Abstract
Objectives: to evaluate the cardio- and cerebrovascular events in outpatients suffering from Chronic Inflammatory Rheumatology diseases, affected by Rheumatoid Arthritis and Spondylo-Enthesitis-related Arthritis, before and after the availability of biological anti-TNFα drugs in the Piedmont region of Italy.
Methods: Data collected from a ten-year time period in three outpatient rheumatology facilities in the Piedmont region of Italy were analysed for cardio- and cerebrovascular events. In the middle of this time period, biological drugs were introduced for the outpatients.
Results: The cohort consisted of 579 rheumatology patients, of which 52.8 % were affected with Rheumatoid Arthritis and 47.2 % with spondylo-enthesitis-related arthritis. The rate of cardiovascular and cerebrovascular events was significantly lower in patients treated with biological drugs compared to those treated with synthetic anti-rheumatic drugs.
Conclusions: in this population the availability of biological anti-TNFα drugs was associated with fewer cardiovascular and cerebrovascular events in rheumatology outpatients.
Key words
arthritis, cardiovascular events, cerebrovascular events, antiTNFα
Introduction
Rheumatoid Arthritis (RA) and Spondylo-Enthesitis-related Arthritis (SpA) are systemic chronic flogistic diseases which in their natural history led to progressive damage and articular deformity, with worsening of quality of life, reduced articular functionality and patients’ inability to work [1-5]. In the last two decades, the therapeutic paradigm has deeply shifted. Indeed, it has been demonstrated that early treatment with synthetic Disease Modifying Antirheumatic Drugs (sDMARD), in monotherapy or combined, followed by the use of biological drugs (bDMARD) when the clinical response is inadequate, is associated with a notably better clinical and functional outcome [6-8]. Epidemiological studies have highlighted that Rheumatoid Arthritis reduces life expectancy by around 10-15 years and that cardiovascular events, above all myocardial infarction and heart failure, represent the leading cause of deaths (around half) [9-12]. Autoptic studies on Rheumatoid Arthritis patients, performed before biological drugs were available, had shown the presence of cardiovascular pathologies in around 60 % of the patients [13,14]. The prevalence of cardiovascular events in Rheumatoid Arthritis patients was similar to that observed in type II diabetes patients [15-18]. The high prevalence of cardiovascular pathology in patients affected with Chronic Arthritis is probably due to both the increase of traditional risk factors for atherosclerosis and the presence of a chronic flogistic state that induces an acceleration of the atherosclerosis process [19-21]. In physiological conditions, the vascular endothelium is involved in maintaining antithrombotic and anticoagulant homeostasis. In a flogistic state the endothelium becomes a target for cytokines, autoantibodies, and immune complexes; as a result, pro-atherogenic and pro-thrombotic modifications take place [22]. In particular, during Chronic Arthritis, the release of pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) produced in the synovial tissue seems to induce dyslipidemia, insulin resistance, and pro-atherogenic modifications in the vascular endothelium [23]. Indeed, hyperinsulinemia, insulin resistance and reduction in glucose tolerance have been observed in patients affected with Rheumatoid Arthritis, with a correlation to the severity of flogosis [24,25]. An effective drug treatment on flogosis and disease activity could be expected to reduce cardiovascular risk in patients affected with Chronic Arthritis and, actually, Rheumatoid arthritis patients treated with methotrexate had shown a reduced mortality due to cardiovascular events, but not due to other causes [26].
In the last 10 years, the use of TNFα antagonist biotechnological drugs has led to a significant reduction of cardiovascular risk, despite the fact that these drugs are contraindicated in the presence of heart failure [27-31]. The treatment with TNFα antagonist biotechnological drugs in Rheumatoid Arthritis patients has been demonstrated to significantly reduce the disease activity and the endothelium dysfunction; in addition, these drugs have shown to improve other risk factors for atherosclerosis, reducing insulin resistance (through modulation of adiponectin) and increasing High-Density Lipoprotein [32]. Moreover, in Hypertension Treatment European Guidelines, the C-reactive protein (CRP) has been included as a risk factor, predictive of cardiovascular events [33-38].
