A phase II study to evaluate the efficacy and safety of a Bidirectional intraperitoneal and systemic induction chemotherapy (BISIC) were performed in patients with Peritoneal metastasis (PM) from gastric cancer in neoadjuvant setting. Sixty-one patients were treated with oral administration of S1 (60 mg/m2/day) for 14 consecutive days, followed by 7 days rest, plus intraperitoneal (i.p.) and intravascular (i.v.) administration of docetaxel and cisplatin (30 mg/m2 each) on day 1 and on day 8. The treatment course was repeated every 3 weeks for 3 times. Positive cytological results in 38 patients before BISIC became negative in 27 (71.1%) patients after BISIC. After BISIC, 44 patients received laparotomy and CRS, and complete cytoreduction was achieved in 28 of 44 patients (64%). During BISIC, side effects of grade 3 and 4 were found in 6 (9.9%) patients. After CRS, 7 (15.8%) and 3 (6.8%) patients developed Grade 3 and 4 complications. The overall operative mortality rate was 4.5% (2/44). Histologic effects on primary tumors were found in 87.9% (29 /33 tumors). Complete histologic disappearance of PM was observed in 10 (22.7%) of 44 patients. Median survival time (MST) was 15.1 months, with a one and two-year survival of 62.4%, and 44.0%. BISIC therapy is safe and effective in gastric cancer patients with PM.
chemotherapy, BISIC, peritoneal metastasis, gastric cancer, neoadjuvant setting
The prognosis of gastric cancer patients with peritoneal metastases (PM) is extremely poor. Systemic chemotherapy or Cytoreductive surgery (CRS) alone does not improve the long-term survival of patients with PM. All the patients died of disease within 8 years after systemic chemotherapy with or without gastrectomy [1]. During recent two decades, a new therapeutic approach based on the combination of Cytoreductive surgery (CRS) and Perioperative intraperitoneal chemotherapy (PIC) has been developed [2,3]. During operation, the macroscopic disease is completely removed by the peritonectomy techniques and the residual micrometastases are treated with Hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC). Complete removal of primary tumor, regional lymph nodes and PM is essential for the long-term survival. Unfortunately, complete removal of PM is sometimes difficult when patients have diffuse involvement of the peritoneum. Accordingly, neoadjuvant intraperitoneal chemotherapy was developed to diminish PM before CRS [4]. Yonemura et al. reported the results Neoadjuvant intraperitoneal/systemic chemotherapy (NIPS), and patients with PM was treated by systemic chemotherapy of S-1 and intraperitoneal (i.p.) docetaxxel and cisplatin. After treatment by NIPS, the rate of complete cytoreduction increased, and allover survival after NIPS in combination with CRS was improved [5]. However, the results after NIPS are still dismal.
Recently, we developed a combination chemotherapy named Bidirectional intraperitoneal and systemic induction chemotherapy (BISIC) for the PM from gastric cancer in neoadjuvant setting. The regimen consists of i.p. and i.v. docetaxel and cisplatin once a 3 week interval to daily systemic chemotherapy of S-1 for 14 days. The present study demonstrated the results of a phase II clinical trial of BISIC to evaluate the efficacy and tolerability in gastric cancer patients with PM.
The eligibility criteria were as follows: histological proven primary and recurrent gastric cancer with PM, age younger than 75 years old, Eastern Cooperative Oncology Group performance status of zero to two, adequate bone marrow, liver, and renal function, and expected survival period of longer than 3 months. Patients were excluded if they had metastasis to distant organ sites, other active concomitant malignancies, or other severe medical conditions.
Written informed consent was obtained from all patients. This study was carried out in accordance with the Declaration of Helsinki. This study was approved by the Ehics Committee of Kishiwada Tokushukai Hospital, with ethical approval number H19. Sixty-one patients who were newly diagnosed as advanced gastric cancer or recurrent gastric cancer were enrolled in this study when PM was histologically confirmed.
A peritoneal port system (Hickman Subcutaneous port; BARD, Salt Lake City, UT, USA) was introduced into the abdominal cavity under local anesthesia, and the tip was placed on the cul-de-sac. Then, a peritoneal wash cytology was performed after 500 ml of physiological saline was injected into the peritoneal cavity. Papanicolaou staining was done before and at the end of each course.
