Abstract
Ab initio calculations were carried out to analyze the interactions between a molecule of B2H4 with H2O, CH3OH, NH3, NH2CH3, NH(CH3)2 and N(CH3)3 molecules at the MP2/aug-cc-pvdz computational level. B2H4 through its bridged hydrogens (Hb) could act as a hydrogen bond donor while its B-B act as hydrogen bond acceptor. Thus, interaction of B2H4 with the mentioned molecules resulted in formation of Hb…X or B-B…H hydrogen bond complexes. In contrast, Ht atoms of B2H4 have not enogh strength to form Ht…H dihydrogen bond complexes with the above molecules. Results showed that the B-B...H interactions are stronger than its Hb...X counterpart. The obtained structures were analyzed by the natural bond orbital (NBO) and Atoms in Molecules (AIMs) methodologies.
Key words
Hydrogen bond complexes, Borane, B2H4
Introduction
Borane complexes have been studied extensively and have even been the subject of Nobel Prize work by Brown [1,2]. It has been the subject of proton affinity experiments in chemical ionization mass spectrometers. Among non-covalent interactions which have been known in boron chemistry, both dihydrogen bonding and hydrogen bonding types are particularly significant [4-21].
B2H4, designated as diborane(4), probably is the best known electron-deficient analogue of ethylene [22-26]. The molecule B2H4 bears 10 valence electrons for chemical bonding. There are two standard two electron terminal B–H bonds, thus accounting for a total of four electrons. This leaves a total of six electrons to share between the two bridging H atoms and the two B atoms. Consequently, there are two 3c-2e curved ‘banana’ B–H–B bridging bonds. According to the above illustrations, B2H4 has two types of terminal (Ht-B) and bridging (B-Hb-B) hydrogen atoms which differ in nature and characteristics. The bridging hydrogens of B2H4 are participating in electron deficient ‘three-center, two-electron bonds’ thus, they bear enough partial positive charge to act as hydrogen bond donor (HBD) to form Hb…X (X= N, O) hydrogen bonds with electron donating molecules [13,14,17-21,26]. On other hand, recent studies are showing that B-B bond also could act as HBA in the interactions of borane clusters with HBD species to form H…B-B hydrogen bonds [13,20,26].
From a fundamental point of view, the present work aims to extend the knowledge of the intrinsic activity of Ht, Hb and B-B bond of diborane(4) as hydrogen bond acceptor or hydrogen bond donor towards other molecules. For this propose, we investigated interaction of B2H4 toward H2O, CH3OH and NHn(CH3)3-n, n= 0-3 derivatives thorough theoretical calculation.
Computational methods
Calculations were performed using the Gaussian 03 system of codes [27]. The geometries of the isolated B2H4 and H2O, CH3OH and NHn(CH3)3-n molecules as well as their complexes were fully optimized at the mp2/aug-cc-pVDZ computational level. Harmonic vibrational frequency calculation confirmed the structures as minimal and enabled the evaluation of zero point energy (ZPE). The counterpoise procedure was used to correct the interaction energy for basis set superposition error [28]. The AIM package was used to obtain bond properties and molecular graphs [29,30]. The natural bond orbitals (NBO) method implemented within the Gaussian 03 set of codes was applied to perform NBO analysis [31].
Results and discussion
Interaction of B2H4 with H2O and CH3OH molecules gave the B2H4-H2O and B2H4-CH3OH complexes which have hydrogen bond interactions between B-B bond as HBA and OH functions of H2O and CH3OH as HBD. Results are demonstrting that later complex has greater stability than the former one.
The association of B2H4 and NHn(CH3)3-n (n=0-3) derivatives led to the formation of the 1:1 hydrogen bond complexes which has been denoted as B2H4-NH3, B2H4-NH2Me, B2H4-NHMe2 and B2H4-NMe3, Figure 1. In these complexes hydrogen bond interactions has been found between a bridging proton of the B2H4 as a proton donor and nitrogen atom of amine as a proton acceptor (Hb…N). According to the data given in Table 1, stabilities of B2H4-NHn(CH3)3-n complexes increased with enhancing basicity of amines in the following order: B2H4-NMe3 >B2H4-NHMe2 > B2H4-NH2Me > B2H4-NH3.
