Many experimental models are applied to expand the arsenal of molecules that can inhibit or stimulate angiogenesis. Walker 256 carcinosarcoma was discovered when analyzing mammary gland adenocarcinomas in female rats and has been maintained in the laboratory since then. It has fast and aggressive growth, enabling experimental evaluation of the response to antiangiogenics. We analyzed the use of gum arabic, a natural substance obtained from the sap of the trunks and branches of Acacia senegal trees, and eugenol, one of the main components of clove essential oil, against Walker tumors in rats. Both substances alone have been experimentally found to have antioxidant activity, as well as anticancerogenic and cytotoxic effects, but no study has yet tested the effect of both substances simultaneously. Specifically, we evaluated the effect of gum arabic and eugenol on the progression and angiogenesis of Walker tumor cells implanted in the subcutaneous tissue of Wistar rats. Sixty female rats, with average weight of 150 grams, were distributed in 10 groups of 6 animals each, with five control groups and five treatment groups. The treatment groups received Walker tumor implants on the first day of the experiment, followed by administration of the test substances by gavage for 5 days. The control groups received the same substances, except the inoculum was distilled water. The substances administered were distilled water, celecoxib, gum arabic and eugenol, the last of which was used in pure form. We evaluated the tumor dimensions and macroscopic and microscopic appearance (hematoxylin-eosin staining). The study of angiogenesis was performed by immunohistochemistry (CD-31), and the quantification was achieved by evaluation of the microvascular density by the SAMM software applied to images of the areas with greatest microvascular density. The animals that received only distilled water suffered from greater invasion of the dorsal subcutaneous tissue and musculature by the tumor in relation to the other groups. The tumor volume was smaller in the groups that received gum arabic or eugenol alone and those receiving gum arabic together with eugenol and celecoxib, but the microvascular density was smaller only in the group that received celecoxib alone. Thus, only celecoxib inhibited angiogenesis. Gum arabic and eugenol, in the doses administered, reduced the tumor growth, but did not inhibit angiogenesis of the Walker tumor cell implants.
angiogenesis inhibitors, walker 256 carcinoma, gum arabic eugenol
Angiogenesis is a process consisting of multiple steps leading to the formation of new blood vessels from existing capillaries. In 1971, Judah Folkman reported the importance of vascularization for the growth of tumors, postulating that a tumor might die if it were prevented from obtaining its own blood supply. Therefore, inhibition of tumor vascularization can be a promising way to treat various types of cancer [1]. The appropriate dose of the antiangiogenic agent can lead to normalization of the tumor vasculature, and thus improve the effect of chemotherapy.
Because of the rising incidence of cancer morbimortality in Brazil and the rest of the world, it is necessary to find new alternatives to fill gaps in the current treatment arsenal.
The Walker 256 carcinosarcoma was discovered in 1928 in mammary gland adenocarcinomas of pregnant female albino rats. Since then, these tumors have been maintained in the laboratory in musculature implants of rats [2]. Their rapid development and aggressiveness enables experimentally evaluating the response to antiangiogenics.
Gum arabic is a product obtained from the sap of the trunks and branches of Acacia Senegal trees [3]. Various studies have shown its potent antioxidant effects in humans, by diminishing oxidative stress [4]. The relationship between oxidative stress and angiogenesis is well established, as is that between oxidative stress and tumor hypoxia [5].
Eugenol is one of the principal components of clove essential oil. It reportedly has anticancerogenic, cytotoxic and antitumor activities [6], producing a greater cancer cell apoptosis rate than other commercially available drugs [7].
The angiogenesis inhibitors currently available to treat malignant neoplasms are not effective against all tumors, so it is necessary to investigate new possibilities. The objective of this work was to evaluate the effect of gum arabic and eugenol, alone and combined, on the progression and angiogenesis of Walker tumors implanted in Wistar rats, through immunohistochemistry, as well as the tumor growth and possible metastasis.
Subjects
This study was approved by the Committee on Ethical Use of Animals (CEUA) of Ceará Federal University (UFC). We used 60 female Wistar rats, supplied by the bioterium of UTC’s Department of Surgery. The animals were kept in groups of 6 per cage, in 10 cages, and were clinically assessed throughout the experiment.