S1 was administered orally twice daily at a dose of 60 mg/m2/day for 14 consecutive days, followed by 7 days rest. Docetaxel and cisplatin were administered i.p. at a dose of 30 mg/m2 on day 1. Docetaxel and cicplatin was diluted in 500 ml of normal saline and administered through the implanted peritoneal port system. The same dose of docetaxel and cisplatin were administered i.v. on day 8 after standard premedication. The treatment course was repeated every 3 weeks for 3 times.
After three cycles of BISIC, patients who had the following criteria are excluded as the candidates for CRS: 1) evidence of para-aortic lymph node involvement and distant hematogenous metastasis confirmed by Computed tomography (CT), or Magnetic resonance imaging (MRI), 2) patients with progressive disease after BISIC, or 3) patients with severe co-morbidities or poor general condition.
Objective tumor responses on PM and primary tumors were evaluated by CT and gastroesophageal endoscopy before and after BISC. Histologic effects on primary tumors and PM were evaluated according to the general rules for gastric cancer treatment [6]. Histological response after chemotherapy is classified into 4 categories. EF-0 shows no histologic response or response less than one third of the tumor tissue. A histologic EF-1 means that the degeneration of cancer is detected in the tumor tissue raging from one third to less than two thirds of the tumor tissue. EF-2 shows the degeneration of cancer tissue in wider than two thirds of the tumor tissue, while an EF-3 means the complete disappearance of the cancer cells.
Patients were evaluated to assess the extent of disease, before entry into the study and after 3 cycles of BISIC, by physical examination, Computed tomography (CT), upper gastrointestinal series, endoscopic examination and peritoneal cytologic status. Blood cell count, liver and renal function test, electrolytes and urinalysis were done once every two weeks. The response of primary tumors and PM were evaluated after 3 cycles of this treatment according to the response criteria of the Japanese Research Society for Gastric Cancer [6]. The National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3 was applied to evaluate adverse drug reactions. Postoperatie complication was assessed based on Common Terminology Criteria for Adverse Events v 4.0.
The primary end point was 1-year Overall survival (OS) rate. Secondary end points were the overall response rate (ORR), histological effects and safety.
All patients were followed and no patients were lost to follow-up. Outcome data were obtained from medical records and patients’ interview. All statistical analyses were performed using SPSS software statistical computer package version 17 (SPSS Inc., Chicago, USA).
From June 2012 to December 2013, 61 patients were enrolled in this study (Table 1). Only 47 patients with measurable target lesions were assessed for Overall response rate (ORR). The ORR was 29% with 14 patients showing partial response. In 33 patients with primary tumor, 29 patients (88%) showed partial response.
The chemotherapy was discontinued due to adverse events in 2 patients, and due to disease progression in 15 patients. In 44 patients, CRS was done 3 to 4 weeks after the last cycle of chemotherapy.
Before BISIC, cytology had been positive in 38 (67.8%) of 57 patients. These 38 positive cytological results before BISIC became negative in 27 (71.1%) patients after BISIC (Table 2).
After BISIC, 44 patients received laparotomy and CRS. At laparotomy, mean Peritoneal cancer index (PCI) [7] was 8.5, ranging from 0 to 27.
Total gastrectomy was performed in 25 patients. A variety of supplemental procedures were performed to achieve complete cytoreduction. Total colectomy, right hemicolectomy, left hemicolectomy and transverse colectomy were done in 9, 8, 2, and 2 patients, respectively. Colectomies combined with low anterior resection were done in 10 patients. Segmental resection of small bowel and small bowel mesentery was done in 24 patients. Hysterectomy combined with bilateral salpingo-oophorectomy was performed in 23 patients. For parietal peritonectomy, pelvic peritonectomy, subdiaphragmatic peritonectomy and peritonectomy of para-colic gutter were done in 31, 19 and 44 patients. Mean operation time was 227 min (120~380 min), and mean blood loss was 1344 ml (100~3500 ml).
