Abstract
Metabolic Syndrome (MS) is one of the most commonly used names for a clinical entity that brings together or associates different disorders that increase the risk of cardiovascular disease. Cannabis sativa has been used as an herbal medicine for tens of centuries. This study aimed to analyze the effects of cannabis oil administration on cannabinoid-induced tetrad, systolic and diastolic blood pressure, and metabolic parameters in normal female rats fed a sucrose-rich diet (SRD). Female Wistar rats were fed with the one of following diets for 21 days: Reference Diet (RD): standard commercial laboratory diet, SRD, and SRD+Cannabis oil (SRD+Ca): oral administration of 1 mg/kg body weight of cannabis oil daily. The full spectrum cannabis oil contains a total cannabinoid CBD:THC, 2:1 ratio.
Cannabis oil administration in the SRD significantly increased analgesia and decreased locomotion. Systolic and diastolic blood pressures decreased during the experimental protocol. In the SRD+Ca group, serum triglyceride levels decreased significantly, without changes in cholesterol and glucose levels. In addition, serum uric acid, AST, ALT and AP levels were significantly decreased. In the liver, the abnormalities of the histological sections observed in the SRD group and triglycerides and cholesterol content improved remarkably with the cannabis oil administration. Our results suggest that the administration of full-spectrum cannabis oil, could be useful as a strategy to prevent some of the alterations present in MS, including hypertension, dyslipidemia, and liver damage. Furthermore, the analgesic effect of cannabis oil could be observed in female rats fed an SRD.
Keywords
cannabis oil; female rats, Metabolic Syndrome, liver, cannabinoid-induced tetrad, blood pressure, metabolic parameters.
Abbreviations
ALT: Alanine Aminotransferase, AP: Alkaline Phosphatase, AST: Aspartate Aminotransferase, CBD: Cannabidiol, MS: Metabolic Syndrome, SRD: Sucrose-rich Diet, RD: Reference Diet, SRD+Ca: SRD+Cannabis Oil, THC: Tetrahydrocannabinol, Tg: Triglyceride.
Introduction
In recent years, different metabolic disorders, including obesity, insulin resistance, hypertension, and dyslipidemia, among others, have favored the development of Metabolic Syndrome (MS) and are considered risk factors for type 2 diabetes mellitus and cardiovascular disease. MS has attained the status of a global epidemic [1,2]. Our research group has significant experience and has made interesting contributions to the study of the metabolic alterations present in MS, using an experimental model induced nutritionally by the administration of a Sucrose-Rich Diet (SRD) and a sedentary lifestyle in male Wistar rats [3-5].
Currently, few natural pharmaceutical strategies are available to treat MS. The use of plants as medicines has predated human history. A medicinal plant is any plant that contains substances with therapeutic potential in one or more parts. Recently, one notable medicinal plant that has continued to gain attention over the years is Cannabis sativa. Cannabis sativa L., an annual plant belonging to the Cannabaceae family, is native to Asia. It has been used as an herbal medicine for tens of centuries to treat various diseases and symptoms [6-8]. Some of the possible metabolic effects of medicinal cannabis have been investigated, although the results are still controversial. Current studies have focused on two cannabinoids, CBD and THC, with great therapeutic potential in inflammatory diseases, diabetes, and diabetic complications [9-11]. Our group has previously reported that cannabis oil (CBD:THC, 2:1 ratio) improved systolic and diastolic blood pressure, dyslipidemia, steatosis, and liver damage in male Wistar rats fed a SRD [12]. Given that MS is an epidemic that increases the risk of type 2 diabetes, cardiovascular disease, atherosclerosis, and death, it is essential to analyze the usefulness of cannabis and its components in MS.
Therefore, in this study, we investigated the effects of a full-spectrum cannabis oil, CBD:THC, 2:1 ratio, on the cannabis tetrad, systolic and diastolic blood pressure, serum metabolic parameters and liver steatosis in experimental MS induced by SRD-fed female rats. To our knowledge, this is the first study to examine the metabolic effects of the oral administration of cannabis oil (CBD:THC, 2:1 ratio) in SRD-fed female rats.
Materials and methods
Cannabis oil Preparation and Characterization
Cannabis oil was obtained from dried inflorescences of the Cannabis sativa CAT1 variety grown at the Environmental Research Center (CIM-CONICET-UNLP) (RESOL-2021-3236-APN-MS), as previously described by Degrave et al [12]. Cannabis oil contains 0.60 mg/mL CBD and 0.43 mg/mL THC, at a CBD:THC, 2:1 ratio. Finally, adequate dilution was performed to obtain the working oil at a concentration of 1 mg/mL.
Animals and Diets
Female Wistar rats (n=18) were purchased from the Veterinary Sciences Institute of Litoral (ICIVET- Litoral). Faculty of Veterinary, National University of Litoral (Esperanza, Santa Fe, Argentine) and were maintained with unrestricted access to water and food under controlled temperature (22 ± 1 °C), humidity, and air flow conditions, with a fixed 12-h light/dark cycle (light on 07.00 AM to 7.00 PM). Adequate measures were taken to minimize pain or discomfort in the rats, and the smallest number of animals possible was used. This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals and approved by the Institutional Ethics Committee of the Faculty of Biochemistry and Biological Sciences.