To perform a global evaluation of patients with Chronic Arthritis, taking into account the disease progression and cardio- and cerebrovascular co-morbidities, a review of ten-year clinical records was conducted in three outpatient rheumatology units in the Piedmont region of Italy.
Materials and Method
Data collected from a ten-year period, on patients affected with Rheumatoid Arthritis (RA) and Spondylo-Enthesitis-related Arthritis (SpA) followed in three outpatient rheumatology facilities in Biella, Novara and Turin-1 territory, Piedmont region of Italy, were analyzed looking for lifetime cardio- and cerebrovascular events. Biological drugs were made available to these facilities in the middle of this time period. The cardiovascular events searched were cardiac (angina pectoris, acute myocardial infarction, atrial fibrillation, heart failure) and cerebrovascular (stroke, chronic vascular encephalopathy). The demographic and clinical characteristics of the population have been described, including the drug therapy taken.
Summary statistics were done, and Pearson's Chi-squared Test for Count Data or a Proportion Test were applied on the counts or proportions.
Results
Data were collected from 579 patients, with a 1.7 female/male ratio. 52.8 % of the subjects were affected with RA and 47.2 % with SpA. 47.5 % of the patients were in treatment with biological drugs (bMARD) and 52.5 % with synthetic drugs (sDMARD). Hypertension was recorded in 42.8 % of cases. Cardiovascular events were registered in 22 % of cases, 56.7 % of which were cardiac and 43.3 % cerebrovascular. In both pathologies, the female gender rate is higher, with a statistically significant difference only in the RA group (M:F = 1.3: 3.37, 70 males and 236 females) compared to the SpA group (M:F = 1:1.2, 124 males and 152 females) (Figure 1).The mean age of RA patients was 71 years, with a mean duration of disease of 17 years; the SpA patients had a lower mean age (64 years) with a mean duration of disease of 10 years.
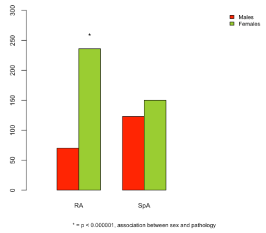
Figure 1. Sex distribution according to pathology group.
At least one event was present in 21.9 % of patients (127 patients) of which 56.6 % were RA patients and 43.4 % SpA ones. 78 % of patients did not have any cerebro- or cardiovascular events recorded: 51.7 % were RA patients and 48.3 % SpA patients.
There was a male prevalence (63 %) among SpA patients that had shown a cerebro- or cardiovascular event, and this feature was more evident in the bDMARD therapy group (p = 0.002).
The distribution of patients according to the presence of cerebro- or cardiovascular events and type of treatment was 11.1 % (bDMARD) and 88.9 % (sDMARD). In bDMARD patients, the level of events was clearly lower in both RA and SpA, with a high statistical significance (p < 0.000001) (Figure 2).
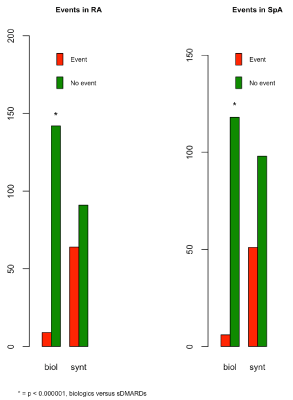
Figure 2. Cerebro- and cardiovascular events with biological and synthetic drugs in RA and SpA patients.
Among patients with events, 14.6 % had presented more than one, 5.5. % in therapy with sDMARD, and 0.74 % in treatment with bDMARD.
In the RA group, angina pectoris and acute myocardial infarction were prevalent among cardiac diseases, while multi-ischemic encephalopathy was the most frequent among cerebrovascular events. In the SpA group, the most common cardiac events were acute myocardial infarction, atrial fibrillation, and angina pectoris, while stroke was the most frequent among cerebrovascular events.
Regarding other risk factors, hypertension did not correlate with cardio- or cerebrovascular events, but there was a high percentage of missing data which could limit the interpretation of this finding.
Having a single event was the most frequent pattern (83.5 %), both with sDMARD and bDMARD.
Discussion and Conclusions
Biotechnological drugs have changed the therapeutic approach to Chronic Inflammatory Arthritis (RA and SpA), modifying the clinical progression and clearly improving patients’ quality of life.