Experiment
On the first day of the experiment, groups I, II, III, IV and V (control) received inoculum of distilled water (0.1 mL) on the back, while groups VI, VII, VIII, IX and X received Walker tumor cell implants on the back. In the five ensuing days, the substances were administered by gavage in both groups, in a case-control experimental design (Figure 1)
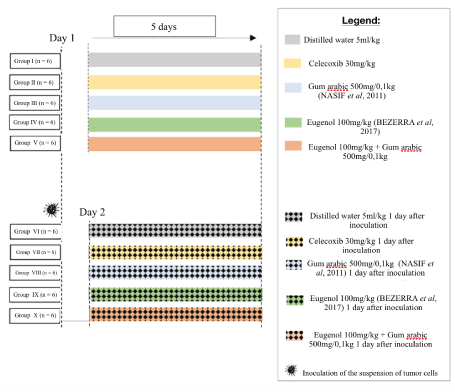
Figure 1. Experimental design
Walker tumor cells and inoculum administration on the animals’ backs
The Walker tumor cells are maintained weekly in the bioterium of the Drug Research and Development Center (NPDM). A solution was prepared of Ringer’s lactate (10 mL) and gentamicin (100 uL at a concentration of 40 mg/mL) and divided in two Falcon tubes. Then 5 ml of this solution was placed in a Petri dish and kept on ice. Next, three animals with Walker tumors were euthanized by anesthetic overdose (according to the National Council on Animal Control and Experimentation - CONCEA). The tumor was removed from each animal (Figure 2) and placed in a Petri dish and cut into small pieces with surgical scissors and tweezers until forming a macerate with solid and liquid parts (Figure 3). The solid was discarded and the liquid part was collected in a syringe and emptied into a Falcon tube. From this material, 0.7 mL was inoculated intramuscularly in the calf of one of the hind legs, at a concentration of 106 tumor cells/mL, of five animals, which were placed individually in labeled cages.
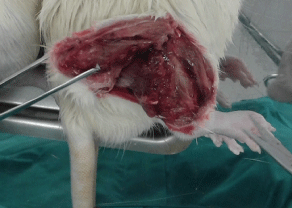
Figure 2. Removing the tumor from the hind calf
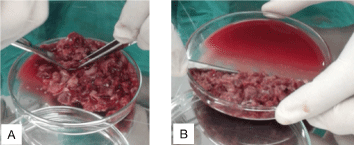
Figure 3. (A) Parts of the tumor being macerated (B) Solid content pending disposal
After seven days, the five animals were euthanized and the tumor was removed to count the cells, to attain a concentration of 2.0 x 106 tumor cells/mL. Then a solution was prepared of 20 uL of tumor liquid with 20 uL of trypan blue in an Eppendorf tube, and placed in a Neubauer chamber for counting in two fields on the diagonal. The value was multiplied by 106 to establish the estimated concentration of tumor cells/mL and diluted to adjust the concentration to the volume of 0.1 mL, for inoculation.
The next step was to implant the Walker 256 tumor cells in the subcutaneous tissue along the dorsal midline of each animal. The animals were previously anesthetized with ketamine at 100 mg/kg weight and xylazine at 10 mg/kg weight. Soon thereafter, trichotomy was performed on the back of each animal (Figure 4), to avoid any contamination or infection due to the inoculation, and antisepsis was performed (Figure 4). Next, the inoculate was demarcated (Figure 4), followed by inoculation of the suspension of tumor cells (Figure 4) at 4 cm from the animal’s tail.
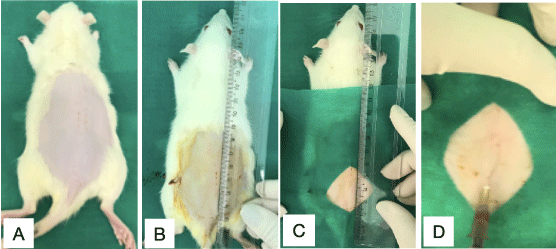
Figure 4. Procedure for inoculation of tumor cells
(A) Trichotomy (B) (C) Antisepsis and marking of the inoculation area. (D) Inoculation
On the seventh day, the animals were anesthetized for surgical resection of the skin and subcutaneous tissue of the back (Figure 5) with an electric scalpel (to avoid leakage from the blood vessels). After removal of the material for examination, the animal was euthanized with anesthetic overdose.
