Abstract
TP63 is a key regulator of epithelial development and homeostasis, but its role in cancer progression remains unclear. In this study,we assessed the usefulness of ΔNp63 (predominant isoform of TP63) as a prognostic biomarker of upper tract urothelial carcinoma(UTUC) that is a relatively uncommon cancer and is often associated with poor outcome.
We investigated the immunoreactivity of ΔNp63in radical nephroureterectomy specimens on tissue microarrays containing samples from 83 patients with UTUC. There were no significant associations between ΔNp63expression and tumor grade/stage, disease progression, or cancer-specific survival (CSS). However,in subgroup analysis of 32 patients who experienced disease recurrence after radical nephroureterectomy and subsequently received platinum-based chemotherapy showed that high ΔNp63 expression was associated with better CSS (P<0.05). Our study indicated that ΔNp63 expression could be a significant prognostic biomarker anda promising factor for predicting chemo-sensitivity in patients with UTUC.
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy andaccounts for only a small number of urothelial carcinoma[1-3]. Because patients with UTUC often exhibit only mildsymptoms, it is difficult to diagnose at the early stage. Indeed, about 60% of UTUCsare invasive at diagnosis compared with 15-25% of bladder tumors[4,5].Radical nephroureterectomy is offered as a standard treatment for localized tumors. Systemic chemotherapy isadministered for patients who have metastasis at diagnosis or experience recurrence after surgery, but the prognosis is poor in most cases[6].Although pathological stage and tumor grade are associated with tumor progression and poor survival[7,8], it is difficult for physicians guided only by these histopathological factors to predict prognosis anddecide whether to offer adjuvant chemotherapyin a precise manner forindividual UTUC patients. A greater understanding of the biological behavior of tumors is necessary for the realization of precision medicine andimprovement of patient outcomes.
TP63 is a member of the TP53 family, and this gene is composed of 15 exons, spanning over 270,000bp on chromosome 3q27[9,10]. The Tp63 gene has two transcriptional start sites: one contains an N-terminal transactivation (TA) domain (TAp63), and the othersdon’t (ΔNp63). Both genes can be alternatively spliced to generate proteins with three types of C-termini(α, β, and γ). TP63 is constitutively expressed in the nuclei of epithelial cells and acts as a key regulator of development and homeostasis of epithelium, butthe role of TP63 in cancer progression has not been fully understood.In human urothelial tissues and urothelial carcinomas, ΔNp63 is predominantly expressed compared to TAp63 [11-13]. Loss of ΔNp63 expression is associated with more advanced disease and acquisition of epithelial-mesenchymal transition[13-16], while it’s expression in muscle-invasive bladder cancer correlates with a worse prognosis [11,17]. Fukushima et al demonstrated loss of ΔNp63 expression during muscle-invasive recurrence progressed from low-grade papillary noninvasive bladder tumor, accompanied by N-cadherin up-regulation, indicating that ΔNp63 could suppress invasion of urothelial bladder carcinoma cells [18].
Thus TP63 likely plays a complex role in tumor formation and progression of urothelial cancer. In this study, we investigate the prognostic significance of ΔNp63 expression in UTUC patients.
Material and methods
Patients and tissue samples
A tissue micro-array (TMA) of UTUC specimens was constructed with spotted triplicate tumor samples from 83 patients who underwent radical nephroureterectomy performed with curative intent between 1999 and 2011 at Osaka General Medical Center, Osaka, Japan. Appropriate approval was obtained from the local institutional review board before construction and use of the TMA, and written informed consent was obtained from all patients. Clinicopathological characteristics of the patients were obtained from medical records and follow-up data. Tumor progression was defined as the development of recurrence at the site of radical nephroureterectomy, lymph node metastasis, and/or visceral metastasis. Metachronous or synchronous lower tract recurrence (e.g., in the bladder) was not defined as tumor progression. Patients were followed up from initial diagnosis to the appearance of the event of interest or the end of the study. Patients who did not present the event of interest by the end of the study were censored from time-to-event analyses.