Complete cytoreduction (CC-0) was achieved in 28 (64%) of 44 patients. During BISIC, side effects of grade 3 and 4 were found in 4 (6.6%) and 2 (3.3%) patients (Table 3). The frequent grade 3/4 toxic effects included leucopenia (4.9%) and fatigue (3.3%). After CRS, 4 (9.0%) and 3 (6.8%) patients developed Grade 3 and 4 complications (Table 4). The most frequent complications are bleeding in 3. The overall operative mortality rate was 4.5% (2/44), and the cause of death was multiple organ failure due to leakage and bleeding. Grade 4 complications were found in 3 patients, and 3 patients underwent operation for the postoperative bleeding in two patient and bowel fistula in one patient.
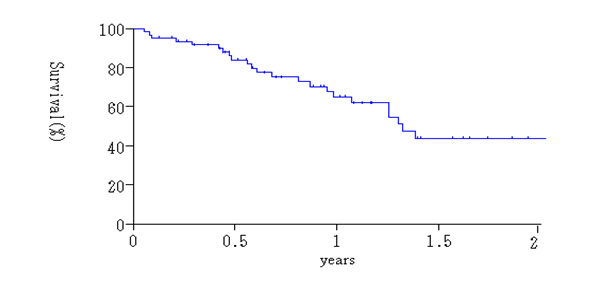
Histologic effects on primary tumors were found in 29 (87.9%) of the 33 tumors, and EF-1, -2, -3 response in the primary tumors were detected in 19 (57.6%), 9 (27.3%) and 1 tumor (4.0%), respectively (Table 5). Complete histologic disappearance of PM was observed in 10 (22.7%) of 44 patients (Table 5). Thirty-six patients were alive at the time of analysis. The survival curve for all patients is shown in Figure 1. Median Survival Time (MST) was 12.2 months, with a one and two-year survival of 62.4%, and 44.0%.
Table 1.Patient’s characteristics
| Sex |
|
| Male |
39 |
| Female |
32 |
| Age, years |
|
| Median |
54 |
| Range |
31-75 |
| ECOG performance status |
|
| 0 |
45 |
| 1 |
16 |
| Primary or recurrence |
|
| Primary |
38 |
| Recurrence |
23 |
| Histologic type |
|
| Differentiated |
4 |
| Poorly differentiated |
57 |
| Macroscopic type |
|
| Type 3 |
16 |
| Type 4 |
45 |
| Treatments |
|
| CRS# after BISIC& |
44 |
| BISC alone |
17 |
# CRS: Cytoreductive Surgery
& BISIC: Bidirectional Intraperitoneal Systemic Induction Chemotherapy
Table 2. Peritoneal cytological status before and after BISIC
| |
Three cycles after BISIC |
|
| Before BISIC |
negative |
positive |
|
| negative |
18 |
1 |
19 |
| positive |
27 |
11 |
38 |
| |
45 |
12 |
57 |
Table 3. Numbers of patients with toxic effects during BISIC
| Toxicity |
Grade (CTCAE v.3.0) |
| |
1 |
2 |
3 |
4 |
5 |
| Leukopenia |
1 |
1 |
3 |
|
|
| Fatigue |
2 |
4 |
1 |
1 |
|
| Diarrhea |
5 |
|
|
|
|
| Mucositis |
|
2 |
|
|
|
| Meningitis |
|
|
|
1 |
|
| Port infection |
|
|
|
1 |
|
Table 4. Mortality and morbidity after CRS
| |
Grade |
| |
1 |
2 |
3 |
4 |
5 |
| Bleeding |
|
|
|
2 |
1 |
| Intestinal fistula |
|
|
|
1 |
|
| Leakage |
|
|
|
|
1 |
| Pancreatic fistula |
|
|
2 |
|
|
| Wouddehiciency |
|
|
1 |
|
|
| Abscess |
|
|
1 |
|
|
Table 5. Histological effects after BISIC
2021 Copyright OAT. All rights reserv
| Lesions |
EF-0 |
EF-1 |
EF-2 |
EF-3 |
| Primary tumors (N=33) |
4 (12.1%) |
19 (57.6%) |
9 (27.3%) |
1 (4.0%) |
| Peritoneal metastasis (N=44) |
6 (13.6%) |
19 (43.2%) |
9 (20.5%) |
10 (22.7%) |
After the comprehensive treatment for PM, complete cytoreduction is the strongest prognostic factor for the long-term survival [7-9]. However, survival of patients with PCI score higher than the threshold value is poor, even if they received complete cytoreduction. Diagnostic laparoscopy showed that complete ctroreduction was supposed to achieve only in fewer than 30 % of gastric cancer-patients with PM who had not been treated with neoadjuvant chemotherapy [10]. Accordingly, PCI levels higher than the threshold value should be reduced within the threshold level by Neoadjuvant chemotherapy (NAC) before CRS,
Systemic chemotherapy is usually used as NAC. However, the effects of systemic chemotherapy on PM are limited, and no long-term survivors were reported after systemic chemotherapy. Takeyoshi et al. reported the median overall survival by weekly paclitaxel with doxifluridine was 215 days, and 1-year survival rate was only 29.2% [11]. The reason is considered that the peritoneal cavity acts as a sanctuary against systemic chemotherapy, because of the existence of a Blood-peritoneal barrier (BPB). BPB consists of stromal tissue between mesothelial cells and submesothelial blood capillaries [12]. Accordingly, only a small amount of systemic drugs are capable of penetrating this barrier.
