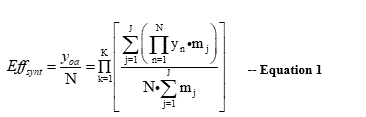A synthesis efficiency algorithm is essential for the evaluation and control of complex drug syntheses in chemistry and life sciences. It must also be based on concrete and reliable criteria. Such an algorithm has been developed to precisely evaluate multicomponent reactions (MCRs) as well as even highly complex systems and determine their synthesis efficiency Effsynt. The efficiency algorithm is also able to discriminate different pathways within the same synthesis in terms of efficiency. The mathematical operations are highly suitable for electronic data processing (EDP). This algorithm can also be used as a basis for fair cost assessment of complex chemical syntheses.
synthesis control, synthesis efficiency, synthesis evaluation, efficiency algorithm, algorithm discrimination, algorithm control, complex syntheses, multicomponents
During the current socio-economic transformation and the ongoing upheavals in the sectors of information technology, energy, electromobility and biochemistry, which are in some cases extremely competitive, the focus is increasingly on the ecologic and economic aspects of chemistry as applicable to humans. Comprehensive efforts are being undertaken in this field, including in large workshops, e.g. [1]. In drug chemistry, the synthetical standard is highly developed with regards to the effectiveness on diseases and its selectivity is as good as possible. The synthetical part of chemical drug production is still generally stringently conventional, generating an enormous consumption of resources and exorbitant costs! This is strongly contrary to the fundamental philosophy and the requirements of green chemistry. To change this economic and ecological downside, great efforts have been made on the one hand through the highly successful use of biochemical and molecular biological techniques. On the other hand, the in some ways advantageous system of organic chemistry can be changed into a highly effective and highly efficient synthesis system when synthesis steps are radically reduced [2,3]. This is not achievable with a conventional chemical synthesis setup of reactions involving 1 or 2 reaction partners. Using 3 or more reactants, however, creates a breakthrough into a completely novel synthesis strategy (Scheme 1). This kind of chemistry is known as multicomponent reaction chemistry (MCR) [4,5], and has already been successful in various drug syntheses [2-9].
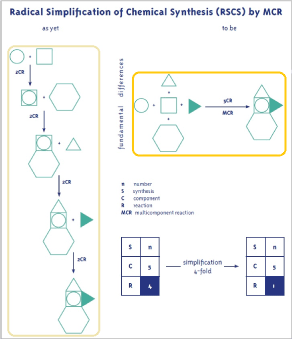
Figure 1. Radical simplification of chemical synthesis by MCR chemistry.
Scheme 1 already demonstrates 4-fold simplification. This corresponds to approximately 75% less resources, energy, work etc. being used and thus 75% less overall costs. This is a convincing argument for using MCR chemistry [2,3]. Most chemical drug syntheses are highly complex and therefore require numerous synthesis steps n. A precise and reliable tool is required here, based on concrete criteria overall yields yoa and number of synthesis steps N that can be easily measured, delivering the synthesis efficiency Effsynt. This has been developed for highly complex syntheses, resulting in the efficiency algorithm in Equation 1 [10]. The mathematical operations are highly suitable for electronic data processing (EDP).
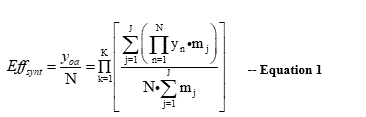
K = number of main reaction steps
j = number of branches
N = number of synthesis steps
m = number of weightings
y = weighted yields
The US Food and Drug Administration approved a record number of new drugs in 2018, with 59 scientific highlights reaching the market [11]. Five of these drugs, 1-5, may gain a broad use in applications due to their effects on common and severe diseases.