Immunohistochemistry
Immunohistochemical staining was performed on 5-μm sections from the UTUC TMA.Sections were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval in 10 mM citrate buffer (pH 6.0) at 121°C?for 15 min before staining, and endogenous peroxidase activity was quenched with H2O2. Sections were then incubated with a primary antibody against ΔNp63 (p40, Calbiochem, Merck Millipore, Darmstadt, Germany). After incubation with a biotinylated antibody and treatment with the Vectastain ABC Kit (Vector Laboratories?Burlingame, Burlingame, USA), peroxidase activity was visualized with Vector NovaRED (Vector Laboratories, Burlingame, USA) according to the manufacturer’s instructions. The sections were counterstained with hematoxylin.All of the stained sections were manually scored by researchers who were blinded to sample identity.
Scoring system
Nuclear staining of ΔNp63 was evaluated for both intensity and extent (percentage of positive cells). An “H score” was assigned to each TMA spot as the sum of the products of the intensity (0, negative; 1, weakly positive; 2, moderately positive; and 3, strongly positive) and the extent of immunoexpression (0 to 100%), obtaining a value from 0 to 300, as previously described[19]. The final H score for each case was defined as the average score of triplicate TMA spots and was used during statistical analyses. For statistical analysis, the patients were divided into two groups according to the H score (High group: H score > the median, and Low group: H score ≤ the median).
Statistical analysis
Statistical analyses were performed using JMP® Pro 13.2.0 (SAS Institute Inc., Cary, NC). Patient characteristics were compared using the Mann-Whitney U test and χ2-test. The survival rates were determined using the Kaplan-Meier method and compared with the log-rank test.
Results
Table 1 shows the characteristics of the 83 patients. This cohort consisted of 47 male and 36 female patients, 48 to 87 years of age (median: 71 years), with low-grade urothelial carcinoma (in 11 patients) and high-grade urothelial carcinoma (in 72 patients). The carcinomas corresponded to non-muscle-invasive tumors (pTa or pT1) in 31 patients and muscle-invasive tumors (pT2, pT3, or pT4) in 52 patients. Pathological lymph node metastasis (pN+) was noted in 11 patients. The follow up period (measured from the date of radical nephroureterectomy to the time of last follow-up visit) ranged from 2 to 139 months. The median cancer specific survival (CSS) time was 46 months. During this period, 34 patients (41.0%) experienced local or distant tumor recurrence, and 22 (26.5%) experienced bladder recurrence (Figure 1).
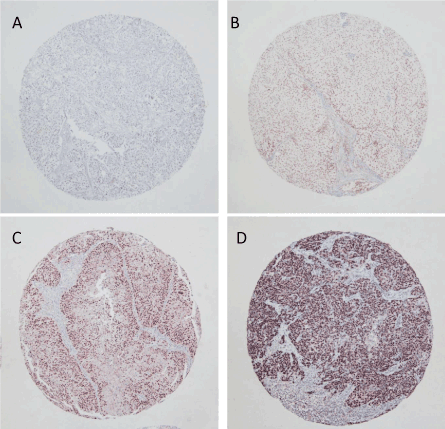
Figure 1:Typical patterns of immunohistochemical expression of ΔNp63 (A: negative, B: weakly positive, C: moderately positive, D: strongly positive) in upper tract urothelial carcinomatissues. Original magnification: ×200.
Representative patterns of immunoexpression are depicted in Fig. 1. ΔNp63 staining was strong in 22 cases (26.5%), moderate in 33 (39.8%), weak in 17 (20.5%) and absent in 11 (13.2%). ΔNp63 immunoreactivity was not associated with pathological stage or tumor grade. To evaluate the prognostic values of ΔNp63 expression in UTUC, we performed Kaplan-Meier analysis coupled with the log-rank test. ΔNp63 expression was not associated with cancer-specific mortality or disease recurrence in lymph node or distant metastasis in overall cohort (Figures 2A,2B). However, in subgroup analysis of 32 patients (including 8 patients who underwent adjuvant platinum-based chemotherapy) who experienced the disease recurrence after radical nephroureterectomy andsubsequently received platinum-based chemotherapy, high ΔNp63 expression was associated with better CSS (Figure 2C).