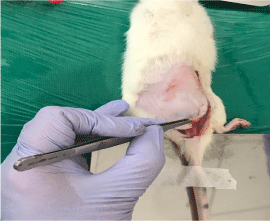
Figure 5. Resection of the skin and dorsal subcutaneous tissue
Tumor aspect and growth
After removal of the subcutaneous tissue from the back, the tumor appearance was evaluated regarding invasion, necrosis and adherence, using a pachymeter to measure the main dimensions (Figure 6).
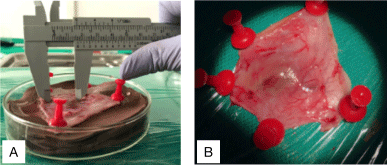
Figure 6. Measuring the tumor dimensions
(A) Measuring the tumor dimensions with a pachymeter (B) Microscopic aspects of the tumor
The tumor volume was calculated through the following expression [8]:
Tumor volume (cm3) = (D.d2) x 0.5, where: |
D: is the major tumor diameter, in cm
d: is the minor tumor diameter, in cm
Macroscopic and microscopic evaluation of metastasis
After euthanasia of the rats, the lungs, liver, spleen and kidneys were removed for macro and microscopic analysis by fixing in 10% formol. In the case of microscopic examination, tissue sections were stained with hematoxylin and eosin. The animals’ carcasses were incinerated.
Evaluation of tumor angiogenesis by immunohistochemistry
The exclusion criteria for the immunohistochemistry analysis were no tumor development and presence of tumors with extensive necrosis. All 30 animals were included. The silanized slides were deparaffinized in an oven for one hour at 65 ºC, immersed in xylene, rehydrated in a decreasing alcohol series, washed with tap water and submitted to antigen recovery with a solution of Tris-EDTA at pH 9.0 in a water bath at 85 ºC for 30 minutes. After cooling to room temperature, the sections were washed in phosphate buffer solution (PBS - 0.1 M, pH 7.3) in two baths of five minutes each. Then the endogenous peroxidase was blocked with a 3% solution of H2O2 (hydrogen peroxide) diluted in PBS for 30 minutes (Hsu et al., 1981).
After two washings of five minutes in PBS, the slides were incubated overnight with the antibody Abcam CD-31 (1:100). Then they were allowed to cool to room temperature, and the sections were washed twice in PBS baths for five minutes each, followed by incubation with anti-rabbit/anti-rat polymer (Dako® EnVision Dual Link System HRP) for 30 minutes. After two further washings for five minutes in PBS, the slides were incubated with 3,3- diaminobenzidine (DAB) for five minutes. The reaction was stopped with distilled water and the slides were counter-stained with 7% Harris modified hematoxylin for 10 seconds, washed in tap water, dehydrated, diaphanized and mounted with Enthellam® (Andrade et al., 2007). The positive control was rat kidney tissue and the negative control was performed by suppression of the primary antibody response with incubation of the antibody diluent.
Quantification of tumor angiogenesis
Digital images of the histological slides were captured in standardized form using a light microscope (BX4, Olympus Optical Co Ltd – Japan) coupled to a digital camera (C7070 Wide Zoom, Olympus Imaging America Inc. – USA). Initially we scanned the tumor using low magnification (40 x), for the purpose of identifying the zones of greatest vascular density, or hot spots (Weidner, 1995). For each tumor, we selected three hot spots. Then we captured color images at 200x of the fields related to the three hot spots. The images were stored in Windows Bitmap format (BMP), with dimensions of 600 x 444 pixels, according to the RGB: (Red, Green, Blue) color model.
The images were analyzed by a system for analysis of morphometry denoted by SAMM (Figure 7), part of a program developed for that purpose [9]. The software was previously calibrated to recognize the spectrum of colors related to the structures of interest (microvasculature), according to the staining technique used. This procedure enables the software to automatically identify and segment the blood vessels (separating them from the other components of the preparation), both in the entire image and in the region of interest defined by the user. After the segmentation, the program determines the microvascular density of the field studied. For that purpose, it calculates the density of the area, defined by the quotient between the area occupied by the microvasculature and the total area of the field under analysis.
