(n=0) |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
in range |
70 – 180 mg/dL
(n=72) |
45/72
(62.5%) |
71/72
(98.6%) |
72/72
(100%) |
-0.4 |
3.4 |
8.4
mg/dL /% |
hyper |
≥ 180 mg/dL
(n=28) |
27/28
(96.4%) |
28/28
(100%) |
28/28
(100%) |
-2.3 |
3.8 |
8.1% |
Table 3. Summary of blood glucose monitoring system results from assessment of analytical accuracy according to ADA Diabetes Medial Care guidelines and additional performance metrics
Performance evaluation by user
Test subjects were 18–78 years of age, displayed an equal gender distribution, and a broad educational level, with the majority having completed vocational training (Figure 1). The performance evaluation by the user demonstrates a high degree of usability, mirrored in consistent user-friendliness and high user acceptance ratings in design, functionality, and operability. Without prior training with the BGMS all users were able to operate the meter, not requiring any trial runs. In total, between 92% - 100% of test subjects responded with ‘‘very positive / strongly agree’’ and ‘‘positive / agree’’ (summarized in Figure 3). Notably, test strip sample uptake, readability and comprehensibility of measurement results received 100% positive ratings. Some shortcomings have been identified, however. Potential areas of conflict between the test group’s demographics and the device’s design resulted in decreases in user satisfaction. Changing batteries and/or recharging the device was consistently criticized as being difficult by older subjects (92%), followed by comprehensibility of error messages (93%) and comprehensibility of display/menu/symbols (94%).
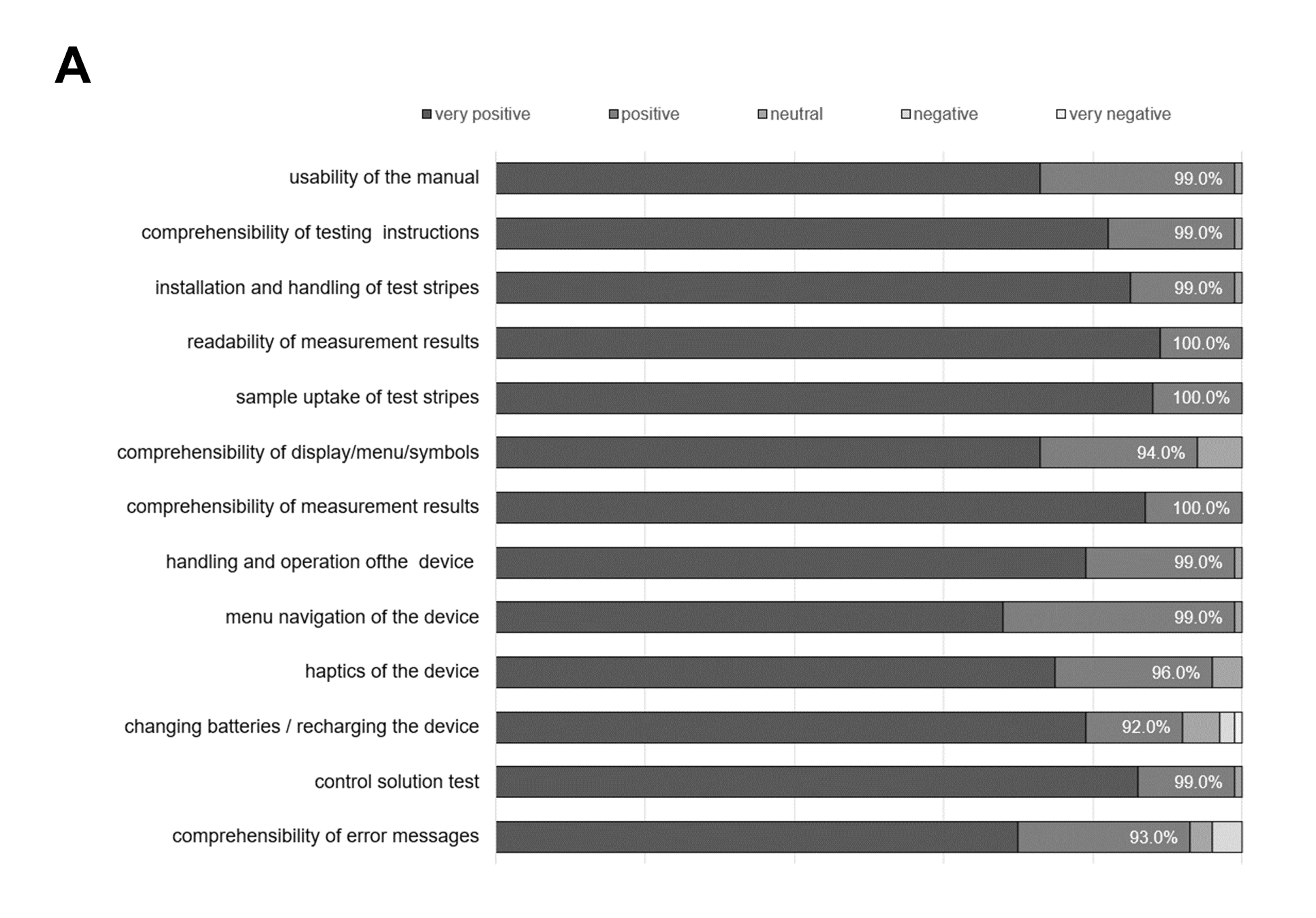
Figure 3. Results of the performance evaluation by the user ease-of-use questionnaire. Percentages represent the proportion of subjects responding with ‘‘very positive / strongly agree’’ and ‘‘positive / agree’’
Discussion
Assessment and control of the glycemic status is one of the crucial elements of diabetes management [2,7]. Reliable, i.e., accurate and precise information about variations of blood glucose levels enables both physicians and patients to assess the effect of procedures, diet or treatment decisions on recovery and maintenance of physiologically acceptable blood glucose levels. Monitoring is instrumental for adjustments in medications and diet in order to achieve optimal glycemic control that reduces the risks of short-term and long-term complications [2,11]. Advances in CGM have simplified monitoring for patients with diabetes, enabling a near real-time assessment that, despite its name, is almost continuous. Next to current glucose levels, it is direction and magnitude of change that are of particular importance to assess the glycemic variability and to predict and prevent impending glucose excursions [12,13]. However, in the light of the still present limitations in technology or implementation [3-5], conventional BGMS remain a very relevant component of diabetes management and are still advised by guidelines and manufacturers as a backup system [14-17] to address issues like connection problems, detached sensors, or conflicting symptoms.