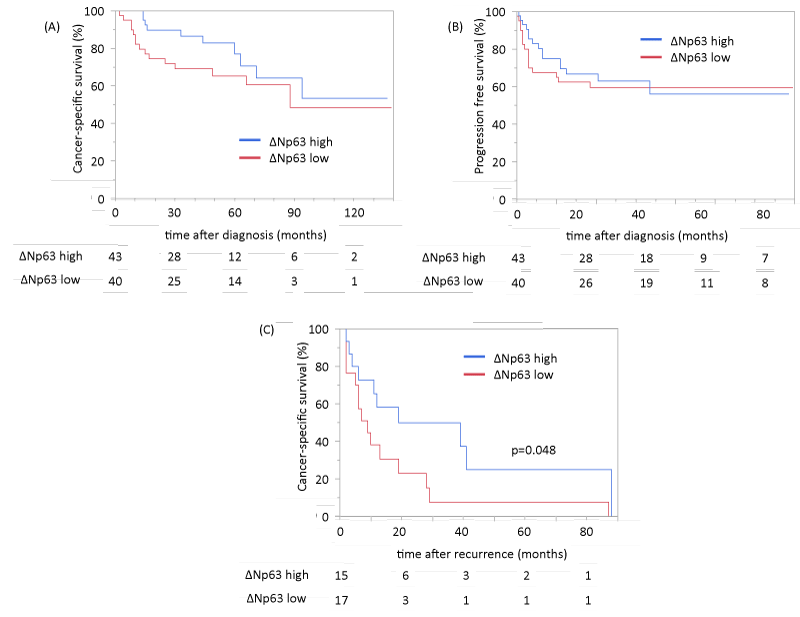
Figure 2: Cancer-specific survival rate and progression-free recurrence survival rate in patients with UTUC according to the expression levels of ΔNp63 (A: cancer-specific survival of overall cohort, B: progression-free survival of overall cohort, C: cancer-specific survival of patients who experienced recurrence after nephroureterectomy and received platinum-based chemotherapy).
Discussion
In this study, we showed the expression of ΔNp63 in radical nephroureterectomy specimens was significantly correlated with CSS in patients whoexperienced recurrence after surgery and received platinum-based chemotherapy, whilethe expression of ΔNp63 has no association with cancer-specific mortality or disease recurrence in lymph node or distant metastasis in overall cohort.
However, there have been many studiesdemonstrating the association of ΔNp63 expression with disease aggressiveness and prognosis of urothelial bladder cancer[11-18],while the expression of ΔNp63 shows no significant association with cancer-specific mortality in our UTUC cohort. This is possibly due to differencesin treatment strategy (e.g., lymphadenectomy, perioperative chemotherapy), embryology and cancer biology between upper tract and bladder tumor. Further studies are needed to clarify this discrepancy.
The expression of ΔNp63 in radical nephroureterectomy specimens showed significant correlation with CSS of patients who experienced recurrence after surgery and subsequently received platinum-based chemotherapy. In muscle invasive bladder cancer, Choi et al reported that “basal tumors” characterized by p63 activation has good response to neoadjuvant chemotherapy [20]. ΔNp63 could thus be a useful marker forpredicting chemo-sensitivity in UTUC patients who have recurrence or metastasis after radical nephroureterectomy.
This study has some limitations. First, we used TMA samples instead of whole tumor sections and the heterogeneity of staining could affect the evaluation of the expression levels. However, several studies have shown that multiple TMA spots adequately represent the expression of an entire section in the assessment of immunohistochemical markers [21]. Second, this retrospective data lacks clinical information (e.g., blood test findings, performance status, and detail information of chemotherapy), it was difficult to investigate the association of chemo-sensitivity with ΔNp63 expression in patients with UTUC thoroughly.