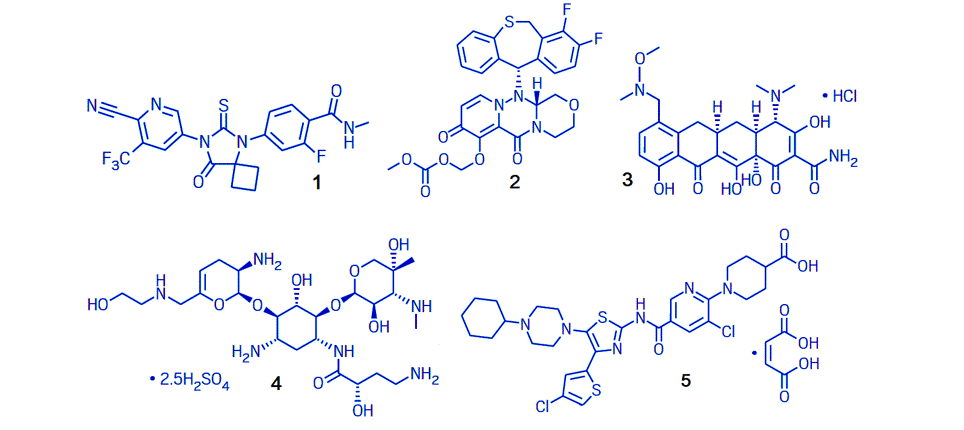
1 |
Erleada |
Apalutamide |
Prostate cancer |
Johnson + Johnson |
2 |
Xofluza |
Baloxavir marboxil |
Influenza |
Genentech |
3 |
Seysara |
Sarecycline |
Acne vulgaris |
Paratec Pharmaceutical |
4 |
Zemdri |
Plazomicin |
Urinary tract infections |
Achaogen |
5 |
Doptelet |
Avatrombopag |
Chronic kidney disease |
Dova Pharma |
To achieve the most efficient syntheses for the drugs 1-5, each Effsynt has to be determined by using the efficiency algorithm of Equation 1, then compared with other alternative syntheses and with different configurations of the synthesis setups within the same synthesis.
As insufficient data (all yields yn and synthesis steps N) for 1-5 is available in literature, the case study (Scheme 4 in [10]) will principally show the method for using the algorithm in Equation 1. Logically, the efficiency algorithm illustrates the synthesis setup in Scheme 2 as three reaction sets 1-3, namely as Schemes 2-4 and showing the complex synthesis of the case study as three separate flow diagrams.
Random numbers were added to the Part A-J with both parallel reactions V-D and X-G in Scheme 2. The overall yield yoa was then calculated incrementally with the algorithm in Equation 1. Expediently, a main reaction (set-1) to which the parallel reactions are linked (set-2, set-3) is set up. The weighted arithmetical mean of the yields for each reaction set is derived at the connection forms. The overall yield yoa of the total synthesis is determined through the sequential operation of the main reaction sets. The latter can be calculated using the following calculation method. Finally, the value of yoa is divided by the total synthesis steps N to obtain the synthesis efficiency Effsynt.

Figure 2. Efficiency algorithm calculation of Effsynt for forming J via synthesis setup-1.
Calculation execution
Split the main reaction set, set-1, at the connection points and with the parallel reactions into set-1, set-2 and set-3, then use operation ∑(∏yn)j to calculate the weighted mean yield values for both branches C,D and E,G. The latter values, together with the values from P[∑(∏yn)j]k, deliver the overall yield yoa. Dividing this by the total synthesis step number N = 8 provides the synthesis efficiency Effsynt according to Equations 2-4.
parallel reactions set-2 to main reaction position (C), operation ∑(∏yn)j
y(C,D) = 1/2*[y(A-C)*1+y(V-D)*1] -Equation 2
= 1/2*[0.80*1+0.67*1] = 1/2*1.47 = 0.735
parallel reactions set-3 to main reaction position (E), operation ∑(∏yn)j
y(E,G) = 1/4*[y(C-E)*1+y(X-G)*3] -Equation 3
= 1/4*[0.77*1+(0.83*0.79*0.72)*3] = 1/4 [0.77+1.416] = 0.547
sequential main reactions set-1, operation ∏[∑(∏yn)j]k
y(A-J) = y[(C,D)*(E,G)*(E-J) ] -Equation 4
= 0.735*0.547*0.92*0.88
yoa = 0.326 (32.6%)
This synthesis consists of N = 8 synthesis steps, so the synthesis efficiency is
Effsynt = yoa/N = 0.3255/8 = 0.0407 (4.07%)
General order is almost identical to Scheme 3 synthesis setup-2 above. The parallel reaction branch A-E includes a further parallel reaction branch V-D and so A-E is also a (sub)main reaction set (Equation 6).
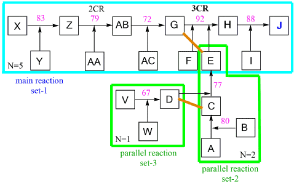
Figure 3. Efficiency algorithm calculation of Effsynt for forming J via synthesis setup-2.