|
Glucose concentration range |
Lot |
30-50 mg/dL |
51-110 mg/dL |
111–150 mg/dL |
151-250 mg/dL |
251-400 mg/dL |
BGM measurement (reference glucose) |
41.6 mg/dL
(42.4 mg/dL) |
89.9 mg/dL
(93.7 mg/dL) |
135.6 mg/dL
(142.5 mg/dL) |
192.9 mg/dL
(203.5 mg/dL) |
288.3 mg/dL
(307.4 mg/dL) |
1 |
0.7 mg/dL |
1.6 mg/dL |
2.0% |
1.6% |
1.5% |
2 |
0.8 mg/dL |
1.5 mg/dL |
1.5% |
1.7% |
1.5% |
3 |
0.8 mg/dL |
1.6 mg/dL |
1.5% |
1.9% |
2.2% |
Table 1. Measurement Repeatability Results for Contour Care. For samples with blood glucose concentrations < 100 mg/dL results are given as SD, for glucose concentrations ≥ 100 mg/dL results are given as CV
Decisions on effective therapeutic interventions necessitate measurements that represent the individual's actual glycemic state. In this light, while compliance with the relevant standards is a mandatory prerequisite for market release and use of BGMS, the degree of adherence to those standards can vary substantially and is therefore better understood as a spectrum along which a more nuanced definition of quality may be required. Ultimately, the highest possible measurement accuracy is in the patient's best interest for successful therapy decisions.
Numerous studies on Contour BGMS confirm full compliance with the requirements of DIN EN ISO 15197:2013, as well as the more stringent 2015 revision [18-21]. Available literature on performance evaluation by the user also highlights that the majority of subjects found the BGMS easy to use [21-23].
A prior evaluation by the authors already attested to the test device’s excellent system accuracy [8]. This report confirms these previous findings and expands by including an assessment of its precision as well as accuracy in the hands of lay patients of varying technical expertise and no prior experience with the device as confounding factors. Given the significant impact of device usability on adherence to diabetes management protocols, incorporating human factors and usability engineering principles into the development and evaluation of BGMS is of particular importance [24-27].
Conclusion
These consistent findings of excellent results and full, or even exceeding, compliance evidently characterize the entire Contour family. Therefore, in demonstrating impeccable results even among inexperienced users, the BGM Contour Care represents an effective and reliable solution for routine self-monitoring.
Conflict of interest
There are no conflicts of interest.
Funding
All authors are employees of the Institute of Diabetes Karlsburg GmbH, Germany, which carries out studies evaluating blood glucose meter systems on behalf of various clients. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Acknowledgement
The authors would like to thank the personnel of the Institute of Diabetes GmbH for providing technical help, intellectual input and feedback in conducting the studies, and preparing the manuscript. Sponsors were permitted to review and comment on the manuscript, final decision on content was retained by the authors.