In the Piedmont region in the North of Italy, biological drugs have been available to outpatient rheumatology units since 2006. The clinical and therapeutic management of these types of patients led to a reduction in direct and indirect costs when compared to inpatient management. In addition, the continuity in the medical assistance of these chronic patients allowed the physician to have a better control of flogosis and rheumatological outcomes, as well as cerebro- and cardiovascular co-morbidities, which are the most frequent causes of death in chronic inflammatory arthritis patients. Indeed, the main objective of the work described in the present paper was to study the association between cerebro- and cardiovascular events and therapies taken by RA and SpA outpatients in three Piedmont region facilities, during a ten-year time period. This time period has allowed for the evaluation of the association between cerebro- and cardiovascular events and therapies, with possible modification of treatment during the period, starting with sDMARD and, since 2006, with bDMARD. In our population, RA patients were slightly more prevalent (52.8 %) than SpA patients (47.2 %), and more female patients were present, as expected according to scientific literature [39] (Figure 1).
Gender distribution remained globally the same, even in patients that had a cardiovascular event, but in SpA patients with cardiovascular events there were more males, and even more in biological drug treated ones (p = 0.002), as if the males were afforded less protection by biological drugs. It has to be noted that 78 % of all patients did not have any cardiovascular events and, among those with cardiovascular events, 14.2 % had the event before the diagnosis of arthritis. The secondary prevention of cardiovascular events in patients that had already had an event was more effective in patients who were treated with biological drugs (only 3 % of the total had a second event) than in patients treated with sDMARD (13 % of the total had another cardiovascular event). Our data suggests that the use of biologics could reduce cardiovascular risk.
Even with limitations due to this type of data collection, this paper can suggest the potential role of anti-TNFα drugs in reducing cardiovascular and cerebrovascular risk.
Further studies with prospective methodology, on different cohorts and of longer duration should be done to discover the actual incidence and prevalence of cardio- and cerebrovascular events during the treatment of Chronic Inflammatory Arthritis with anti-TNFα agents and/or with sDMARDs.
Acknowledgements
LL, IA, MD, MF contributed to the study design, data acquisition and interpretation. LL and LU drafted the paper and analysed and interpreted the data. Editorial/medical writing support was provided by Ugo Lancia at Fullcro S.r.l. and was funded by Pfizer.
References
- Grosso V, Caporali R (2014) Treat-to-target nell’artrite reumatoide: quali obiettivi. ARS 2: 3-10.
- McInnes IB, O’Dell JR (2010) State of the art: rheumatoid arthritis. Ann Rheum Dis 69: 1898-1906. [crossref]
- Minisola G (2013) Le patologie reumatiche croniche invalidanti e la loro rilevanza epidemiologica. Sala degli Atti Parlamentari. Roma.
- Malattie Reumatiche e Lavoro (2008) Indagine Osservatorio Sanità e Salute.
- I Rapporto sociale sull’Artrite Reumatoide (2008) Indagine Conoscitiva ANMAR-SIR-CENSIS.
