Background: The use of Basiliximab as an induction immunosuppression agent in lung transplantation is well-established and is associated with a decreased incidence of acute rejection and improved survival. However, variation exists in the timing of administration of Basiliximab with regard to reperfusion of the lung allograft.
Methods: Serum blood samples were obtained from 30 patients undergoing lung transplantation at a single lung transplantation center between February 2017 and January 2018. The samples were evaluated for 12 different cytokines, including IL-2. Primary graft dysfunction (PGD) scores were calculated at four time points in the first 72 hours following surgery. These cytokine levels and PGD scores were then compared with regard to when Basiliximab was administered.
Results: 20 patients received induction immunosuppression with Basiliximab (7 patients before reperfusion, 13 patients after reperfusion). Cytokine levels were not different between the groups. Patients administered Basiliximab after reperfusion had significantly lower PGD scores immediately and at 24 hours postoperatively compared to patients receiving Basiliximab before reperfusion (differences in mean PGD scores p<0.001 and p=0.004, respectively). Patients administered Basiliximab prior to reperfusion had a statistically significant reduction in mean PGD scores over the first 72 hours postoperatively (p=0.045).
Conclusion: When Basiliximab was administered before allograft reperfusion in lung transplantation, initial PGD scores were higher, but there was a statistically significant progressive reduction in postoperative PGD scores over the first 72 hours following allograft implantation. There were no significant differences in the rates of acute rejection or infection at 6 months regardless of Basiliximab administration timing.
Successful organ transplantation requires the use of immunosuppressant medications in order to prevent rejection of the donor allograft in the recipient. Lung transplantation presents a unique challenge in this regard because the lung donor allograft is continuously exposed to the external environment with each breath that the recipient patient takes. In terms of the initiation of immunosuppression at the time of transplantation, there remains variability in the use of induction immunosuppression and timing of the induction between transplant centers. The goal of induction immunosuppression is to reduce the incidence of acute cellular rejection (ACR), as the observed rate of ACR within the first year after transplantation is notably higher and seen in more than 50% in patients who do not receive induction immunosuppression therapy [1]. This is very important clinically because it is known that even one episode of minimal ACR increases the subsequent risk of the development of bronchiolitis obliterans in lung transplant recipients [2].
The use of Basiliximab, an IL-2 receptor antagonist, as an induction immunosuppression agent in lung transplantation is well-established and is associated with a decreased incidence of acute rejection and improved survival [3,4]. However, the timing of administration of Basiliximab with regard to pre- and post-reperfusion of the lung allograft is not standardized, and the effect of this timing has not been fully elucidated. The logistics of administration of induction immunosuppression to a particular patient varies depending on the risks and benefits of such induction therapy. Therefore, for practical reasons, the timing of its administration changed over time in our center with the pulmonary transplant team taking over responsibility for its administration in order to prevent double dosing. With this change, it became not uncommon for patients to receive induction immunosuppression after the transplant procedure. There were no overt adverse events observed with this change in practice. However, in light of the manufacturer recommendation [5] to give Basiliximab within two hours before the start of the transplant procedure, once it has been determined that induction immunosuppression is to be utilized in a particular lung transplant patient, we now leave the timing of administration of the Basiliximab to the discretion of the operating surgeon based on personal operating practices. In the majority of patients, this equates to administration of the induction immunosuppression drug after reperfusion of the lung allograft while the patient is still in the operating room. Given this important difference in surgeon practice, it afforded the opportunity for a detailed investigation into this difference in terms of patient outcomes. As such, this study aims to evaluate clinical differences in primary graft dysfunction scores and cytokine levels in the immediate postoperative period in patients undergoing lung transplantation and receiving induction immunosuppression with Basiliximab before and after allograft reperfusion.