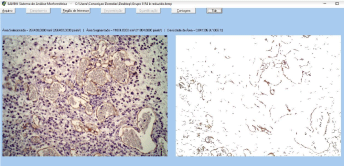
Figure 7. SAMM environment
Identification and segmentation of immunostained vessels and cells, to calculate the density of the area (microvascular density): total area occupied by the microvasculature divided by the total area of the field under analysis
Statistical analysis
For the purpose of identifying and removing discrepant values and to avoid inconsistent results due to such discrepancies, the main variables, namely the variation of body weight, tumor volume and microvascular density, were initially analyzed by the Tukey method, which defines as outliers those measures that are less than Q1 − 1.5 × IQR and higher than Q3 + 1.5 × IQR, where Q1 and Q3 correspond, respectively, to the first and third quartiles, while IQR denotes the interquartile range (IQR = Q3 − Q1). Then these variables were analyzed by the Shapiro-Wilk test to verify the normality of the distribution. For descriptive statistics, the mean and standard deviation (SD) were calculated and parametric tests were applied for analytical statistics. Thus, comparisons between the groups distilled water (DW), celecoxib (CEL), gum arabic (GA), eugenol (EUG) and the association of gum arabic with eugenol (GA+EUG), concerning the variables mentioned above, were carried out using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test to check for differences between paired groups. In all analyzes, two-tailed tests were applied and the significance level was set at 0.05 (5%), thus considering P < 0.05 as statistically significant. GraphPad Prism version 8.0 (GraphPad Software, San Diego, California, USA) was used to perform statistical procedures and plot the graphs.
Exclusion of animals
No animals were excluded from the study – there was no mortality (Table 1).
Table 1. Relative variation of body weight, tumor volume and microvascular density measured in the animals of the distilled water (DW), celecoxib (CEL), gum arabic (GA), eugenol (EUG) and gum arabic plus eugenol (GA+EUG) groups. Comparisons of the five groups were performed by one-way analysis of variance (ANOVA) complemented with the Tukey multiple comparison test, to verify pairwise difference between the groups
Parameter |
DW
MMean ± SD |
CEL
MMean ± SD |
GA
MMean ± SD |
EUG
MMean ± SD |
GA+EUG
MMean ± SD |
Significance
(ANOVA) |
Relative variation of body weight (%) |
2.74 ± 2.42
(n = 6) |
2.01 ± 2.01
(n = 6) |
3.98 ± 2.54
(n = 5) |
3.25 ± 3.59
((n = 5) |
1.48 ± 0.73
(n = 5) |
P = 0.5071 |
Tumor volume
(mm3) |
2.02 ± 1.10
(n = 6) |
0.69 ± 0.26a
(n = 5) |
1.07 ± 0.48
(n = 5) |
0.98 ± 0.41
(n = 6) |
1 .10 ± 0.55
(n = 6) |
P = 0.0242 |
Microvascular density (%) |
14.11 ± 2.57
(n = 6) |
8.18 ± 3.93b
(n = 6) |
11.52 ± 2.85
(n = 6) |
15.18 ± 2.83c
(n = 5) |
14.56 ± 1.84d
(n = 5) |
P = 0.0028 |
Source: Authors. SD: standard deviation. The letters a(P=0.0193) and b(P=0.0145) denote statistically significant differences in relation to the DW group, while the letters c(P=0.0052) and d(P=0.0117) indicate statistically significant differences in relation to the CEL group (Tukey test)
Evaluation of the appearance and growth of the Walker tumors
After removing the dorsal subcutaneous tissue from each animal, we evaluated the macroscopic tumor appearance and measured the dimensions with a pachymeter. The animals that received distilled water (group VI) had the greatest invasion of dorsal subcutaneous tissue and musculature.