References
- Poolsup N, Suksomboon N, Rattanasookchit S (2009) Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: An update. Diabetes Technol Ther 11: 775-784. [Crossref]
- Renard E (2005) Monitoring glycemic control: The importance of self-monitoring of blood glucose. The Am J Med 118: 12-19. [Crossref]
- Block CD, Vertommen J, Manuel-y-Keenoy B, Gaal LV (2008) Minimally-invasive and non-invasive continuous glucose monitoring systems: Indications, advantages, limitations and clinical aspects. Curr Diabetes Rev 4: 159-168. [Crossref]
- Wollersheim T, Engelhardt LJ, Pachulla J, Moergeli R, Koch S, et al. (2016) Accuracy, reliability, feasibility and nurse acceptance of a subcutaneous continuous glucose management system in critically ill patients: A prospective clinical trial. Ann Intensive Care 6: 1-3. [Crossref]
- Kruger DF, Anderson JE (2021) Continuous glucose monitoring (CGM) is a tool, not a reward: Unjustified insurance coverage criteria limit access to CGM. Diabetes Technol Ther 23: S45-S55. [Crossref]
- DIN EN ISO 15197:2015-12, In vitro diagnostic test systems - Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197:2013); EN ISO 15197:2015 DIN Media GmbH
- American Diabetes Association (2021) 15. Diabetes care in the hospital: Standards of medical care in diabetes—2021. Diabetes Care 44: S211-S220. [Crossref]
- Kenning M, Puchert A, Salzsieder E (2024) Comparative system accuracy of blood glucose monitoring systems—Advocacy for a new accuracy assessment metric. J Diabetes Sci Technol 19: 270-271. [Crossref]
- Parkes JL, Slatin SL, Pardo S, Ginsberg BH (2000) A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 23: 1143-1148. [Crossref]
- Klonoff DC, Lias C, Vigersky R, Clarke W, Parkes JL, et al. (2014) The surveillance error grid. J Diabetes Sci Technol 8: 658-672. [Crossref]
- Karter AJ (2006) Role of self-monitoring of blood glucose in glycemic control. Endocr Pract 12: 110-117. [Crossref]
- Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL (1987) Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 10: 622-628. [Crossref]
- Kovatchev B (2019) Glycemic variability: Risk factors, assessment, and control. J Diabetes Sci Technol 13: 627-635. [Crossref]
- Holt RI, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, et al. (2021) The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 44: 2589-2625. [Crossref]
- American Diabetes Association (2022) Introduction: Standards of medical care in diabetes—2022. Diabetes Care 45: S1-S2. [Crossref]
- Fact sheet: Continuous glucose monitoring [Internet]. National Diabetes Service Scheme. 2025. Verfügbar unter: https://www.ndss.com.au/wp-content/uploads/fact-sheets/fact-sheet-continuous-glucose-monitoring.pdf
- Use CGM and BGM together to manage your diabetes [Internet]. 2025 [zitiert 6. Mai 2025]. An accurate BGM is an important component for self-management by CGM users. Verfügbar unter: https://www.diabetes.ascensia.com.au/bgmforcgm/
- Klaff L, Shelat P, Zondorak D, Wayland-Smith A, Vernes P, et al. (2021) Accuracy and user performance of a new blood glucose monitoring system. J Diabetes Sci Technol 15: 1382-1389. [Crossref]
- Dunne N, Viggiani MT, Pardo S, Robinson C, Parkes JL (2015) Accuracy evaluation of CONTOUR® PLUS compared with four blood glucose monitoring systems. Diabetes Ther 6: 377-388. [Crossref]
- Klaff LJ, Brazg R, Hughes K, Tideman AM, Schachner HC, et al. (2015) Accuracy evaluation of contour next compared with five blood glucose monitoring systems across a wide range of blood glucose concentrations occurring in a clinical research setting. Diabetes Technol Ther 17: 8-15. [Crossref]
- Bailey T, Wallace JF, Greene C, Pardo S, Brown D, et al. (2015) Accuracy and user performance evaluation of the Contour® Next Link 2.4 blood glucose monitoring system. Clin Chim Acta 448: 139-145. [Crossref]
- Caswell M, Frank J, Viggiani MT, Pardo S, Dunne N, et al. (2015) Accuracy and user performance evaluation of a blood glucose monitoring system. Diabetes Technol Ther 17: 152-158. [Crossref]
- Bailey TS, Wallace JF, Pardo S, Warchal-Windham ME, Harrison B, et al. (2017) Accuracy and user performance evaluation of a new, wireless-enabled blood glucose monitoring system that links to a smart mobile device. J Diabetes Sci Technol 11: 736-743. [Crossref]
- Toletti G, Boaretto A, Di Loreto C, Fornengo R, Gigante A, et al. (2024) Enhancing diabetes therapy adherence: A comprehensive study on glucometer usability for type 2 diabetes patients. Front Clin Diabetes Healthc 5: 1328181. [Crossref]
- Heinemann L, Drossel D, Freckmann G, Kulzer B (2016) Usability of medical devices for patients with diabetes who are visually impaired or blind. J Diabetes Sci Technol 10: 1382-1387. [Crossref]
- Berkman ND, Davis TC, McCormack L (2010) Health literacy: What is it?. J Health Commun 15: 9-19. [Crossref]
- Marciano L, Camerini AL, Schulz PJ (2019) The role of health literacy in diabetes knowledge, self-care, and glycemic control: A meta-analysis. J Gen Intern Med 34: 1007-1017. [Crossref]
