A 20-years-old healthy female developed new-onset cardiac abnormalities discovered on a routine primary care visit, when she received her 2nd dose of the HPV vaccine. The patient had no significant past medical history apart from hypothyroidism, a single episode of febrile seizure at the age of 2 and receiving the first dose of HPV vaccine 3 weeks prior. In previous routine medical visits by various healthcare providers there was no indication of an irregular heartbeat or an arrhythmia. There was no family history of heart disorders or sudden cardiac death. During this visit to her new adult primary care doctor, a baseline physical examination revealed irregular heart rhythm. An ECG was performed showing frequent premature ventricular complexes and ST abnormalities (Figure 1). The patient had another abnormal ECG a week later during a follow up visit, which similarly demonstrated premature aberrantly conducted complexes and a marked ST abnormality. An echocardiogram was negative for any structural heart anomalies. Finally, a week following her third vaccination with the HPV vaccine, the patient started to experience dizziness, joint pain and unusual fatigue. Less than 3 weeks later, she was found dead from a cardiac arrest during her night sleep. A full autopsy analysis revealed no anatomical, histological, toxicological, genetic or microbiological findings that might be linked to a potential cause of death.
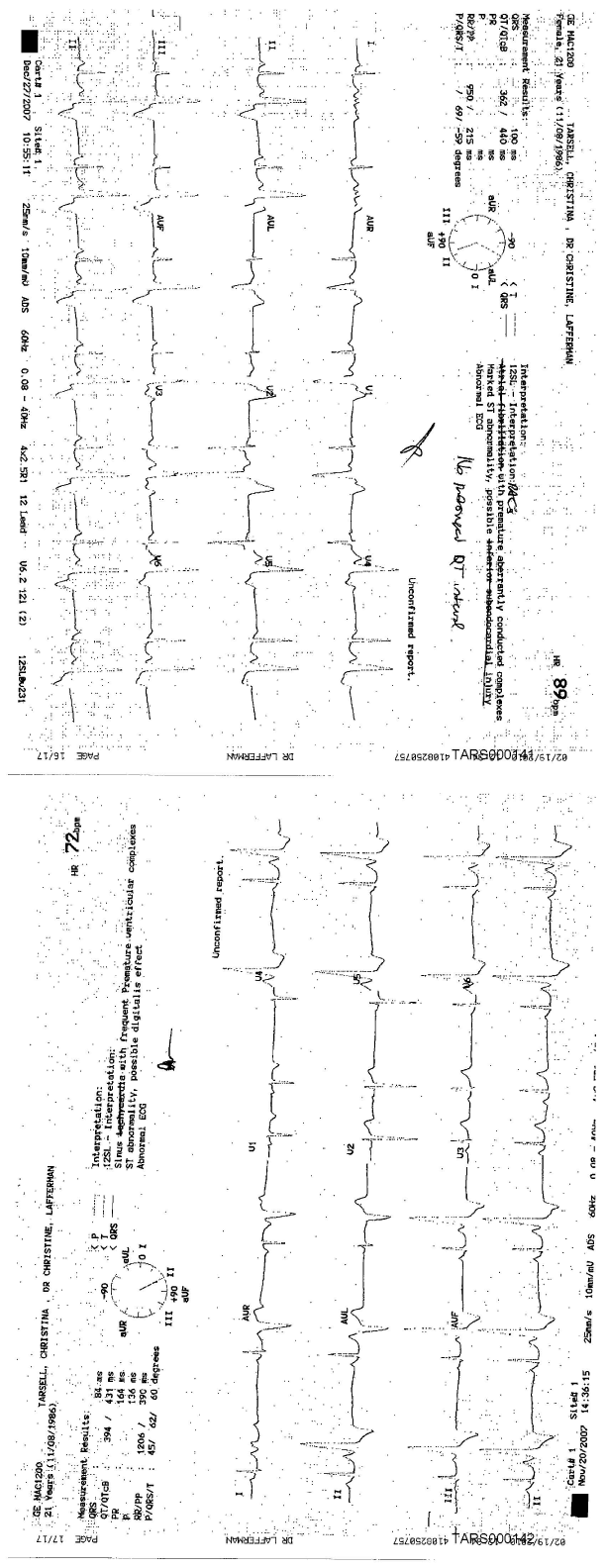
Figure 1. ECG showing frequent premature ventricular complexes and ST abnormalities
HPV Vaccine, sudden death, cardiac arrest, ASIA syndrome, molecular mimicry, Nocturnal cardiac arrhythmia
The first vaccine was created back in 1798, when Edwards Jenner inoculated individuals with fluid from the blisters of smallpox disease [1]. Thereafter, the use of vaccination spread globally, leading to eradication of lethal infectious. However, over the years, worries have been raised regarding the safety of certain vaccines.