Table 1. The SEuncorr, BSSE, ∆ZPE, and SEcorr (corrected with BSSE and ∆ZPE) in kcal mol-1 calculated at MP2/aug-cc-pVDZ.
Complex |
SEuncorr |
BSSE |
∆ZPE |
SEcorr |
B2H4-H2O |
-4.02 |
0.96 |
1.66 |
-1.40 |
B2H4-CH3OH |
-4.53 |
1.16 |
1.08 |
-2.29 |
B2H4-NH3 |
-2.10 |
0.75 |
0.83 |
-0.52 |
B2H4-NH2Me |
-3.15 |
1.15 |
0.80 |
-1.20 |
B2H4-NHMe2 |
-3.96 |
1.47 |
0.71 |
-1.78 |
B2H4-NMe3 |
-4.32 |
1.73 |
0.61 |
-1.98 |
Values of SEuncorr were determined as follows: SEuncorr = E(B2H4∙∙∙Y) – [E(B2H4) + E(Y)] with Y = H2O, CH3OH, NH3, NH2CH3, NH(CH3)2 and N(CH3)3;
Values of SEcorr were determined as follows: SEcorr = SEuncorr + ∆ZPE + BSSE.
The results due to the intermolecular bond lengths are given in the Table 2 and Figure 1. In the B2H4-H2O and B2H4-CH3OH complexes, the B1-B4 bond has elongation (0.0015); but, other bonds of B2H4 are shortened (from -0.0009 to -0.0053) upon complex formation. Moreover, a 0.0061 lenthening was observed for O-H bond in these complexes.
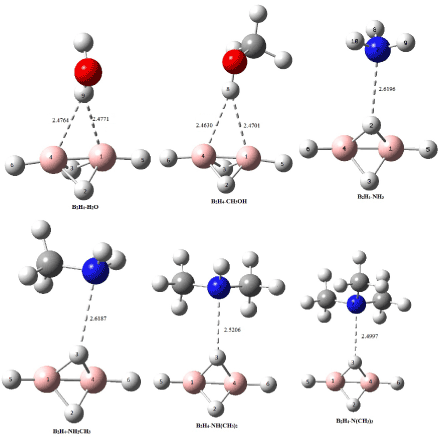
Figure 1. Schematic representation of optimized complexes at MP2/aug-cc-pVDZ computational level. Distances are in Å.
Table 2. Bonds length of free B2H4 and their variation during intermolecular interactions at MP2/aug-cc-pVDZ.
|
B2H4 |
B2H4-H2O |
B2H4-CH3OH |
B2H4-NH3 |
B2H4-NH2Me |
B2H4-NHMe2 |
B2H4-NMe3 |
Bond |
d |
Dd |
Dd |
Dd |
Dd |
Dd |
Dd |
B1-H5 |
1.1827 |
-0.0009 |
-0.0010 |
0.0004 |
0.0004 |
0.0004 |
0.0004 |
B4-H6 |
1.1828 |
-0.0009 |
-0.0009 |
0.0003 |
0.0000 |
0.0004 |
0.0004 |
B1-B4 |
1.4908 |
0.0015 |
0.0015 |
-0.0024 |
-0.0015 |
-0.0020 |
-0.0020 |
B1-H2 |
1.3586 |
-0.0033 |
-0.0024 |
-0.0046 |
-0.0077 |
-0.0029 |
-0.0028 |
B1-H3 |
1.3587 |
-0.0028 |
-0.0018 |
-0.0023 |
-0.0069 |
-0.0035 |
-0.0040 |
B4-H2 |
1.3593 |
-0.0039 |
-0.0041 |
-0.0055 |
+0.0024 |
-0.0036 |
-0.0035 |
B4-H3 |
1.3592 |
-0.0033 |
-0.0053 |
-0.0028 |
-0.0028 |
-0.0040 |
-0.0044 |
O-H |
0.9658 |
0.0061 |
0.0061 |
|
|
|
|
N….H |
|
- |
- |
2.6196 |
2.6187 |
2.5206 |
2.4997 |
H…B1 |
|
2.4771 |
2.4701 |
|
|
|
|
H…B4 |
|
2.4764 |
2.4630 |
|
|
|
|
On the other hand, the N…Hb distances in the B2H4-NHn(CH3)3-n complexes are in the range of 2.6196 to 2.4997 Å. These distances could be considered as weak bonding interactions between the two components. Comparison of the Hb…N distances showed that the obtaine trend was in agreement with the stability of these complexes.