All the animals were weighed on the first and last day of the experiment to calculate the weight variation in percent (Figure 8). The relative body weight variation (ΔP%), also expressed in percent, was determined in the animals of the distilled water (DW), celecoxib (CEL), gum arabic (GA), eugenol (EUG) and gum arabic plus eugenol (GA+EUG) groups, calculated according to the expression: ΔW%=[(WD1–WD6)/WD1].100, where WD1 and WD7 denote the body weight measured on the first and seventh days, respectively. Data are expressed as mean and standard deviation of the measurements. Comparisons among the five groups were carried out by one-way analysis of variance (ANOVA) complemented by the Tukey multiple comparison test between the paired groups. There were no statistically significant differences of the DW, CEL, GA, EUG and DW+EUG groups regarding relative variation of body weight (ANOVA: F= 0.8531; P= 0.5071).
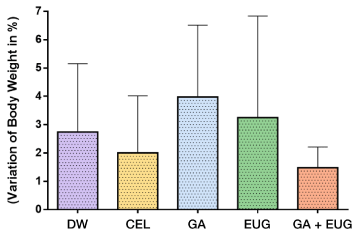
Figure 8. Variation of body weight in %
Source: Authors
The tumor volume in the CEL group was significantly smaller (*P=0.0193) than in the DW group. The tumor volume was also smaller in the groups that received gum arabic, eugenol and gum arabic with eugenol (groups VIII, IX and X, respectively) (P < 0.05) (Figure 9).
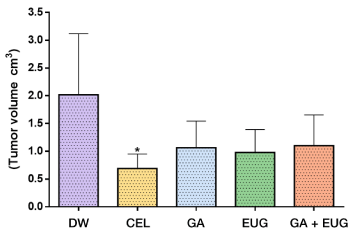
Figure 9. Tumor volume in cm3
Source: Authors *P < 0.05 versus group treated with distilled water
Evaluation of tumor angiogenesis
After analysis by the SAMM software of the three zones identified as having greatest vascular density, the program identified, segmented the blood vessels and determined the microvascular density of the sites studied, corresponding to the quotient between the area occupied by the microvasculature and the total area of the field analyzed.
The microvascular density, expressed as a percentage, was the ratio between the area of the microvasculature and total area of the field analyzed, multiplied by 100 (Figure 10).
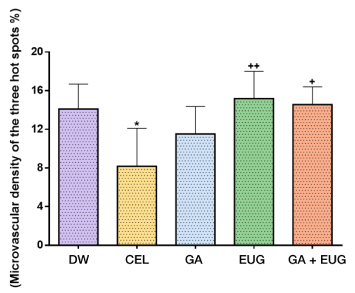
Figure 10. Microvascular density of the three hot spots
Source: Authors *P =0.0145 versus the group treated with DW. ++(P=0.0052) and +(P=0.0117) indicate statistically significant differences in relation to the CEL group
Evaluation of metastases
To detect metastases, we macroscopically and microscopically evaluated lung, liver, spleen and kidney tissue sections, stained with hematoxylin and eosin. Only the animals in the groups that received celecoxib, gum arabic and eugenol (groups VII, VIII and IX, respectively) presented metastases. In group VII, one animal presented metastasis in the liver; in group VIII, one animal had signs of metastasis in the liver and another in a kidney and in group IX, one animal also presented metastasis in the liver and one in a kidney.
Since 1971, when Judah Folkamn called attention to the importance of tumor vascularization for the growth of tumors, many studies of antiangiogenics have been conducted, seeking promising targets for treatment of various types of cancer [1]. It is known that the inhibitors of angiogenesis available in the market for treatment of malignant neoplasms are not effective for all tumor types, so the search for new possibilities is necessary.
This study was designed to evaluate the effect of eugenol and gum arabic on the progression and angiogenesis of Walker tumors in Wistar rats, which were chosen for their ease of obtainment, maintenance, feeding and hygiene in cages, resistance to infections, and low cost [10]. They also have a short biological cycle, a high degree of genetic similarity with humans (sharing around 80% of DNA) [11], docile behavior and size sufficient for use in surgical procedures [12] to accompany the growth of Walker tumors.
Our experimental model followed that described by [13], utilizing dorsal tumor implantation. There are many experimental models that can be used to investigate angiogenesis. We chose the Walker tumor model because it is available at UFC, making it economically possible. Besides this, the model is well known in studies of tumor behavior [14] and angiogenesis [15], besides having high virulence, with a tumor take rate of almost 100%, and rapid growth, enabling evaluation in a short time span [16].