Vaccine-associated adverse events are mainly acute and transient; other reactions, such as autoimmune phenomena, are uncommon [2]. Post-vaccination autoimmunity, although uncommon, is well described and include conditions such as Guillain–Barre syndrome, immune thrombocytopenic purpura, Postural Orthostatic Tachycardia Syndrome (POTS) and other autoimmune manifestations [3].
HPV is a group of viruses belonging to a family of double-stranded circular DNA viruses, capable of infecting epithelial cells of the skin, oral and genital mucosa. HPV-16 & HPV-18 are responsible for about 70% of cervical cancers worldwide, HPV-6 and HPV-11 are the most common causes of genital warts [4].
There are three types of HPV vaccines available as of date: the bivalent Cervarix (aimed against serotypes 16 and 18), the quadrivalent Gardasil (aimed against serotypes 6, 11, 16 and 18) and the 9-valent vaccine (aimed against serotypes 6, 11, 16, 18, 31, 33, 45, 52 and 58) [5]. Vaccination with HPV vaccines was found to be effective, providing a long-lasting protection against HPV infection and premalignant lesions [6].
Herein, we intend to review current data regarding the relationship between HPV vaccination and susceptibility to sudden cardiac death.
The first larger post-licensure analysis of side effets using the Vaccine Adverse Event Reporting System (VAERS) database [7] identified 32 deaths among 12,424 HPV Vaccine-related reports received during the period from June 1, 2006 to December 31, 2008. Out of these 32 deaths, at least 6 were cardiac-related deaths, confirmed by autopsy reports and medical records. The rate of these cardiac deaths did not produce a significant safety signal.
The median time from the last HPV vaccination to death was 14.5 days, a time-frame consistent with our case, in which the death occurred less than three weeks after HPV vaccine administration. We have conducted a search in the VAERS database in order to evaluate the current number of death cases related to HPV vaccination. We were surprised to find out a total number of 292 cases (Table 1), out of them there were 2 cases of cardiac death and 11 more cases of sudden death.
Table 1. A search in the VAERS database in order to evaluate the current number of death cases related to HPV vaccination, updated on 2.5.2017
Symptoms |
Vaccine |
Events reported |
Percent |
Brain death |
HPV (Gardasil) |
2 |
0.68% |
Brain death |
HPV (Gardasil 9) |
1 |
0.34% |
Death |
HPV (Gardasil) |
228 |
78.08% |
Death |
HPV (Gardasil 9) |
4 |
1.37% |
Death |
HPV (No brand name) |
36 |
12.33% |
Death |
HPV (Cervarix) |
12 |
4.11% |
Sudden cardiac death |
HPV (Gardasil) |
2 |
0.68% |
Sudden death |
HPV (Gardasil) |
11 |
3.77% |
However, it is obvious that VAERS has limitations, since the postmarket reporting of side effects is discretionary and the reports are collected from a population of unknown size. Consequently, it is not possible to estimate the frequency of adverse events or to establish a cause and effect relationship via VAERS and similar passive-reporting systems. Moreover, cardiac arrhythmias are not currently listed ore fully recognized as a possible adverse reaction to vaccines [8]. In many cases cardiac-related manifestations are vague and non-specific and hence readily misdiagnosed or underappreciated [9].
Another major limitation of the VAERS analysis by Slade, et al. [7] should be mentioned. Namely, the authors used the distributed and not the administered doses as the denominator when calculating the rate of adverse events. Based on adverse event data from countries that track the administered doses, the rate of adverse events are likely underestimated by five to tenfold [10]. Thus, the actual number of adverse events including cardiac-related fatalities in association with HPV vaccine could be much higher than currently reported.