In B2H4-NH3, the NH3 molecule interacts with H2 atom of B2H4. Data given in Table 2 showed that the bridging B-H-B bond, as well as B1-B4 bond, have contraction (-0.0046, -0.0055, -0.0023,-0.0028 and -0.0024 for B1-H2, B4-H2, B1-H3, B4-H3 and B1-B4 bonds, respectively); while, the terminal B1-H5 and B4H6 bonds showed small elongation upon complexation.
In B2H4-NH2CH3, interaction occured between NH2CH3 molecule and the bridging H3 atom of B2H4. In this complex, B1-H2, B1-H3, B4-H3 and B1-B4 bonds showed contraction (-0.0077, -0.0069, -0.0023, and -0.0015, respectively); while, the terminal B1-H5 bond and the bridging B4-H2 bond revealed elongation after complexation.
In B2H4-NH(CH3)2 and B2H4-N(CH3)3, the interactions were occured between the bridging H3 atom of B2H4 and the amine molecules. In these complexes, B1-H2, B1-H3, B4-H2, B4-H3 and B1-B4 bonds showed contraction (from -0.0020 to -0.0044); while, the terminal B1-H5 and B4-H6 bonds showed small elongation after complexation.
The selected vibrational stretching frequencies (cm‑1) with the corresponding intensities (km mol-1) for the studied complexes are listed in Table 3. The unsymetric stretching frequenciy of B1-H5 and B4-H6 showed a 5 cm-1 blue shift in B2H4-H2O and in B2H4-CH3OH complexes, which is in line with the shortening of the related bonds. The sym-B1-H2-B4 band showed 7, 5, 15 and 15 cm-1 blue shift in B2H4-NH3 , B2H4-NH2CH3, B2H4-H2O and B2H4-CH3OH complexes, respectively; while, it revealed -6 and -9 cm-1 red shift in B2H4-NH(CH3)2 and B2H4-N(CH3)3 complexes.
Table 3. Unscaled vibrational frequencies (cm‑1) with corresponding intensities (values given in parenthesis, km mol-1) for B2H4 and its complexes at MP2/aug-cc-pVDZ.