In contrast, i.p. chemotherapy offers potential therapeutic advantages over systemic chemotherapy by generating very high locoregional intensity of drugs in the peritoneal cavity [13]. Coccolini F et al. reported that i.p. chemotherapy + CRS is associated with improved overall survival [14].
For the i.p. chemotherapy, degree recommended drugs which stay long time in the peritoneal cavity after i.p. administration [15]. Molecular weights of paclitaxel, docetaxel, gemcitabine, 5-fluorouracil and doxorubicinis are high. After i,p, administration of these drugs, the ratio of the area under the drug concentration-time curve (AUC) in the peritoneal cavity and AUC in plasma (AUCp/AUCs) is higher than those after i,p, administration of other drugs [15]. Among these drugs, AUCp/AUCs were much larger for paclitaxel and docetaxel than for other drugs [16]. They are administered as micellar preparation, Taxol and Taxotere, which consists of paclitaxel in Cremophor and docetaxel in Polysorbate-80. Paclitaxel and docetaxel are slowly released from micellar compounds, and the relatively higher intraperitoneal concentration of the paclitaxel and docetaxel are maintained for a long time [16]. From these results, Yonemura et al. developed Neoadjuvant intraperitoneal/systemic chemotherapy (NIPS) [17]. NIPS can eradicate PFCCs before CRS, and may prevent the attachment of PFCCs on the surgical wound at CRS. In addition, complete histologic response on PM was found in 37% of patients after NIPS [17].
More recently, alternate administration of systemic and intraperitoneal chemotherapy was developed [18] and we designated the method Bidirectional Intraperitoneal and Systemic Induction Chemotherapy (BISIC). BISIC creates a wider treatment area than single treatment by a bidirectional diffusion gradient. In the present study, positive cytology before BIPSC became negative in 79% of patients after 3 cycles of BISIC. Histologic response rates on PM after BISIC and NIPS were 86.4% (38/44) and 60% (88/147), respectively [17] and the histologic response rate after BISIC was significantly higher than that after NIPS. In addition, one-year survival of patients after BISC and NIPS were 62% and 36% [17]. These results may indicate that BISIC has more effective for survival than NIPS. An analysis of long-term survival after BISC is awaited.
During BISIC, side effects of grade 3 and 4 were found in 9.9% (6/61) among 61 patients. The frequent grade 3/4 toxic effects included leucopenia (4.9%) and fatigue (3.3%). All patients recovered after appropriate infusion therapy. Ishigami et al. developed a new BISIC consists of weekly i.v. and i.p. paclitaxel combined with S1 [18]. However, grade 3/4 toxic effects were found in 38% of patients, including neutropenia (38%) and anemia (10%), and were higher than our results.
These results indicate that NIPS and BISIC are effective therapies for the eradication of PFCCs and for the reduction of PCI score. BISIC has more powerful in histologic response on PM than NIPS.
Accordingly, BISIC considered being a safe method as compared with the results of previous reports of systemic chemotherapy. However, BISIC might increase the mortality rate after CRS. After CRS, 4 (9.0%) and 3 (6.8%) patients developed Grade 3 and 4 complications, and mortality was experienced in 4.5% (2/44) of patients.
Glehen reported a higher complication rate of 47% in patients who underwent extensive cytoreductive surgery (gastrectomy combined with the removal of more than 2 peritoneal zones) [19]. The magnitude of surgery, the number of resected organs, the number of anastomosis, and the operation time are considered to have contributed to the significantly higher complication rate. To avoid futile aggressive treatments, the preoperative and intraoperative stringent selection of patients must be emphasized.