HPV-16 DNA - stimulated secretion of tumor necrosis factor
In addition to VAERS data, there is at least one relevant case reported in the medical literature [11] which relates to a previously healthy 18 year old girl who suffered a sudden death during her night sleep, six months after her 3rd HPV vaccine injection [11]. Although her death occurred many months after the last dose of HPV vaccine, her symptoms began shortly after the 1st dose and included a range of non-specific complaints, including headaches, dizziness spells, memory lapses and difficulty thinking. After receiving her 2nd injection, she also developed intermittent arm weakness, fatigue, signs of peripheral neuropathy, and palpitations. These symptoms persisted until her untimely death. Full autopsy analysis revealed no findings that might be linked to a potential cause of death. However, HPV-16 L1 gene DNA fragments were detected in the post-mortem blood and spleen tissue analysis. These were identical in sequence the fragments previously found in16 separate HPV vaccine vials. These 16 vials were from different vaccine lots and originated from different countries, including the U.S., Russia, Bulgaria and India, which indicates a widespread contamination process during HPV vaccine manufacture [12]. Moreover, these fragments detected in the HPV vaccine were bound to the aluminum adjuvant used in the vaccine formulation, which likely provided protection against endogenous nucleases [13]. This may be the explanation for their persistence in the blood over 6 months following injection. Interestingly, although the World Health Organization webpage specifically state that HPV vaccine is a highly purified vaccine and contain no DNA fragments [14-16], the findings of such DNA residuals in HPV vaccine vials [12], and in the tissues of the deceased vaccinated girl, show that the methods of purifications are not very efficient.
The HPV-16 L1 gene DNA fragments detected in the postmortem blood and splenic tissue in this case are presumably present in the nucleated cells, probably macrophages. It has been shown that the injection of free HPV-16 L1 plasmid DNA Intramuscularly in mice can activate the immune system by inducing a strong CD8 T cell response [17]. Furthermore, the presence of DNA fragments in macrophages may cause release of various cytokines, including tumor necrosis factor (TNF)- α [18], a recognized myocardial depressant [19] and marker for sudden cardiac death [20-22]. Interestingly, in a study of 8 cases of sudden infant deaths, all of occurred during sleep, Emura, et al. [22] found elevated levels of TNF- α and other pro-inflammatory cytokines in peripheral blood smear preparations that were significantly above normal thresholds. Because of this, Emura, et al. concluded that cytokine abnormality may be one of the underlying mechanisms in sudden infant death syndrome [22].
Molecular mimicry
In addition, there are other factors that might contribute to determine adverse cardiovascular events including sudden death following HPV vaccination. Kanduc [23] found a shared pattern between 34 pentamers from the HPV viral capsid protein and human protein. These proteins, when altered, have been shown to play a major role in arrhythmias, cardiovascular diseases and sudden death. For example, 9 out of the 34 viral pentamers belong to the human protein, Titin, a key component in the assembly and functioning of striated muscles. Defects in Titin may cause ventricular cardiomyopathy characterized by a high risk of cardiac failure and sudden cardiac death. Other significant matches include components of intercellular desmosome junctions such as plakophilin-2, desmoplakins, and desmocollin-2. Defects in these desmosomal proteins have been reported in arrhythmogenic right ventricular cardiomyopathy [24,25] which as mentioned above, has previously been linked to sudden cardiac death during sleep [26-28]. The voltage-dependent L-type calcium channel subunit alpha-1C has also been shown to match with the HPV-16 L1 sequence. This protein in known to be a altered in the Brugada syndrome, an important arrhythmogenic disorder associated with high-risk nocturnal arrhythmias [29,30].
Extending the peptide matching analyses to L1 proteins from the four strains (HPV 6, 11, 16, and 18) (Table 2), it emerges an even more impressive immunocrossreactive potential that specifically threatens the cardiac functions. Space precludes a detailed peptide-by-peptide discussion. Suffice to say that the peptide overlap between HPV L1 antigens and human Titin escalates to 41 pentapeptides (excluding multiple occurrences).