Compound Bond |
B2H4 |
B2H4-H2O |
B2H4- MeOH |
B2H4-NH3 |
B2H4-NH2Me |
B2H4-HMe2 |
B2H4-NMe3 |
n |
n |
Dn |
n |
Dn |
n |
Dn |
n |
Dn |
n |
Dn |
n |
Dn |
B1 - B4 |
1343(3) |
1335(2) |
-8 |
1335(2) |
-8 |
1345(2) |
2 |
1342(2) |
-1 |
1343(1) |
0 |
1342(2) |
-1 |
sym-B1-H2-B4 |
2129(18) |
2144(15) |
15 |
2144(15) |
15 |
2136(27) |
7 |
2134(34) |
5 |
2123(13) |
-6 |
2120(16) |
-9 |
usy-B1-H2-B4 |
2136(44) |
2151(33) |
15 |
2149(29) |
13 |
2147(13) |
11 |
2147(21) |
11 |
2141(40) |
5 |
2141(40) |
5 |
us-B1-H5,B4-H6 |
2811(36) |
2816(25) |
5 |
2816(23) |
5 |
2805(42) |
-6 |
2805(37) |
-6 |
2803(35) |
-8 |
2802(34) |
-9 |
sym-B1-H5,B4-H6 |
2851(0.2) |
2855(0) |
4 |
2853(0) |
2 |
2847(0) |
-4 |
2846(0.2) |
-5 |
2844(0) |
-7 |
2843(0) |
-8 |
N…H |
|
- |
|
- |
|
105(20) |
|
110(11) |
|
119(8) |
|
94(1) |
|
H….B-B |
|
138(12) |
93 |
126(0) |
|
|
|
|
|
|
|
|
|
symH-O-H |
|
3711(247) |
-40 |
|
|
|
|
|
|
|
|
|
|
CH3O-H |
3804(4)a |
|
|
|
|
|
|
|
|
|
|
|
|
3938(67) b |
3898(141) |
|
3714(358) |
-127 |
|
|
|
|
|
|
|
|
3841(34) |
|
|
|
|
|
|
|
|
|
|
|
|
a nsym O-H;b nunsym O-H
Moreover, the unsym-B1-H2-B4 band showed 5 to 15 cm-1 blue shift in these complexes. Also, the O-H band in B2H4-H2O and B2H4-CH3OH complexes showed -40 and -127 cm-1 red shifts with respect to free H2O and CH3OH molecules, respectively. The B1-B4 vibrational absorbtion bands in B2H4-H2O and in B2H4-CH3OH complexes showed -8 cm-1 red shift, which is in agreement with its lengthening due to the complex formation. In contrast, in B2H4-NHn(CH3)3-n complexes, this bond was less affected by complex formation; thus, the observed shifts were negligible. In agreement with the lengthening of B1-H5 and B4-H6 bonds, their unsymetric stretching frequencies, which observed at 2811 cm-1 in free B2H4, showed -6 to -9 cm-1 red shift in the B2H4-NHn(CH3)3-n complexes.
Aim analysis
The atoms in molecules (AIM) theory is applied here to analyze the characteristics of the H…N and H…B-B interactions through the location of Bond Critical Points (BCP) with (3,-1) coordinates in the Hessian matrix fitted to the intermolecular contact area [29,30]. In Table 4, the results of the QTAIM topological parameters, namely as electronic density (ρ), Laplacian (Ñ2ρ) and the ratios between the kinetic (G) and potential (U) electron energy density are listed [32]. These last ones are embodied into the QTAIM formalism as follows:
Table 3. Unscaled vibrational frequencies (cm‑1) with corresponding intensities (values given in parenthesis, km mol-1) for B2H4 and its complexes at MP2/aug-cc-pVDZ.
Compound Bond |
B2H4 |
B2H4-H2O |
B2H4- MeOH |
B2H4-NH3 |
B2H4-NH2Me |
B2H4-HMe2 |
B2H4-NMe3 |
n |
n |
Dn |
n |
Dn |
n |
Dn |
n |
Dn |
n |
Dn |
n |
Dn |
B1 - B4 |
1343(3) |
1335(2) |
-8 |
1335(2) |
-8 |
1345(2) |
2 |
1342(2) |
-1 |
1343(1) |
0 |
1342(2) |
-1 |
sym-B1-H2-B4 |
2129(18) |
2144(15) |
15 |
2144(15) |
15 |
2136(27) |
7 |
2134(34) |
5 |
2123(13) |
-6 |
2120(16) |
-9 |
usy-B1-H2-B4 |
2136(44) |
2151(33) |
15 |
2149(29) |
13 |
2147(13) |
11 |
2147(21) |
11 |
2141(40) |
5 |
2141(40) |
5 |
us-B1-H5,B4-H6 |
2811(36) |
2816(25) |
5 |
2816(23) |
5 |
2805(42) |
-6 |
2805(37) |
-6 |
2803(35) |
-8 |
2802(34) |
-9 |
sym-B1-H5,B4-H6 |
2851(0.2) |
2855(0) |
4 |
2853(0) |
2 |
2847(0) |
-4 |
2846(0.2) |
-5 |
2844(0) |
-7 |
2843(0) |
-8 |
N…H |
|
- |
|
- |
|
105(20) |
|
110(11) |
|
119(8) |
|
94(1) |
|
H….B-B |
|
138(12) |
93 |
126(0) |
|
|
|
|
|
|
|
|
|
symH-O-H |