This study enrolled 30 patients undergoing lung transplantation at a high-volume academic lung transplantation center between February 2017 and January 2018. The study was Institutional Review Board approved, and informed consent was obtained from patients prior to lung transplantation. As part of this study, serum blood samples were collected prospectively on all study patients. The serum blood samples were taken at four different time points around the time of lung transplantation: 1) preoperatively, 2) post-reperfusion of the lung allograft in the operating room, 3) at 24 hours postoperatively, and 4) at 72 hours postoperatively. Levels of the following cytokines were evaluated in all of these blood samples: IFN-γ, IL1-α, IL1-β, IL-2, IL-4, IL-6, IL-8, IL-10, MCP-1, TNF-α, EGF, and VEGF. Primary graft dysfunction (PGD) scores were recorded at four time points following transplantation: immediately upon completion of the transplant, 2) at 24 hours postoperatively, 3) at 48 hours postoperatively, and 4) at 72 hours postoperatively.
The administration of Basiliximab was reviewed in a retrospective manner. The decision about whether or not to proceed with Basiliximab as induction therapy was determined by our institution’s multidisciplinary lung transplant team. Induction immunosupression was held in patients with cytomegalovirus mismatch or in those with concomitant multidrug resistant respiratory infections and clinical concern for potential infectious complications. In the operating room, the exact timing of Basiliximab administration during the course of the operation was at the discretion of the operating surgeon. All patients included in this study received allografts from donation after brain death (DBD) and allograft from donors following cardiac death (DCD) or EVLP (Ex-vivo lung perfusion) were excluded. PGD scores were calculated based the International Society for Heart and Lung Transplantation (ISHLT) consensus definition for PGD [6]. 6-month rates of acute cellular rejection and infection were also studied in a retrospective manner. Overall, the cytokine levels and PGD scores were then compared with regard to when Basiliximab was administered and how this corresponded with the incidence of acute rejection and infection in this lung transplant patient population.
Statistical analysis of the study data was performed using the Student’s t-test with two tailed P values of 0.05 or less as indicators of significance.
Among the 30 patients undergoing consecutive transplantation, 20 received induction immunosuppression with Basiliximab (7 before reperfusion of the allograft and 13 after reperfusion) (Table 1). Two patients required cardiopulmonary bypass (6.7%), 1 patient in each group receiving induction therapy. None of the patients were on extracorporeal membrane oxygenation (ECMO) preoperatively. With regard to cytokine levels, no significant differences in the levels of any of the following cytokines were observed between these two different groups: IFN-γ, IL1-α, IL1-β, IL-2, IL-4, IL-6, IL-8, IL-10, MCP-1, TNF-α, EGF, and VEGF (Figure 1).
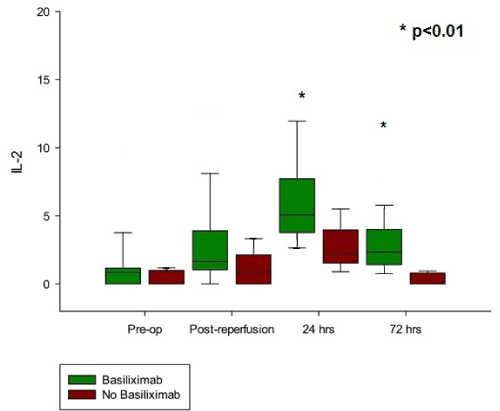
Figure 1. Effect of Basiliximab on IL-2 cytokine levels in lung transplant patients
Table 1. Basiliximab administration before and after reperfusion of allograft, patient characteristics
Patient Characteristics |
Pre-Reperfusion |
Age (years) |
Gender |
Diagnosis |
Type of Transplant |
CMV status R/D |
1 |
66 |
Male |
ILD |
Single |
R+/D+ |
2 |
74 |
Female |
COPD |
Single |
R-/D+ |
3 |
49 |
Male |
ILD |
Single |
R+/D+ |
4 |
48 |
Female |
Cystic fibrosis |
Bilateral |
R+/D+ |
5 |
56 |
Female |
COPD |
Single |
R-/D- |
6 |
61 |
Male |
ILD |
Bilateral |
R+/D+ |
7 |
54 |
Male |
ILD |
Bilateral |
R-/D+ |
|
|
|
|
|
|
Post-Reperfusion |
|
|
|
|
|
1 |
65 |
Female |
COPD |
Bilateral |
R-/D- |
2 |
66 |
Female |
Bronchiectasis |
Bilateral |
R-/D- |
3 |
57 |
Male |
COPD |
Bilateral |
R+/D+ |
4 |
73 |
Male |
ILD |
Single |
R+/D+ |