The animals were initially fed a standard powdered commercial rodent diet (GEPSA FEED, Buenos Aires, Argentina). Rats 130-140 g (young adults) were randomly divided into three experimental groups for 21 days: 1) rats fed a standard powdered rodent commercial diet (reference diet, RD, n = 6). 2) Rats fed a semisynthetic sucrose-rich diet (SRD, n = 6). 3) Rats fed SRD plus orally administered cannabis oil (SRD+Ca, n = 6) at a dose of 1 mg/kg body weight daily during the experimental protocol (21 d). The diet compositions are detailed in a study by Degrave et al [12]. The rats began and finished the experimental protocol in the same stage of the estrous cycle (diestrus or proestrus). Individual body weight was recorded daily. Food intake of the animals in each group were assessed twice a week throughout the experimental period (21 days). At the end, the food was removed at 07.00 a.m. and experiments were performed between 07.00 and 09.00 a.m. Animals were anesthetized with intraperitoneal sodium pentobarbital (60 mg/kg body weight). Blood samples were collected from the inferior vena cava and rapidly centrifuged, and serum was either immediately assayed or stored at -20 °C until use. The liver of each rat was totally removed, weighed, and sectioned for different subsequent assays. Liver samples were fixed in 10% (v/v) buffered formalin for 24 h at room temperature and embedded in paraffin for histology analysis or frozen and stored at the temperature of liquid N2.
Determination of Blood Pressure
Blood pressure was measured in the three dietary groups in conscious animals during the experimental period using a CODA™ Monitor of tail-cuff non-invasive blood pressure system (Kent Scientific Corporation, Torrington, CT, USA) as previously described [13].
Cannabinoid-Induced Tetrad Test
Rats were evaluated for hypolocomotion (open field test), hypothermia (body temperature), cataleptic (bar test) and analgesic (hot plate test) effects, as previously described in Degrave et al. [12].
Analytical Methods
Serum triglyceride, cholesterol, uric acid and glucose levels were measured by spectrophotometric methods using commercial enzymatic kits according to the manufacturer’s protocols (Wiener Lab, Argentina). The activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (AP) enzymes were measured by spectrophotometric methods using commercial enzymatic kits according to the manufacturer’s protocols (Wiener Lab., Argentina). Triglycerides and total cholesterol contents in liver were extracted with chloroform-methanol (2:1) mixture. Aliquots were evaporated and total cholesterol and triglycerides were analyzed using the enzymatic methods mentioned previously.
Liver histology
A semi-automatic rotary microtome (Leica ®M2025) was used to obtain paraffin-embedded (5 µm thickness) liver cross-sections that were stained with hematoxylin–eosin (H&E) to provide an overall view of the tissue. The images of the stained sections were recorded with a Spot Insight V3.5 color video camera, attached to a microscope (Olympus BH2, Tokyo, Japan). At least ten fields were recorded in each section by using a Dplan 20X objective to evaluate the histological features of tissue.
Statistical Analysis
Results were expressed as mean ± SEM. Statistical comparisons were made transversely between different dietary groups. Data were tested for variance using Levene’s test and normality by Shapiro-Wilk’s test. The statistical difference between groups (RD, SRD and SRD+Ca) was determined by one-way ANOVA followed by post-hoc Newman-Keuls' test. P values lower than 0.05 were statistically significant (SPSS 17.0 for Windows, SPSS INC. Chicago, Illinois).
Results
Body weight, food intake, and time course of systolic and diastolic blood pressure.
Initial body weight, final body weights and final food intake were similar between the animals in the different experimental groups (Table 1). Time course of systolic and diastolic blood pressure was evaluated throughout the experimental period in the three dietary groups. At weeks 1, 2, and 3, SRD-fed rats showed a significant (P<0.05) increase in systolic and diastolic blood pressure compared with the RD-fed group. Cannabis Oil administration (SRD+Ca) decreased both parameters significantly (P<0.05) at week 1, although they did not reach the reference values. At weeks 2 and 3, a significant reduction (P<0.05) was observed in both parameters compared with the SRD group, reaching reference values.