Conclusion
We demonstrated that ΔNp63 expression immunohistochemically determined in a set of TMA consisting of radical nephroureterectomy specimens could be a novel biomarker for CSS and response to chemotherapy in patients who had recurrence after surgery.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
- Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH (2017) Association between demographic factors and prognosis in urothelial carcinoma of the upper urinary tract: a systematic review and meta-analysis. Oncotarget 31: 7464-7476. [Crossref]
- Miyazaki J, Nishiyama H1 (2017) Epidemiology of urothelial carcinoma. Int J Urol 24: 730-734.[Crossref]
- Raman JD, Messer J, Sielatycki JA, Hollenbeak CS (2011) Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int 107: 1059-1056.[Crossref]
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, et al. (2011) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. EurUrol 2011; 59: 997-1008.[Crossref]
- Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, et al. (2009) Upper Tract Urothelial Carcinoma Collaboration. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 115: 1224-1233.[Crossref]
- Seisen T, Krasnow RE, Bellmunt J, Rouprêt M, Leow JJ, et al. (2017) Effectiveness of Adjuvant Chemotherapy After Radical Nephroureterectomy for Locally Advanced and/or Positive Regional Lymph Node Upper Tract Urothelial Carcinoma. J ClinOncol 35: 852-860.[Crossref]
- Huben RP, Mounzer AM, Murphy GP (1988)Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer 62: 2016-2020.[Crossref]
- Leitner CV, Ederer IA, de Martino M, Hofbauer SL, Lucca I, et al. (2016) Dynamic prognostication using conditional recurrence and progression estimates for patients with nonmuscle invasive bladder cancer. J Urol 196: 46-51.[Crossref]
- McKeon F (2004) p63 and the epithelial stem cell: more than status quo? Genes Dev 18: 465-469.[Crossref]
- Murray-Zmijewski F, Lane DP, Bourdon JC (2006) p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ 13: 962-972.[Crossref]
- Choi W, Shah JB, Tran M, Svatek R, Marquis L, et al. (2012) p63 expression defines a lethal subset of muscle-invasive bladder cancers. PLoS One 7: e30206. [Crossref]
- Tran MN, Choi W, Wszolek MF, Navai N, Lee IL, et al. (2013) The p63 protein isoform?Np63a inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J BiolChem 288: 3275-3288. [Crossref]
- Koga F, Kawakami S, Kumagai J, Takizawa T, Ando N, et al. (2003) Impaired Delta Np63 expression associates with reduced beta-catenin and aggressive phenotypes of urothelial neoplasms. Br J Cancer88: 740-747.[Crossref]
- Koga F, Kawakami S, Fujii Y, Saito K, Ohtsuka Y, et al. (2003) Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res 9: 5501-5507.[Crossref]
- Zigeuner R, Tsybrovskyy O, Ratschek M, Rehak P, Lipsky K, et al. (2004) Prognostic impact of p63 and p53 expression in upper urinary tract transitional cell carcinoma. Urology 63: 1079-1083.[Crossref]
- Urist MJ, Di Como CJ, Lu ML, Charytonowicz E, Verbel D, et al. (2002) Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol 161: 1199-1206.[Crossref]
- Karni-Schmidt O, Castillo-Martin M, Shen TH, Gladoun N, Domingo-Domenech J, et al. (2011)Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am J Pathol 178: 1350-1360.[Crossref]
- Fukushima H, Koga F, Kawakami S, Fujii Y, Yoshida S, et al. (2009) Loss of DeltaNp63alpha promotes invasion of urothelial carcinomas via N-cadherin/Src homology and collagen/extracellular signal-regulated kinase pathway. Cancer Res 69: 9263-9270.[Crossref]
- Munari E, Fujita K, Faraj S, Chaux A, Gonzalez-Roibon N, et al. (2013) Dysregulation of mammalian target of rapamycin pathway in upper tract urothelial carcinoma. Hum Pathol 44: 2668-2676.[Crossref]
- Choi W, Porten S, Kim S, Willis D, Plimack ER, et al. (2014) Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25: 152-165. [Crossref]
- Camp RL, Neumeister V, Rimm DL (2008) A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J ClinOncol 26: 5630-5637.[Crossref]