Table 2. Peptide sharing between HPV L1 and human proteins that, when altered, are associated to sudden death
Peptide sequence |
HPV strain |
Human protein associated to sudden death |
AGAVG |
16 |
ACADM. Medium-chain specific acyl-CoA dehydrogenase, mitochondrial. ACADM defects associate with fasting hypoglycemia, hepatic dysfunction and encephalopathy, often resulting in death [39] |
LGVGI
GSSRL |
16
18 |
ACADV. Very long-chain specific acyl-CoA dehydrogenase, mitochondrial. One major phenotype is a childhood form, with high mortality and high incidence of cardiomyopathy [40] |
PGSCV |
18 |
AKAP9. A-kinase anchor protein 9. AKAP9 defects may cause long QT syndrome, a heart disorder characterized by a prolonged QT interval and ventricular arrhythmias. They cause syncope and sudden death in response to exercise or emotional stress, and can present with a sentinel event of sudden cardiac death in infancy [41] |
LCSIT
|
6,11 |
ANK2. Ankyrin-2. Involved in long QT syndrome, A heart disorder characterized by a prolonged QT interval on the ECG and polymorphic ventricular arrhythmias. They cause syncope and sudden death in response to exercise or emotional stress, and can present with a sentinel event of sudden cardiac death [42] |
GTVCK
LQAGL
QAGLR |
11
16
18 |
CAC1C. Voltage-dependent L-type calcium channel subunit alpha-1C. Defects in CAC1C are the cause of 1) Timothy syndrome, a disorder characterized by multiorgan dysfunction including lethal arrhythmia; 2) Brugada syndrome 3, characterized by the association of Brugada syndrome with shortened QT intervals. Ventricles beat so fast that the blood is prevented from circulating efficiently in the body. When this situation occurs, the individual will faint and may die in a few minutes if the heart is not reset [43, 44] |
RPSDS
|
6, 11 |
CACB2. Voltage-dependent L-type calcium channel subunit beta-2. Involved in a heart disease characterized by the association of Brugada syndrome with shortened QT intervals. Ventricles beat so fast that the blood is prevented from circulating efficiently in the body and the individual will faint and may die in a few minutes [44, 45] |
AGAVG
NKFGL |
16
18 |
CMC2. Calcium-binding mitochondrial carrier protein Aralar2. A form of citrullinemia characterized primarily by elevated serum and urine citrulline levels; characterized by neuropsychiatric symptoms including abnormal behaviors, loss of memory, seizures and coma. Death can result from brain edema [46] |
SVTTS |
6 |
CSRP3. Cysteine and glycine-rich protein 3. Associated with dilated and hypertrophic phenotypes of cardiomyopathy ventricular dilation and impaired systolic function, resulting in congestive heart failure and arrhythmia. Patients are at risk of premature death. The symptoms include dyspnea, syncope, collapse, palpitations, and chest pain. They can be readily provoked by exercise [47, 48] |
SDVPI
TKTKK
STSET |
6
11
16 |
ECHB. Trifunctional enzyme subunit beta, mitochondrial. Altered ECHB can lead to hypoglycemia, cardiomyopathy, sensorimotor axonopathy. Sudden infant death may occur. Most patients die from heart failure [49] |
LQPPP; QPPPG |
16 |
FEV. Protein FEV. Functions in the maintenance of the central serotonergic neurons. FEV defects associate with susceptibility to sudden infant death. Pathogenic mechanisms precipitating an infant sudden death remain elusive [50] |
RVNVG; VNVGM
VHTPS; HTPSG
GVEVG
LILHY |
6,11
11
16
18 |
FLNC. Filamin-C. Hypertrophic ventricular cardiomyopathy. Symptoms include dyspnea, syncope, collapse, palpitations, and chest pain, that can be readily provoked by exercise. High risk of cardiac failure and sudden cardiac death [51]
|
PSTAP |
11 |
GATA5. Transcription factor GATA-5. Involved in atrial fibrillation, characterized by disorganized atrial electrical activity and ineffective atrial contraction promoting blood stasis in the atria and reduces ventricular filling. It can result in palpitations, syncope, thromboembolic stroke, and congestive heart failure, arrhythmia. Patients are at risk of premature death [52] |
RTSVG; TSVGS |