- Verstappen SM, Jacobs JW, Bijlsma LW (2003) Five-year follow-up of rheumatoid arthritis patients after early treatment with disease modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheum 48: 1797-1807. [crossref]
- Lard LR, Visser H, Speyer I, Vander Horst-Bruinsma IE, Zwinderman AH, et al. (2001) Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different strategies. Am J Med 111: 446-451. [crossref]
- van Aken J, Lard LR, le Cassie S, Hazes JM, Breedveld FC, et al. (2004) Radiological outcome after four years of early versus delayed treatment strategy in patients with recent onset rheumatoid arthritis. Ann Rheum Dis 63: 274-279. [crossref]
- Sarzi-Puttini P, Atzeni F, Gerli R, Bartoloni E, Doria A, et al. (2010) Cardiac involvement in systemic rheumatic diseases: an update. Autoimmun Rev 9: 849-852. [crossref]
- Nurmohamed MT (2009) Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev 8: 663-667. [crossref]
- Soltesz P, Kerekes G, Der H, Szücs G, Szántó S, et al. (2011) Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmune Rev 10: 416-425. [crossref]
- Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, et al. (2005) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population–based cohort study. Arthritis Rheum 52: 402-411. [crossref]
- Kapetanovic MC, Lindqvist E, Geborek P, Saxne T, Eberhard K (2011) Long-term mortality rate in rheumatoid arthritis patients with disease onset in the 1980s. Scand J Rheumatol 6: 433-438. [crossref]
- Koivuniemi R. Paimela L, Soumalainen R, Leirisalo-Repo M (2013) Cardiovascular diseases in patient with Rheumatoid Arthritis. Scand J Rheumatol 42: 131-135. [crossref]
- Van Halm VP, Peters MJ, Voskuyl AE, Boers M, Lems WF, et al. (2008) Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease, a cross sectional study. The CARRE Investigation. Ann Rheum Dis 68: 1395-1400. [crossref]
- Gonzalez A, Maradit KH, Crowson CS, Ballman KV, Roger VL, et al. (2008) Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 67: 64-69. [crossref]
- Assous N, Touzé E, Meune C, Kahan A, Allanore Y (2007) Cardiovascular disease in rheumatoid arthritis: single center hospital-based cohort study France. Joint Bone Spine 74: 66-72. [crossref]
- Wållberg-Jonsson S, Johansson H, Ohman ML, Rantapää-Dahlqvist S (1992) Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol 26: 2562-2571. [crossref]
- Ridker PM (2009) Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost 7: 332-339. [crossref]
- Dessein PH, Semb AG (2013) Could cardiovascular disease risk stratification and management in rheumatoid arthritis be enhanced? Ann Rheum Dis 72: 1743-1746.
- Arts EE, Popa C, Den Broeder AA, Semb AG, Toms T, et al. (2015) Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis 74: 668-674. [crossref]
- Manzato E (2001) La malattia coronarica, Approccio integrato al paziente con malattia vascolare, Edizioni Scientifiche.
- McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, et al. (2006) Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with lupus systemic erithematosus and rheumatoid arthritis. Arthritis and Rheum 54: 2541-2549. [crossref]
- Navarro-Millan I, Charles-Schoeman C, Yang S, Bathon JM, Bridges SL Jr, et al. (2013) Changes in lipoprotein associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 65: 1430-1438. [crossref]
- Curtis JR, John A, Baser O (2012) Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-TNF therapy. Arthritis Care Res 64: 1282-1291. [crossref]
- Choi HK, Hernan MA, Seerger JD, Robins JM, Wolfe F (2002) Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 359: 1173-1177. [crossref]
- Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, et al. (2011) Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 50: 518-531. [crossref]
- Barnabe C, Martin BJ, Ghali WA (2011) Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 63: 522-529. [crossref]
- Dixon WG, Watson KD, Lunt M, Hyrich KL, British Society for Rheumatology Biologics Register Control Centre Consortium, et al. (2007) Reduction in the incidence of myocardial infarction in patients’ rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 56: 2905-2912. [crossref]
- Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, et al. (2011) Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 70: 482-487. [crossref]
- Choy E, Sattar N (2009) Interpreting lipid levels in the context of high–grade inflammatory state with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis 68: 460-469. [crossref]
- Tam LS, Tomlinson B, Chu TT, Li TK, Li EK (2007) Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol 26:1495-1498. [crossref]
- del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44: 2737-2745. [crossref]
- Libby P, Crea F (2010) Clinical implications of inflammation for cardiovascular primary prevention. Eur Heart J 31: 777-783. [crossref]
- Emerging Risk Factors Collaboration, Kapotage S, Di Angelantonio E, Lowe G, Pepys MB, et al. (2010) C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375: 132-40. [crossref]
- Winyard PG Tatzber F, Esterbauer H, Kus ML, Blake DR, et al. (1993) Presence of foam cells containing oxidized low-density lipoprotein in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis 52: 677-680. [crossref]
- Pasceri V, Yet ET (1999) A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation 100: 2124-2126. [crossref]
- Koenig W, Khuseyinova N (2007) Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 27: 15-26. [crossref]
- Gabriel SE (2001) The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 27: 269-281. [crossref]