The tumors, which are maintained weekly in the laboratory, were removed from the donor animals and used to extract viable cells for inoculation on the backs of the animals at a concentration of 2 x 106 cells/mL.
On the day after inoculation, the substances of each group were administered for five days. Groups VI, VII, VII, IX and X received, respectively, distilled water, celecoxib, gum arabic, eugenol, and gum arabic plus eugenol. On the seventh day, the animals were anesthetized and submitted to surgical resection of the dorsal skin and subcutaneous tissue with an electric scalpel, for macroscopic analysis of the tumor dimensions with a pachymeter and microscopic observation regarding necrosis, invasion and adherence. The tumor microvascular density was analyzed by digital images from the histological slides of tumor tissue, stained for immunohistochemistry (CD-31).
As described in the literature, medicines such as aspirin and non-steroidal anti-inflammatories are used to diminish the inflammation and tumor growth, avoiding the development of solid tumors. The immunological responses act in two ways in the tumor cells, namely reaction against specific tumor antigens and against antigens associated with the tumor, so the therapies developed are based on recognition of antigens and should guarantee that these responses will only induce destruction of tumor cells [17]. Besides the anti-inflammatory effect, some recent studies have revealed that inhibition of the formation of inflammatory mediators can influence the development of tumors, and especially can reduce tumor-mediated body weight loss [18]. Celecoxib was approved by the U.S. Food and Drug Administration (FDA) in 1999 as a chemopreventive agent at a dosage of 400 mg, twice a day. It is the only non-steroidal anti-inflammatory (NSAID) approved for adjuvant treatment of patients with familial adenomatous polyposis (FAP) [19,20]. The antitumor effect of celecoxib seems to occur by dependent and independent mechanisms that inhibit cyclooxygenase 2, a key enzyme for synthesis of prostaglandins [17]. The tumor can release metabolites and produce catabolic factors along with pro-inflammatory cytokines. It is likely that the drug’s effect on metabolic alterations is in part due to the reduction of metabolites and tumor products. The possible anticancerogenic mechanism involves blocking of cell proliferation and angiogenesis, induction of apoptosis and modulation of the immune response, and those mechanisms can depend or not depend on inhibition of COX-2 [19]. Our results corroborate those studies, demonstrating reduction of the tumor volume mainly in the group that received celecoxib, which also reduced the microvascular density and inhibited tumor angiogenesis.
The anti-inflammatory and anti-oxidant properties of gum Arabic [21], known to be present in the tumor angiogenesis process, require better comprehension. Knowledge of its influence on the angiogenesis process can help expand the therapeutic arsenal and promote development of future therapies for complex pathologies, using readily available natural substances. In this study, administration of gum arabic was a determining factor for reduction of tumor volume. In 2010 [22] Nasir and colleagues published a study reporting that gum arabic is a potent inhibitor of tumor growth and inflammation. Our research group recently published a paper reporting the anti-oxidant and antigenotoxic of gum arabic at 2.5% and 5% in mice submitted to colorectal carcinogenesis, with inhibition of aberrant crypts (pre-neoplastic lesions) in mice [21]. [23] observed a reduction of aberrant crypts associated with colorectal carcinoma in Wistar rats treated with 1% gum arabic. Although gum arabic can diminish oxidative stress, leading to possible reduction of angiogenesis, we did not observe this effect in the present study. The animals of group VIII, which received gum arabic, had reduced tumor volume compared to the other groups, but did not present reduction of the mean density or percentage of incidence. There was no inhibition of angiogenesis by the doses administered.
Many of the chemotherapeutic agents used nowadays have negative long-term side effects. A new generation of drugs with greater efficacy and specificity is urgently needed. Eugenol, also a natural compound, has been indicated in various studies as having chemoprotective potential against diverse types of cancer [6], causing greater rates of apoptosis than other drugs available in the market [7]. According to [24,25], eugenol at low doses has specific toxicity against different breast cancer cells, with anticancerogenic properties demonstrated by in vitro and in vivo experiments, indicating it can be used to consolidate adjuvant treatment. Other studies have described its dual effect on oxidative stress, acting either as an anti-oxidant or pro-oxidant agent. Despite the large number of articles about the properties of eugenol, the discrepant results prevent a definitive conclusion about its efficacy and safety. This duality appears to be related to the dose and concentration. For example, at low doses and concentrations, it has been shown to have an anti-oxidant effect, while at high doses and concentrations it has been found to have a pro-oxidant effect. Problems related to the purity of the compound and its volatility during experiments may help explain these contrary results [6]. Therefore, in this study we used it in pure form. The animals in the groups that received eugenol presented smaller tumor volume than the animals that only received distilled water. The histopathological analyses of stained tissue sections of the three hot spots did not find inhibition of microvascular density related to eugenol.