6 |
JPH2. Junctophilin-2. JPH2 is necessary for proper intracellular Ca2+ signaling in cardiac myocytes via its involvement in ryanodine receptor-mediated calcium ion release. Involved in hypertrophic ventricular cardiomyopathy. Symptoms include dyspnea, syncope, collapse, palpitations, and chest pain, that can be readily provoked by exercise. High risk of cardiac failure and sudden cardiac death [53] |
RVFRI
RVFRV; PASPG |
16
18 |
KCND3. Potassium voltage-gated channel subfamily D member 3.Involved in Brugada syndrome, a tachyarrhythmia that can cause the ventricles to beat so fast that the blood is prevented from circulating efficiently in the body. The individual will faint and may die in a few minutes if the heart is not reset [54] |
GTLED
KKRKL |
6, 11, 16
16 |
MYH6. Myosin-6. Involved in hypertrophic ventricular cardiomyopathy; symptoms include dyspnea, syncope, collapse, palpitations, and chest pain. They can be readily provoked by exercise. High risk of cardiac failure and sudden cardiac death [55] |
GTLED
KKRKL |
6, 11,16
16 |
MYH7. Myosin-7. Associated with hypertrophic ventricular cardiomyopath. The symptoms include dyspnea, syncope, collapse, palpitations, and chest pain; high risk of cardiac failure and sudden cardiac death [56] |
GTLED
EKEKQ |
6, 11,16
11 |
MYH7B. Myosin-7B. Associated with left ventricular noncompaction. |
VGEPV |
6, 11 |
MYPC3. Myosin-binding protein C, cardiac-type. Involved in ventricular cardiomyopathy.Symptoms are: dyspnea, syncope, collapse, palpitations, and chest pain. They can be provoked by exercise. Risk of cardiac failure and sudden cardiac death [57] |
VTTSS
KVSGL
PPTTS; RSAPS; TTSSK |
6
16
18 |
MYPN. Myopalladin. Component of the sarcomere that tethers together nebulin (skeletal muscle) and nebulette (cardiac muscle) to alpha-actinin, at the Z lines [58] |
LPPPS |
18 |
NU155. Nuclear pore complex protein Nup155. Involved in atrial fibrillation, a common sustained cardiac rhythm disturbance. Atrial fibrillation is characterized by disorganized atrial electrical activity and ineffective atrial contraction promoting blood stasis in the atria and reduces ventricular filling. It can result in palpitations, syncope, thromboembolic stroke, and congestive heart failure [59] |
MFARH
|
6, 11 |
RN207. RING finger protein 207. Plays a role in cardiac repolarization possibly by stabilizing membrane expression of the potassium channel KCNH2/HERG [60] |
KVVLP
|
6
11 |
RYR2. Ryanodine receptor 2. Calcium channel that mediates the release of Ca2+ and thereby plays a key role in triggering cardiac muscle contraction. Involved in arrhythmogenic right ventricular dysplasia; and in ventricular tachycardia, that may degenerate into cardiac arrest and cause sudden death [61, 62] |
GLQPP
|
16 |
RYR1. Ryanodine receptor 1. Plays a key role in triggering muscle contraction following depolarization of T-tubules. Associated with malignant hyperthermia, accelerated muscle metabolism, contractures, metabolic acidosis, tachycardia and death [63] |
PEKEK;
EKEKQ
KLDDT |
6, 11
11
16, 18 |
SCN8A. Sodium channel protein type 8 subunit alpha. SCN8A alterations may associate with early-onset seizures, features of autism, intellectual disability, ataxia, and sudden unexplained death in epilepsy [64]. |
GRSSI; KRANK; RANKT; RSSIR;SDVPI; VGSSI; VSKAS
GEPVP; KSDVP; KTVVP;
PSDST; SITLS; TVVPK; VENSG; VGEPV;VVDTT; VVPKV; YQYRV
KVNKT; NRSSV; SKSAT; SVSKS; VSKPS
DTTRS
HVEEY
AGLKA; KKYTF; KVSGL PPAPK SEVPL; STANL STILE; TSRLL; VGENV
VVDTT
GLPDT; LELKN; NKFGL; PPPTT;YQYRV; VPPPP |
6
6,11
11
6,11,16
6,18
16
16,18
18 |
TITIN. Titin. Key component in the assembly and functioning of vertebrate striated muscles. Defects in Titin may cause ventricular cardiomyopathy characterized by a high risk of cardiac failure and sudden cardiac death [65] |
EKEKP |
6 |
TRDN. Triadin. Involved in excitation-contraction coupling in the heart and in regulating the rate of heart beats. Involved in ventricular tachycardia that may degenerate into cardiac arrest and cause sudden death. Patients present with recurrent syncope, or sudden death after physical activity or emotional stress [66] |