5 |
65 |
Male |
ILD |
Bilateral |
R-/D- |
6 |
56 |
Male |
ILD |
Single |
R+/D+ |
7 |
66 |
Female |
COPD |
Single |
R+/D+ |
8 |
65 |
Male |
PPH |
Bilateral |
R+/D+ |
9 |
63 |
Male |
COPD |
Bilateral |
R+/D+ |
10 |
69 |
Male |
COPD |
Single |
R-/D- |
11 |
74 |
Male |
ILD |
Single |
R-/D- |
12 |
65 |
Male |
COPD |
Single |
R-/D- |
13 |
65 |
Female |
ILD |
Bilateral |
R+/D+ |
COPD: Chronic Obstructive Pulmonary Disease; ILD: Interstitial lung disease; R: Recipient; D: Donor
The rate of infection in the first 6 months following transplantation was 56.7%, with 17 of 30 patients having documented evidence of bacterial, viral, or fungal infection. Of the 13 patients who received Basiliximab after reperfusion, 6 (46.2%) experienced infection. In the 7 patients who received Basiliximab before reperfusion, 4 (57.1%) experienced infection. Of the 10 patients who did not received any induction immunosuppression, 70.0% (7 of 10 patients) had documented infection. There were no significant differences in the rates of infection at the 6-month post-transplantation mark between these three groups.
The rate of acute cellular rejection in the first 6 months following transplantation in all patients was low at 20% (6 of 30 patients). Of the 7 patients who received Basiliximab before reperfusion, there was one patient who had ACR in the first six months (14.3%). In the 13 patients who received Basiliximab after reperfusion, 3 patients (23%) experienced at least one episode of ACR in the first 6 months following transplantation. Of the 10 patients who did not received any induction immunosuppression, 2 patients (20%) had at least one episode of ACR by 6 months after transplantation. There were no significant differences in the rate of ACR at the 6-month mark between these three groups. The 6-month survival rate was 90%, with 3 patients dying in the study period; one death occurred in each group studied.
With regard to PGD scores (Figure 2): patients who received Basiliximab after reperfusion had significantly lower PGD scores immediately (mean PGD score: 0.77) and at 24 hours postoperatively (mean PGD score: 1.00) compared to patients receiving induction Basiliximab before reperfusion (mean PGD score immediately: 2.57, mean PGD score at 24 hours postoperatively: 2.00) (p<0.001 and p=0.004, respectively). There was no significant difference in PGD scores between these two groups by 72 hours postoperatively (mean 72-hour PGD scores: Basiliximab before reperfusion=1.33, Basiliximab after reperfusion=0.85, p>0.05). There was no significant change in PGD scores over the first 72 hours postoperatively for patients receiving induction Basiliximab post-reperfusion. However, in patients who received Basiliximab prior to reperfusion, there was a statistically significant reduction in mean PGD scores from the immediate postoperative period (mean PGD score of 2.57) to 72 hours postoperatively (mean PGD score of 1.33) (p=0.045).
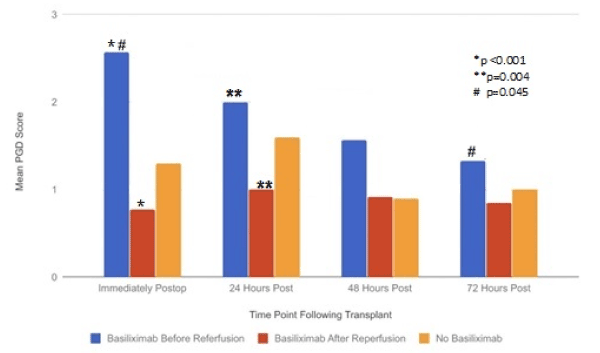
Figure 2. PGD scores over first 72 hours following transplantation
In this study, Basiliximab was given in two different patterns to lung transplant recipients. By doing so, we sought to investigate potential differences in PGD scores in the perioperative period and initial short-term outcomes with respect to the rates of infection and rejection. In this data set, there was a progressive decline in the postoperative PGD scores between immediately post-transplant and 72 hours post-transplant in patients who received Basiliximab before reperfusion but not in those patients who received Basiliximab after reperfusion. Despite this finding, we did not observe a difference in the rate of rejection at 6 months post-transplant regardless of whether the patient received Basiliximab before reperfusion, after reperfusion, or not at all in the transplant period (p=0.221). Likewise, the rate of infection by 6 months was also no different in these same three patient groups (p=0.082).