There was reduction of the tumor volume in the groups that received celecoxib, gum arabic, eugenol, and gum arabic plus eugenol (groups VII, VIII, IX and X, respectively), but the tumor density was only reduced in the group that received celecoxib (group VII), suggesting that the reduction of the tumor volume was not influenced by the microvascular density of the tumor (angiogenesis). The appearance of the tumors was evaluated regarding invasion, necrosis and adherence, and the macroscopic aspects were recorded. The animals in group VI, which received distilled water, had greater invasion of the dorsal subcutaneous tissue and musculature by the tumor in relation to the other groups.
The variation of the animals’ body weight was not statistically significant in any of the groups evaluated.
Gum arabic, celecoxib and eugenol reduced the tumor volume. Gum arabic and eugenol did not influence the tumor progression. Celecoxib was effective in reducing the microvascular density, thus presenting inhibition of tumor angiogenesis.
We are grateful to the staff of the Laboratory for Experimental Surgery, the Drug Research and Development Center, and the Laboratory of Pharmacology and Preclinical Research, all associated with the Medical School of Ceará Federal University (UFC), in Fortaleza, CE, Brazil.
None. This study is part of a master’s degree thesis research of Carolina Carneiro in the Graduate Program in Medical-Surgical Sciences, UFC. Faculty adviser: Prof. Conceicao Aparecida Dornelas.
- MASHREGHI, M. et al. Angiogenesis biomarker and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol, v. 233, n. 4, p. 2949-2965, Apr. 2018. DOI 10.1002/jcp.26049.
- DORNELAS, C. A. et al. Experimental model of Walker 256 carcinosarcoma in rats bladder. Acta Cir. Bras, São Paulo, v. 21, n. 1, p. 38-42, Jan.-Feb. 2006. DOI 10.1590/S0102-86502006000100009.
- GABAS, V. G. S.; CAVALCANTI, O. A. Influência da adição da gum arabic em filmes isolados de polímero acrílico: estudo das propriedades de intumescimento e de permeabilidade. Rev. Bras. Cienc. Farm, São Paulo, v. 39, n. 4, p. 442-448, Oct.-Dec. 2003. DOI 10.1590/S1516-93322003000400012.
- KADDAM, L. et al. Gum Arabic as novel anti-oxidant agent in sickle cell anemia, phase II trial. BMC Hematol, v. 17, n.4, p. 2-6, Mar. 2017. DOI 10.1186/s12878-017-0075-y. eCollection 2017/
- DALEPRANE, J. B.; ABDALLA, D. S. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid Based Complement Alternat Med, v. 2013, p. 175135, Apr. 2013. DOI 10.1155/2013/175135.
- BEZERRA, D. P. et al. The Dual Antioxidant/Prooxidant Effect of Eugenol and Its Action in Cancer Development and Treatment. Nutrients, v. 9, n. 12, p. 1367, Dec. 2017. DOI 10.3390/nu9121367.
- DAS, A. et al. Evaluation of Therapeutic Potential of Eugenol-A Natural Derivative of Syzygium aromaticum on Cervical Cancer. Asian Pac J Cancer Prev, v. 19, n. 7, p. 1977-1985, Jul. 2018. DOI 10.22034/APJCP.2018.19.7.1977.
- SILVA, L. F. G. et al. Experimental tumor model in rats kidney. Acta Cir Bras, v. 17, n. 1, p. 62-66, Jan.-Feb.. 2002. DOI 10.1590/S0102-86502002000100009.
- FECHINE-JAMACARU, F, V. In vivo quantification of corneal angiogenesis using digital image processing. 2006.Tese (Doutorado em Cirurgia) – School of Medicine, Ceará Federal University, Fortaleza, 2006.