TLEDT
PGGTL |
6,11,16
16 |
TRPM4. Transient receptor potential cation channel subfamily M member 4. Involved in atrio-ventricular block causing syncope and sudden death [67] |
NPYFR |
18 |
TSYL1. Testis-specific Y-encoded-like protein 1. Involved in sudden infant death with dysgenesis of the testes syndrome. Features included bradycardia, hypothermia, severe gastroesophageal reflux, laryngospasm, bronchospasm, and abnormal cardiorespiratory patterns during sleep [68] |
The cited investigation by Kanduc [23] and data from Table 2 confirm and extend previous reports describing a high level of homology between microbial antigens and the human proteome [31-34]. Furthermore, they suggest that possible immune cross-reactions deriving from utilization of HPV L1 proteins in current HPV vaccines might be a risk for cardiovascular events. A better understanding of potential antigen cross-reactivity, which at present is abysmally lacking, is necessary to minimise post-vaccination events [23].
The development of vaccines has proven to be a successful and cost-effective for global human health, and they present an essential part of preventive modern medicine.
It is obvious that vaccines are administered to millions of people worldwide, and that not everyone develops serious adverse manifestations. Hence, clearly there are some prior susceptibilities that make some people more at risk of experiencing an adverse reaction to vaccination than others. Among these are genetic factors, personal and familial history of relevant symptoms, hypersensitivity and a prior adverse response to vaccination [35,36]. These factors should be routinely addressed, in order to identify the patients who might be prone to vaccine associated adverse events and give them the best possible care.
- Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y (2009) Vaccines and autoimmunity. Nature Reviews Rheumatology 11: 648.
- Segal Y, Calabrò M, Kanduc D, Shoenfeld Y (2017) Human papilloma virus and lupus: the virus, the vaccine and the disease. Curr Opin Rheumatol 29: 331-342.
- Guimaraes LE, Baker B, Perricone C, Shoenfeld Y (2015) Vaccines, adjuvants and autoimmunity. Pharmacol Res 100: 190-209.
- Tommasino M (2014) The human papillomavirus family and its role in carcinogenesis. Seminars in cancer biology, Elsevier.
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, et al. (2016) Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3-Dose Regimen in Women. JAMA 316: 2411-2421. [Crossref]
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, et al. (2007) Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New England Journal of Medicine 356: 1928-1943.
- Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, et al. (2009) Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 302: 750-757. [Crossref]
- http://www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_ppi.pdf
- Dahan S, Tomljenovic L, Shoenfeld Y (2016) Postural Orthostatic Tachycardia Syndrome (POTS)--A novel member of the autoimmune family. Lupus 25: 339-342.
- Tomljenovic L, Shaw CA (2013) Human papillomavirus (HPV) vaccine policy and evidence-based medicine: Are they at odds? Ann Med 45: 182-193.
- Lee SH (2012) Detection of human papillomavirus L1 gene DNA fragments in postmortem blood and spleen after Gardasil® vaccination-A case report. Advances Biosc Biotech 3: 1214-1224.
- Lee SH (2012) Detection of human papillomavirus (HPV) L1 gene DNA possibly bound to particulate aluminum adjuvant in the HPV vaccine Gardasil. J Inorg Biochem 112: 85-92.
- Lee SH (2013) Topological conformational changes of human papillomavirus (HPV) DNA bound to an insoluble aluminum salt-A study by low temperature PCR. Advances Biol Chem 3: 76-85.
- http://www.who.int/vaccines/en/olddocs/humanpapill.html
- Merck Research LaboratoriesGARDASIL® (2010) Human Papillomavirus [Types 6, 11, 16, 18] Recombinant Vaccine). Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document. Presented to VRBPAC on 17th November 2010.