|
3711(247) |
-40 |
|
|
|
|
|
|
|
|
|
|
CH3O-H |
3804(4)a |
|
|
|
|
|
|
|
|
|
|
|
|
3938(67) b |
3898(141) |
|
3714(358) |
-127 |
|
|
|
|
|
|
|
|
3841(34) |
|
|
|
|
|
|
|
|
|
|
|
|
a nsym O-H;b nunsym O-H
H = G + U |
(1) |
(ħ2/4m)Ñ2ρ = 2G + U |
(2) |
This equation indicates which type of interaction may exist between the two nuclei, wherein, the profile of Ñ2ρ is embodied into the contribution of G and U. If the potential electron energy density is outweighed by the kinetic, the positive profile of Ñ2ρ indicates a depletion of charge density along the inter-nuclear connecting Bond Path (BP) [33]. Furthermore, the atomic connection is recognized as close-shell interaction, which is often designated to H-bonds or other intermolecular weakly bound contacts, such as halogen bonds, dihydrogen bonds, and p-staking [34-40]. Regarding the values gathered in Table 4, it should be highlighted that the positive values of Ñ2ρ ensure that all H-bonds are closed-shell interactions due to the low charge density concentration. The inter-atomic and inter-molecular interactions are also studied in terms of local electron energy density (H) and its components, the local kinetic electron energy density (G), and local potential electron energy density (V) at the BCPs. The relation between these energetic parameters is given in Equation 1.
Also it has been suggested that both  2BCP and the H should be used for characterizing hydrogen bond [41]. The weak hydrogen-bonds means that both
2BCP and the H should be used for characterizing hydrogen bond [41]. The weak hydrogen-bonds means that both  2BCP and H are positive, medium hydrogen-bonds show that
2BCP and H are positive, medium hydrogen-bonds show that  2BCP>0 and H<0; while, strong hydrogen-bonds bearing both
2BCP>0 and H<0; while, strong hydrogen-bonds bearing both  2BCP and H<0. For the investigated complexes (Table 4),
2BCP and H<0. For the investigated complexes (Table 4),  2BCP and H at BCP for H…N and H…B-B interactions are positive. This means that these interactions belong to close shell weak HB interactions.
2BCP and H at BCP for H…N and H…B-B interactions are positive. This means that these interactions belong to close shell weak HB interactions.
2021 Copyright OAT. All rights reserv
The balance between G and V could be used to show the nature of interactions [42]. If  >1, then, the nature of the interaction is purely non-covalent. For all the examined complexes, this ratio was greater than 1, which confirmed the existence of weak interactions between the two systems and nature of the interaction was purely non-covalent.
>1, then, the nature of the interaction is purely non-covalent. For all the examined complexes, this ratio was greater than 1, which confirmed the existence of weak interactions between the two systems and nature of the interaction was purely non-covalent.
Topological parameters ρ and ∇2BCP, also describe the stability of complexes through the identification of charge density centres within the intermolecular bonds. Considering the results of the topological analysis presented in Table 4, good agreement could be found between the values of  2BCP, ρ, and stabilization energies of complexes (Figure 2 and 3).
2BCP, ρ, and stabilization energies of complexes (Figure 2 and 3).
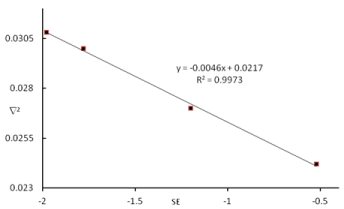
Figure 2. Relationship between the Ñ2BCP (au) and SE(kcal mol-1) for the B2H4-amine complexes.