- SILVA, N. S. F.; SAKATA, R. K.; ISSY, A. M. Correlation between CSF concentration and side effects after spinal morphine injection in rats. Rev Bras Anestesiol, Rio de Janeiro, v. 54, n.1, p. 53-59. Feb. 2004. DOI 10.1590/s0034-70942004000100007.
- MATTARAIA, V.G.M.; MOURA, A.S.A.M.T. Produtividade de ratos Wistar em diferentes sistemas de acasalamento. Cienc. Rural, Santa Maria, v. 42, n. 8, p. 1490-1496, Aug. 2012. DOI 10.1590/S0103-84782012000800026.
- BOTELHO, R. S. T. Elementos da macroscopia em patologia experimental. 2016. Dissertação (Mestrado em Medicina Dentária) – School of Medicine, Universidade de Coimbra, Coimbra, 2016.
- MORANO, J. A. C. O. D. et al. Experimental model of ultrasound thermotherapy in rats inoculated with Walker-236 tumor. Acta Cir Bras, São Paulo, v. 26, p. 53-56, 2011. supl. 1. DOI 10.1590/S0102-86502011000700011.
- CHEKHUN, V. F. et al. Antitumor and genotoxic effects of lactoferrin in Walker-256 tumor-bearing rats. Exp Oncol, v. 40, n. 3, p. 200-204, Oct. 2018.
- GAO, H. et al. Inhibitory effect of endostatin gene therapy combined with phosphorus-32 colloid on tumour growth in Wistar rats. Biosci Rep, v. 36, n. 3, e00353, Jun. 2016. DOI 10.1042/BSR20160117.
- EARLE, W. R. A study of the Walker rat mammary Carcinoma 256, in vivo and in vitro. Am J Cancer. v. 24, p.566-612, 1935.
- GIACOMINI, G, MENEZES, H. Técnicas e perspectivas em imunoterapia do câncer. Revista Saúde e Pesquisa, São Paulo, v.5, n.3, p.567-578, Sep.-Dec. 2012.
- DAVIS, T. W. et al. Inhibition of Cyclooxygenase-2 by celecoxib Reverses Tumor-Induced Wasting. J Pharmacol Exp Ther, v. 308, n. 3, p. 929-934, Mar. 2004.DOI 10.1124/jpet.103.063099.
- GASPARINI, G. et al. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol, v.4, n. 10, p. 605-615, Oct. 2003.DOI 10.1016/s1470-2045(03)01220-8.
- GRÖSCH, S.; MAIER, T. J.; SCHIFFMANN, S. et al. Cyclooxygenase-2 (COX-2) - independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst, v. 98, n. 11, p. 736-747, Jun. 2006. DOI 10.1093/jnci/djj206.
- NASIF, W. A.; LOTFY, M.; MAHMOUD, M. R. Protective effect of gum acacia against the aspirin induced intestinal and pancreatic alterations. Eur Rev Med Pharmacol Sci, v. 15, n. 3, p. 285-292, Mar. 2011.
- NASIR, O. et al. Downregulation of Angiogenin Transcript Levels and Inhibition of Colonic Carcinoma by Gum Arabic (Acacia senegal). Nutr Cancer, v. 62, n. 6, p. 802–810, 2010. DOI 10.1080/01635581003605920.
- AVELINO, A. L. N. et al. Antioxidant and Antigenotoxic Actions of Gum Arabic on the Intestinal Mucosa, Liver and Bone Marrow of Swiss Mice Submitted to Colorectal Carcinogenesis. Nutrition and Cancer, Jun., p. 1-9, 2021. DOI: 10.1080/01635581.2021.1931699.
- BRAGA, V. N. L. et al. Gum arabic and red propolis protecting colorectal preneoplastic lesions in a rat model of azoxymethane. Acta Cir Bras, v. 34, n. 2, e201900207, Feb. 2019. DOI 10.1590/s0102-8650201900207.
- AL-SHARIF, I.; REMMAL, A.; ABOUSSEKHRA, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/surviving down-regulation. BMC Cancer, v. 13, 2013. DOI 10.1186/1471-2407-13-600.