Calculation execution
Split the main reaction set-1 at the connection points with the parallel reactions into set-1, set-2 and set-3, then use operation ∑(∏yn)j to calculate the weighted mean yield values for the two branches D,C and G,E, whereby D,C is a branch of A-E in synthesis setup-2. The latter values, together with the values from ∏[∑(∏yn)j]k deliver the overall yield yoa, dividing this by the total synthesis step number N=8 provides the synthesis efficiency Effsynt according to Equation 5-8.
parallel reactions set-3 to (sub)main reaction set-2 position (C), operation ∑(∏yn)j
y(D,C) = ½*[y(V-D)*1+y(A-C*1) -Equation 5
= ½*[0.67*1+0.80*1] = ½*1.47 = 0.735
sequential (sub)main reactions set-2, operation ∏[∑(∏yn)j]k
y(A-E) = y[(D,C)*(C-E)] -Equation 6
= y[0.735*0.77] = 0.566
parallel (sub)main reactions set-2 to main-reactions set-1 position (G), operation ∑(∏yn)j
y(G,E) = ½[y(AB-G)*1+y(A-E)*1] -Equation 7
= ½[ 0.72+0.566] = ½*1.286=0.643
sequential main reactions set-1, operation ∏[∑(∏yn)j]k
y(A-J) = y[(X-AB)*(G,E)*(G-J) -Equation 8
= 0.83*0.79*0.643*0.92*0.88
yoa = 0.341 (34.1%)
Effsynt = yoa / N = 0,341/8 = 0.0427 (4.27%)
Efficiency balance on synthesis setups in terms of overall yields yoa
Overall yield of synthesis setup-2 (Scheme 3) differs by 1.5% from that of synthesis setup-1 (Scheme 2). Synthesis setup-2 (34.1%) is therefore relatively 4.6% higher than synthesis setup-1 (32.6%). Thus, synthesis setups can play a respectable role in complex chemical syntheses and can easily be determined with the efficiency-algorithm (Equation 1).

Figure 4. Efficiency algorithm calculation of Effsynt of forming J via synthesis setup-3.
The capable efficiency algorithm [10] can be used to control complex drug syntheses, to find the most efficient synthesis by comparison with alternatives, and to precisely evaluate even highly complex systems. The efficiency algorithm is also able to discriminate different pathways within the same synthesis in terms of efficiency. The mathematical operations are highly suitable for electronic data processing (EDP), as is the algorithm itself. The concrete criteria mean that this algorithm is also suitable as a basis for fair cost assessment of complex syntheses in chemistry and life sciences.
- Dr. Michael Weber (2016) Energie-Effizienz Technologien in der Chemischen Industrie. [Online] Available at: http://low-carbon-urban-development-germany-china.org/wp-content/uploads/2016/04/Weber-Sites-Consulting-Energy-Efficient-Technologies-in-Chemical-Industry-DE.pdf
- Eckert H (2017) Synergy effects in the chemical synthesis and extensions of multicomponent reactions (MCRs)-The low energy way to ultra-short syntheses of tailor-made molecules. Molecules 22(3): 349(1-32). [Crossref]
- Eckert H, Wolff-Verlag (2017) Radical and concerted simplification of chemical synthesis. Berlin.
- Zhu J, Bienayme H, Wiley-VCH (2005) Multicomponent reactions, Weinheim. Germany.
- Science of synthesis (2014) Multicomponent reactions I+II. Ed.: Mueller TJJ. Thieme: Stuttgart. Germany.
- Tietze LF, Wiley-VCH (2014) Domino reactions: Concepts for efficient organic synthesis. Weinheim.
- Endo A, Yanagisawa A, Abe M, Tohma S, Kan T, et al. (2002) Total synthesis of ecteinascidin 743. J Am Chem Soc 124: 6552-6554. [Crossref]
- Znabet A, Polak MM, Janssen E, de Kanter FJ, Turner NJ, et al. (2010) A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem Commun 46: 7918-7920. [Crossref]
- Zhu J, Bienayme H (2005) Multicomponent reactions, Wiley-VCH: Weinheim. Germany 24-25.
- Eckert H (2019) Efficiency measurement of complex syntheses in chemistry and life sciences. Journal of Chemical Engineering And Bioanalytical Chemistry 2: 114-117.
- Jarvis LM (2019) The new drugs of 2018. C&EN 97: 32-37.