Several notable outcomes are evident in this study. Importantly, there was no increase in the rate of infection in patients who received Basiliximab at any point during transplantation compared to those who did not receive it for induction therapy. Also, giving Basiliximab before reperfusion did not increase the infection rate compared to those patients who received it after reperfusion. Similarly, no increases in the rate of rejection at 6 months were observed regardless of the pattern of Basiliximab administration. Thus, from both a rejection and infection perspective, there does not appear to be an increased risk to utilizing Basiliximab either before or after reperfusion in patients undergoing lung transplantation, despite current recommendations from the drug manufacturer to administer the first dose of Basiliximab within two hours prior to transplantation [5].
When evaluating our data on Basiliximab administration, the patients who received Basiliximab after reperfusion did have statistically significant lower mean PGD scores in the immediate postoperative period and at 24 hours postoperatively compared to the Basiliximab administration before reperfusion patient cohort. It could be argued that this difference in immediate postoperative PGD values was due to variation in the individual lung allografts themselves before the time of transplantation. However, it is very notable that the mean PGD scores decreased significantly from 2.57 in the immediate postoperative period to 1.33 (p=0.045) by 72 hours post-transplant in those patients who received Basiliximab before reperfusion. This progressive decrease in mean PGD scores was not seen in patients who received induction therapy with Basiliximab after reperfusion of the lung allograft. These differences in early PGD scores can be looked at in two different ways: either the cohort of patients who received Basiliximab before allograft reperfusion had allografts with worse early graft function that was potentially improved following the administration of Basiliximab, or the Basiliximab itself, when given before reperfusion, led to a higher early rate of PGD in the first 24 hours after transplant and then decreased by 72 hours. In our patient cohort, the main driver of increased PGD score in the patients who were administered Basiliximab before lung allograft reperfusion was the chest radiographic changes rather than oxygenation. This is an interesting finding and could be consistent with a possible explanation that the increase in PGD that was observed may be a consequence of Basiliximab administration before reperfusion. Basiliximab is known to cause pulmonary edema after dosing, notably in solid organ transplant patients [7,8]. The proposed mechanism for this pulmonary edema is increased vascular permeability due to increased cytokine release that accompanies blocking of IL-2 by Basiliximab. In our data, however, even in patients who received Basiliximab before reperfusion, the overall cytokine levels were not significantly increased in any of the cytokines that we measured, including IL-2. As such, a different yet to be understood mechanism of pulmonary edema/injury may be at play in these patients. Another potential mechanism for the clinical difference observed in postoperative radiographs is the fact that all of the patients who received Basiliximab after reperfusion were given steroids before administration of Basiliximab. However, in the patients who received Basiliximab before reperfusion, this cohort was not given steroids until after the Basiliximab was administered. As such, the presence of steroids may have decreased the vascular permeability in patients who received Basiliximab after reperfusion, thus possibly being a reason for the decreased rate of infiltrates seen on CXR in these patients, and, in turn, lower PGD scores.
The milieu of cytokines that is a part of the transplantation process is complex. Although we studied the levels of 12 different cytokines, including IL-2, in all 30 patients who underwent lung transplantation in this study, our cytokine data did not demonstrate any significant differences in any of the cytokines between the Basiliximab group across the perioperative transplantation period up to 72 hours post-transplant. We did however note a difference in that patient’s receiving Basiliximab compared to not receiving, at 24- and 72-hours post-transplant period. As expected, lower levels of expression of IL-2 was seen in no Basiliximab group compared to patients who did receive any induction immunosuppression. Notably, levels of IL-2 were in fact observed to increase following administration of Basiliximab and then trending towards baseline preoperative levels at 72 hours of transplantation. This IL-2 trend fits mechanistically with the function of Basiliximab as an IL-2 receptor antagonist, with T-cell activation being blocked, thus leading to an increased level of circulating IL-2. However, this trend was not statistically significant at any time point in the first 72 hours after surgery between the patients receiving Basiliximab.