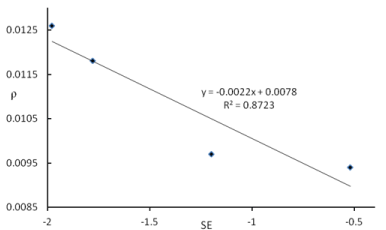
Figure 3. Relationship between the rBCP (au) and SE(kcal mol-1) for the B2H4-amine complexes.
Natural bond orbital analysis
Natural bond orbital (NBO) analysis was performed for the minima found on the studied B2H4 complexes. These complex formations are associated with an orbital interaction between the bonding pairs in the electron donor and the antibonding orbital in the electron acceptor. The quantity of charge transferred from donor to the acceptor (DQ) due to the interaction of donor and acceptor orbitals were 0.0060, 0.0085, 0.0039, 0.0072, 0.0082 and 0.0085 for B2H4-H2O, B2H4-CH3OH, B2H4-NH3, B2H4-NH2CH3, B2H4-NH(CH3)2, and B2H4-N(CH3)3 complexes, respectively. These charge transfers indicated that electron fraction is transferred from HBA to HBD molecules. Thus, charge transfer is not concentrated on the interacting atoms; but, is mostly dispersed among the molecules. Therefore, interpretation of the bond variations and frequency shifts in B2H4 could not be carried out simply.
A useful quantity which might be derived from the results of natural bond orbital analysis is NBO binding energy (E2). The second-order perturbation energy can be taken as an index to judge the strength of the intermolecular bonds.
Table 5, lists the quantity of charge transfer from the donor to the acceptor qCT and the second-order perturbation energy due to the interaction of donor and acceptor orbitals. E(2) allow us to quantitatively evaluate the charge transfer involving the formation of the B2H4 complexes. According to results, the E(2) value of B2H4-CH3OH was greater than B2H4-H2O, confirming the order obtained for the interaction energies of these complexes. Whereas, for the amine complexes some contraversies were seen between the order obtained for their E(2) and the interaction energies.
Table 5. The NBO analysis of studied complexes at MP2/aug-cc-pVDZ.
complexes |
donor® acceptor |
qCTa |
E (2) |
B2H4-H2O |
bd(B1-B4)®σ*(O-H) |
0.0060 |
3.85 |
B2H4-CH3OH |
bd(B1-B4)®σ*(O-H) |
0.0085 |
5.54 |
B2H4-NH3 |
lp(N)®σ*(B1-H2-B4) |
0.0039 |
2.15 |
B2H4-NH2Me |
lp(N)®σ*(B1-H3-B4) |
0.0072 |
1.27 |
B2H4-NHMe2 |
lp(N)®σ*(B1-H3-B4) |
0.0082 |
2.09 |
B2H4-NMe3 |
lp(N)®σ*(B1-H3-B4) |
0.0085 |
2.15 |
Acknowledgement
The researchers greatly appreciate the Research Councils of Lorestan and Sabzevar universities for their partial financial support for this study. Many thanks to L. Sarmadi for some parts of calculations used in this study.
References
- Brown HC, Ramachandran V (1997) In Advances in Boron Chemistry, Siebert, W. Ed. The Royal Society of Chemistry Special Publication, Cambridge, UK. 201-204.
- Lipscomb WN (1977) The boranes and their relatives. Science 196: 1047-1055. [crossref]
- Pierce RC, Porter RF (1973) Low-temperature chemical ionization mass spectrometry of boron hydrides. Proton affinities of diborane and tetraborane(10), J. Am. Chem. Soc. 95, 3849-3855.
- Singh PC, Patwari GN (2006) The C-H···H-B dihydrogen bonded borane-trimethylamine dimer: A computational study, Chem. Phys. Lett. 419, 5-9.