- https://www.ema.europa.eu/en/documents/product-information/gardasil-epar-product-information_en.pdf
- Caulfield MJ, Shi L, Wang S, Wang B, Tobery TW, et al. (2007) Effect of alternative aluminum adjuvants on the absorption and immunogenicity of HPV16 L1 VLPs in mice. Hum Vaccin 3: 139-145. [Crossref]
- Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, et al. (1997) Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur J Immunol 27: 1671-1679.
- Kumar A, Paladugu B, Mensing J, Parrillo JE (2007) Nitric oxide-dependent and -independent mechanisms are involved in TNF-alpha -induced depression of cardiac myocyte contractility. Am J Physiol Regul Integr Comp Physiol 292: R1900-R1906.
- Pomara C, Neri M, Bello S, Pennella A, Turillazzi E, et al. (2010) TNF-alpha and interleukin myocardial expression in a case of fatal sudden cardiac failure during clinic reactivation of systemic lupus erythematosus. Lupus 19: 1246-1249.
- Quinaglia ESJC, Coelho-Filho OR, Andrade JM, Quinaglia T, Modolo RG, et al. (2013) Peri-Infarct Zone Characterized by Cardiac Magnetic Resonance Imaging is Directly Associated with the Inflammatory Activity During Acute Phase Myocardial Infarction. Inflammation.
- Emura I, Usuda H (2011) Biochemical, cytological and histopathological examination of sudden unexpected death in infancy. Pathol Int 61: 469-474.
- Kanduc D (2011) Potential cross-reactivity between HPV16 L1 protein and sudden death-associated antigens. J Exp Ther Oncol 9: 159-165.
- Gerull B, Heuser A, Wichter T, Paul M, Basson CT, et al. (2004) Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 36: 1162-1164.
- Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, et al. (2006) Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet 79: 978-984.
- Corrado D, Basso C, Buja G, Nava A, Rossi L, et al. (2001) Right bundle branch block, right precordial st-segment elevation, and sudden death in young people. Circulation 103: 710-717. [Crossref]
- Cho Y, Park T, Yang DH, Park HS, Chae J, et al. (2003) Arrhythmogenic right ventricular cardiomyopathy and sudden cardiac death in young Koreans. Circ J 67: 925-928. [Crossref]
- Nucifora G, Benettoni A, Allocca G, Bussani R, Silvestri F (2008) Arrhythmogenic right ventricular dysplasia/cardiomyopathy as a cause of sudden infant death. J Cardiovasc Med (Hagerstown) 9: 430-431.
- Matsuo K, Kurita T, Inagaki M, Kakishita M, Aihara N, et al. (1999) The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J 20: 465-470.
- Paul M, Meyborg M, Boknik P, Gergs U, Schmitz W, et al. (2011) Autonomic dysfunction in patients with Brugada syndrome: further biochemical evidence of altered signaling pathways. Pacing Clin Electrophysiol 34: 1147-1153.
- Kanduc D (2012) Peptide cross-reactivity: the original sin of vaccines. Front Biosci 4: 1393-1401.
- Kanduc D (2009) Quantifying the possible cross-reactivity risk of an HPV16 vaccine. J Exp Ther Oncol 8: 65-76. [Crossref]
- Trost B, Lucchese G, Stufano A, Bickis M, Kusalik A, et al. (2010) No human protein is exempt from bacterial motifs, not even one. Self Nonself 1: 328-334. [Crossref]
- Trost B, Kusalik A, Lucchese G, Kanduc D (2010) Bacterial peptides are intensively present throughout the human proteome. Self Nonself 1: 71-74.
- Gatto M, Agmon-Levin N, Soriano A, Manna R, Maoz-Segal R, et al. (2013) Human papillomavirus vaccine and systemic lupus erythematosus. Clin Rheumatol 32: 1301-1307.
- Zafrir Y, Agmon-Levin N, Paz Z, Shilton T, Shoenfeld Y (2012) Autoimmunity following Hepatitis B vaccine as part of the spectrum of 'Autoimmune (Auto-inflammatory) Syndrome induced by Adjuvants' (ASIA): analysis of 93 cases. Lupus 21: 146-152.