- 1. Hong AH, Shin YR, Roh Y, Jeon EK, Song KY, et al. (2013) Treatment outcomes of systemic chemotherapy for peritoneal carcinomatosis arising from gastric cancer with no measurable disease: retrospective analysis from a single center. Gastric Cancer 16: 290-300. [Crossref]
- 2. Glehen O, Yonemura Y, Sugarbaker H (2013) Cytoreductive surgery &periopertaive chemotherapy for peritoneal surface malignancy. Chapter 4; Prevention and treatment of peritoneal metastases from gastric cancer. P79-89. Textbook and Video Atlas. Ed. Paul Sugarbaker PH, Cine-Med Publishing, Inc., North Woodburry, CT, USA
- 3. Yonemura Y, Canbay E, Endou Y, Ishibashi H, Mizumoto A, et al. (2014) Peritoneal cancer treatment. Expert OpinPharmacother 15: 623-636. [Crossref]
- 4. Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, et al. (2006) Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J SurgOncol 32: 661-665.[Crossref]
- 5. Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, et al. (2010) Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J GastrointestOncol 2: 85-97.[Crossref]
- 6. Japanese Research Society for Gastric Cancer (1995) The General Rules for Gastric Cancer Study (1st English ed). Tokyo. KaneharaShuppan.
- 7. Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82: 359-374.[Crossref]
- 8. Sugarbaker PH (1999) Successful management of microscopic residual disease in large bowel cancer. Cancer ChemotherPharmacol 43: S15-25.[Crossref]
- 9. Yan TD, Morris DL (2008) Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg 248: 829-835.[Crossref]
- 10. Valle M, Van der SpeetenGalafalo (2009) Laparoscopic hyperthermic intraperitoneal preoperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J SurgOncol 15:331-334.
- 11. Takeyoshi I, Makita F, Iwazaki S, Ishikawa H, Kakinuma S, et al. (2011) Weekly paclitaxel in combination with doxifluridine for peritoneally disseminated gastric cancer with malignant ascites. Anticancer Res 31: 4625-4630.[Crossref]
- 12. Baron MA (1941) Structure of intestinal peritoneum in man. Am J Anat 69:439-497.
- 13. Markman M (1991) Intraperitoneal therapy in ovarian cancer utilizing agents acjieving high local but low systemic exposure. Reg Cancer Treat 40:256-260.
- 14. Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, et al. (2014) Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J SurgOncol 40: 12-26. [Crossref]
- 15. deBree E, Tsiftsis DD (2007) Experimental and pharmacologic studies in intraperitoneal chemotherapy from laboratory bench to bedside. Advances in peritoneal surface oncology. S. Gonzalez-Moleno ed. Springer: 53-73.
- 16. Miyamoto K, Shimada T, Sawamoto K, Sai Y, Yonemura Y (2012) Disposition kinetics of taxanes in peritoneal dissemination. Gastroenterol Res Pract2012: 963403.[Crossref]
- 17. Yonemura Y (2012) Effects of Neoadjuvant Intraperitoneal/Systemic Chemotherapy (Bidirectional Chemotherapy) for the Treatment of Patients with Peritoneal Metastasis from Gastric Cancer. International Journal of Surgical Oncology. [Crossref]
- 18. Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, et al. (2010) Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 21: 67-70.[Crossref]
- 19. Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, et al. (2010) Peritoneal carcinomatosis from gastric cancer; A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann SurgOncol 17: 2370-2307. [Crossref]
Editorial Information
Editor-in-Chief
Masayoshi Yamaguchi
Emory University School of Medicine
Article Type
Review Article
Publication history
Received: September 24, 2014
Accepted: October 03, 2014
Published: October 07, 2014
Copyright
Copyright: ©2014 Yonemura Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited..
Citation
Yonemura Y, Canbay E, Endou Y, Ishibashi H, Mizumoto A, et al. (2014) A new bidirectional intraperitoneal and systemic induction chemotherapy (BISIC) for the peritoneal metastasis from gastric cancer in neoadjuvant setting. Integr Cancer Sci Therap. 1: doi: 10.15761/ICST.1000106