- Solimannejad M, Alkorta I (2006) Competition between Nonclassical Hydrogen-Bonded Acceptor Sites in Complexes of Neutral AH2 Radicals: A Theoretical Investigation, J. Phys. Chem. A 110, 10817-10821.
- Bakhmutov VI (2008) In Dihydrogen bonds Principles, Experiments, and Applications, A John Wiley& Sons, Inc. Publication. 29-43.
- Morrison CA, Siddick MM (2004) Dihydrogen bonds in solid BH3NH3. Angew Chem Int Ed Engl 43: 4780-4782. [crossref]
- Merino G, Bakhmutov VL, Vela A (2002) Do Cooperative Proton-Hydride Interactions Explain the Gas-Solid Structural Difference of BH3NH3, J. Phys. Chem. A 106, 8491-8494.
- Meng Y, Zhou Z, Duan C, Wang B, Zhong Q (2005) Non-convertional Hydrogen Bonding Interaction of BH3NH3 Complexes: A Comparative Theoretical Study, J. Mol. Struct. (Theochem) 713, 135-144.
- Singh PC, Patwari GN (2006) The C–H...H–B dihydrogen bonded borane-trimethylamine dimer: A computational study, Chem.Phys. Lett. 419, 5-9.
- El-Guerraze AM, El-Nahas A, Jarid C, Serrar H, Esseffar Anane M (2005) Theoretical study of H3AXH3 and H3AYH2 (A= B, Al, Ga; X = N, P, As and Y = O, S, and Se), electrostatic and hyperconjugative interactions roles, Chem. Phys. 313, 159-168.
- Zabardasti A, Kakanejadifard A, Hoseini AA, Solimannejad M (2010) Competition between hydrogen and dihydrogen bonding: interaction of B2H6 with CH3OH and CHnX3-nOH derivatives, Dalton Trans. 39, 5918-22.
- Zabardasti A (2012) Arabpour M. Theoretical Study of Hydrogen and Dihydrogen Bond Interaction of B6H10 with the HF Molecule, Struct. Chem. 23, 473-477.
- Zabardasti A, Arabpour M, Zare N (2013) Theoretical Studies of Intermolecular Interactions of Arachno-pentaborane (11) with HF and LiH, Comput. Theor. Chem. 1008, 27-31.
- Zabardasti A, Zare N, Arabpour M, Kakanejadifard A, Solimannejad M (2013) Theoretical Study of Mixed Hydrogen and Dihydrogen Bond Interactions in Clusters, J. Chem. 1- 7.
- Zabardasti A, Kakanejadifard A, Moosavi S, Bigleri Z, Solimannejad M (2010) Anticooperativity in Dihydrogen Bonded Clusters of Ammonia and BeH4-2, J. Mol. Struc. Theochem. 945, 97-100.
- Derikvand Z, Zabardasti A, Azadbakht A (2015) Theoretical study of intermolecular interactions in CB4H8–HOX (X = F, Cl, Br, I) complexes, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 150, 778-785.
- Zabardasti A, Joshaghani M, Solimannejad M (2010) Competition between Hydrogen and Dihydrogen Bonding: Interaction of B2H6 with HF and LiH, Bull. Chem. Soc. Jpn 83, 1359-1363.
- Zabardasti A, Kakanejadifard A, Zare N, Arabpor M (2011) Theoretical study of dihydrogen bonded clusters of water with tetrahydroborate, Struc. Chem. 22, 691-695.
- Derikvand Z, Zabardasti A, Amini N (2015) Theoretical investigation of H…F and H…H intermolecular interactions of nido-CB4H8 with HF molecule, Struct. Chem. 26, 207-211.
- Zabardasti A, Goudarziafshar H, Salehnassaj M, Oliveira BG (2014) A computational study of hydrogen bonds in intermolecular systems of high complexity: arachno-pentaborane(11)···Y with Y = O2 and N 2. J Mol Model 20: 2403. [crossref]
- Vincent MA, Schaeffer HF (1981) Diborane(4) (B2H4): the boron hydride analog of ethylene, J. Am. Chem. Soc. 103, 5677-5680.