Regardless of the mechanism, the fact that, 1) there was a significant (p=0.045) progressive decline in PGD scores in the first 72 hours following transplantation in patients receiving Basiliximab before reperfusion, and 2) the observation that this cohort had similar PGD scores by the 72-hour mark post transplantation to the PGD scores of the patients who received Basiliximab after reperfusion by the 72 hour mark is clinically important. Daud, et al. [9] showed in their 2008 study that the risk of ultimately developing bronchiolitis obliterans was related directly to worsening PGD score immediately following transplantation. In addition, they found that this association was independent of the rates of acute rejection, lymphocytic bronchiolitis, and community-acquired respiratory infections. This finding this very notable in the patient population in the present study because, although we did not find a difference in the rate of both acute rejection or post-transplant infections, including community-acquired respiratory infections, this does not mean that the progressive lowering of PGD scores that was seen only in the before reperfusion Basiliximab patients will not ultimately translate into a clinical benefit for these patients. The fact that this patient population had statistically significant declines in mean PGD score between the immediate post-transplant time period and 72 hours after implantation of the lung allograft may ultimately translate into a decreased rate of bronchiolitis obliterans in these patients. Further patient follow up and longer-term data are needed in this patient cohort to determine if the association described by Daud, et al. is confirmed in this group of patients.
This study is limited by its retrospective nature and small sample size. Given these limitations, the risk of selection bias is a potential confounder when critically evaluating these data. In addition, longer-term follow up data in this patient population would be very beneficial and improve the data set. As such, a larger randomized, prospective clinical trial on Basiliximab administration timing in lung transplantation is warranted and is the next step to address the limitations of the present small retrospective study and obtain longer-term outcomes.
In conclusion, Basiliximab administration before lung allograft reperfusion was found to be associated with higher PGD scores in the first 24 hours following transplantation. However, giving Basiliximab before reperfusion was also associated with a progressive decline in PGD scores in the first 72 hours following surgery, with PGD scores in these patients being no different at 72 hours than patients who received Basiliximab after reperfusion. Despite the differences in these early postoperative PGD scores, there were no significant differences observed in the rates of acute rejection or infection at 6 months following transplantation whether Basiliximab was administered before or after lung allograft reperfusion. Given that no adverse outcomes were seen with utilizing Basiliximab either before or after allograft reperfusion, both are likely safe methods of administration. However, further study is imperative to investigate if the early progressive decline in mean PGD scores seen in patients treated with Basiliximab before allograft reperfusion will translate into a clinical benefit to lung transplant recipients a longer duration out from the time of transplantation.
- Goldfarb SB, Gaynor JW, Fuller S, Kreindler J, Montenegro LM, et al. (2010) Induction therapy with anti-thymocyte globulin before reperfusion. Ann Thorac Surg 90: 1110-1114.
- Benden C, Faro A, Worley S, Arrigain S, Aurora P, et al. (2010) Minimal acute rejection in pediatric lung transplantation--does it matter? Pediatr Transplant 14: 534-539.
- Swarup R, Allenspach LL, Nemeh HW, Stagner LD, Betensley AD (2011) Timing of Basiliximab induction and development of acute rejection in lung transplant patients. J Heart Lung Transplant 30: 1228-1235.
- Furuya Y, Jayarajan SN, Taghavi S, Cordova FC, Patel N, et al. (2016) The impact of Alemtuzumab and Basiliximab induction on patient survival and time to bronchiolitis obliterans syndrome in double lung transplantation recipients. Am J Transplant 16: 2334-2341.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/basnov010203lb.htm#ind
- Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, et al. (2017) Report of the ISHLT working group on primary lung graft dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 36: 1097-1103.
- Bamgbola FO, Del Rio M, Kaskel FJ, Flynn JT (2003) Non-cardiogenic pulmonary edema during basiliximab induction in three adolescent renal transplant patients. Pediatr Transplant 7: 315-320.
- Lee YJ, Park BS, Park S, Park KM, Park JH, et al. (2018) Basiliximab-induced non-cardiogenic pulmonary edema in a kidney transplant patient. J Korean Soc Transplant 32: 63-68.
- Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, et al. (2007) Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 175: 507-513.