- Mohr RR, Lipscomb WN (1986) Structures and Energies of B2H4, Inorg. Chem. 25, 1053-1057.
- Ruscik B, Schwarz M, Berkowitz J (1989) Molecular Structure and Thermal Stability of the B2H4 and B2H4+ Species, J. Chem. Phys. 91, 4576-4581.
- Shoji Y, Matsuo T, Hashizume D, Fueno H, Tanaka K, et al. (2010) A stable doubly hydrogen-bridged butterfly-shaped diborane(4) compound. J Am Chem Soc 132: 8258-8260. [crossref]
- Alkorta I, Soteras I, Elguero J, Del Bene JE (2011) The boron-boron single bond in diborane(4) as a non-classical electron donor for hydrogen bonding. Phys Chem Chem Phys 13: 14026-14032. [crossref]
- Frisch MJ (2003) Gaussian 03, (Revision B.02), Gaussian, Inc., Pittsburgh, PA.
- Boys SF, Bernardi F (1970) The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors, Mol. Phys. 19, 553-566.
- Ghanty TK (2006) How strong is the interaction between a noble gas atom and a noble metal atom in the insertion compounds MNgF (M=Cu and Ag, and Ng=Ar, Kr, and Xe)? J Chem Phys 124: 124304. [crossref]
- Popelier PLA (2000) In Atoms in Molecules, an Introduction, Prentice Hall, Pearson Education Limited.
- Weinhold F, Landis CR (2012) In Discovering Chemistry With Natural Bond Orbitals, New Jersey: John Wiley & Sons, 132-133.
- Bader RFW (1991) A Quantum Theory of Molecular Structure and its Applications, Chem. Rev. 91, 893-928.
- Grabowski SJ, Ugalde JM (2010) Bond paths show preferable interactions: ab initio and QTAIM studies on the X-H...pi hydrogen bond. J Phys Chem A 114: 7223-7229. [crossref]
- Oliveira BG, Araújo RCMU, Leite ES, Ramos MN (2011) A Theoretical Analysis of Topography and Molecular Parameters of the CFCl3-O3 Complex: Linear and Bifurcate Halogen-Oxygen Bonding Interactions, Int. J. Quantum. Chem. 111, 111-116.
- Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21: 52. [crossref]
- Politzer P, Murray JS, Clark T (2015) σ-Hole bonding: a physical interpretation. Top Curr Chem 358: 19-42. [crossref]
- Oliveira BG, Araújo RCMU, Silva JJ, Ramos MN (2010) A Theoretical Study of Three and Four Proton Donors on Linear HX••• BeH2••• HX and Bifurcate BeH2••• 2HX Trimolecular Dihydrogen-bonded Complexes with X= CN and NC, Struct. Chem. 21, 221-228.
- Oliveira BG, Araújo RCMU, Ramos MN (2008) The (H-δ••• H+δ) Charge Transfer and the Evaluation of the Harmonic Molecular Properties of Dihydrogen-bonded Complexes formed by BeH2 HX with X= F, Cl, CN, and CCH , Struct. Chem. 19, 185-189.
- Capim SL, Santana SR, Oliveira BG, Rocha GB, Vasconcellos MLAA (2010) Revisiting the Origin of the Preferential p-p Stacking Conformation of the (+)-8-phenylmenthyl Acrylate, J. Braz. Chem. Soc. 21, 1718-1726.
- Rozas I, Alkorta I, Elguero J (2000) Behavior of Ylides Containing N, O, and C Atoms as Hydrogen Bond Acceptors, J. Am. Chem. Soc. 122, 11154-11161.
- Oliveira BG (2012) Interplay Between Dihydrogen and Alkali–halogen Bonds: Is There Some Covalency Upon Complexation of Ternary Systems? Theor. Comput. Chem. 998, 173-182.