Table 1: Body weight, food intake, and time course of systolic and diastolic blood pressure throughout the experimental period in female rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis oil (SRD+Ca).
|
RD |
SRD |
SRD+Ca |
Initial body weight (g) |
138,8 ± 1,2 |
138,4 ± 2,7 |
141,67 ± 1,3 |
Final body weight (g) |
178,0 ± 2,5 |
180,3 ± 4,3 |
179,7 ± 2,3 |
Food intake (g/day) |
12,66 ± 0,38 |
12,78 ± 0,29 |
12,13 ± 0,92 |
Diastolic blood pressure (mmHg) |
|
|
|
Week 1 (day 0 to 7) |
81.14 ± 1.25 |
87.00 ± 1,10* |
84.13 ± 1.21# |
Week 2 (day 8 to 15) |
81.15 ± 1.08 |
88.31 ± 0.96* |
81.87 ± 1.22 |
Week 3 (day 16 to 21) |
82.50 ± 0.56 |
90.75 ± 0.67* |
81.40 ± 0.88 |
Sistolic blood pressure (mmHg) |
|
|
|
Week 1 (day 0 to 7) |
115.04 ± 1.24 |
122.51 ± 1.24* |
117.78 ± 1.11# |
Week 2 (day 8 to 15) |
118.79 ± 1.03 |
127.34 ± 1.00* |
119.6 ± 1.62 |
Week 3 (day 16 to 21) |
119.73 ± 0.52 |
128.33 ± 0.81* |
119.31 ± 0.79 |
Values are expressed as mean ± SEM, n=6. *P<0.05 SRD vs RD and SRD+Ca, #P<0.05 SRD+Ca vs SRD and RD when one variable at a time was compared by one-way ANOVA followed by Newman-Keuls´ test.
Table 2: Cannabinoid-induced tetrad test in female rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis oil (SRD+Ca).
|
RD |
SRD |
SRD+Ca |
Analgesia (s) |
3.00 ± 0.57 |
3.2 ± 0.59 |
5.4 ± 0.39# |
Locomotion (cm) |
13.28 ± 1.98 |
25.48 ± 1.36* |
15.73 ± 1.96b |
Catalepsy (s) |
2.25 ± 0.35 |
2.40 ± 0.38 |
2.20 ± 0.32 |
Temperature (°C) |
27.30 ± 0.31 |
27.50 ± 0.56 |
27.32 ± 0.61 |
Values are expressed as mean ± SEM, n=6. *P<0.05 SRD vs RD and SRD+Ca, #P<0.05 SRD+Ca vs SRD and RD when one variable at a time was compared by one-way ANOVA followed by Newman-Keuls´ test.
Cannabis Effects in the Tetrad Assay
Table 2 shows that the incorporation of Cannabis Oil (SRD+Ca) significantly increased (P<0.05) the analgesia. Locomotion, which increased in the SRD group, decreased significantly (P<0.05) in the SRD+Ca group, reaching the reference values. There were no significant differences in the body temperature and catalepsy between the different experimental groups.
Serum Metabolites and Liver Damage Enzyme Activities
Figure 1 shows that at the end of the experimental protocol, serum triglyceride and uric acid levels were significantly higher in SRD-fed rats compared to RD-fed rats. Cannabis oil administration (SRD+Ca) significantly decreased (P<0.05) serum Tg levels, reaching the reference values. Uric acid levels were significantly reduced (P<0.05), although the values were still higher than those of the RD group. No changes in serum glucose and cholesterol levels were observed among the three dietary groups. AST, ALT, and AP serum levels were significantly higher (P<0.05) in SRD-fed rats than in RD-fed rats. In the SRD+Ca group, AST and ALT enzymes decreased significantly (P<0.05), reaching values similar to those observed in the RD group. AP enzyme levels were significantly reduced (P<0.05), although the values were higher than those in the RD group.
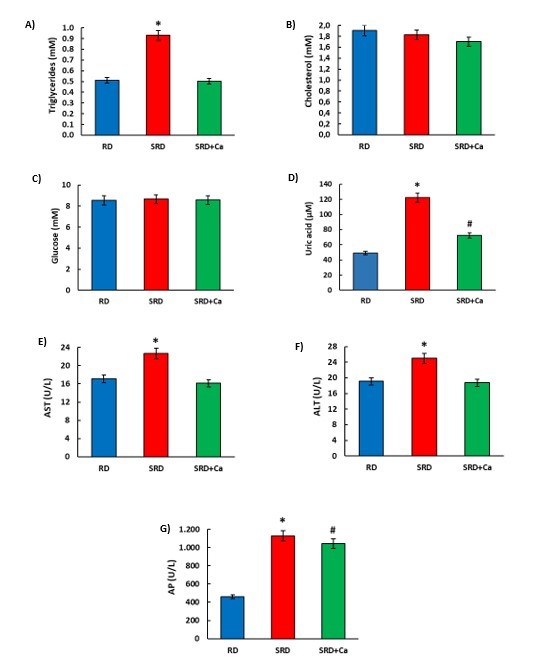
Figure 1: Serum metabolites and liver damage enzyme activities in female rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis oil (SRD+Ca). A) Triglycerides, B) Cholesterol, C) Glucose, D) Uric Acid, E) AST, F) ALT, G) AP. Values are expressed as mean ± SEM, n=6. *P<0.05 SRD vs RD and SRD+Ca, #P<0.05 SRD+Ca vs SRD and RD. when one variable at a time was compared by one-way ANOVA followed by Newman-Keuls´ test.
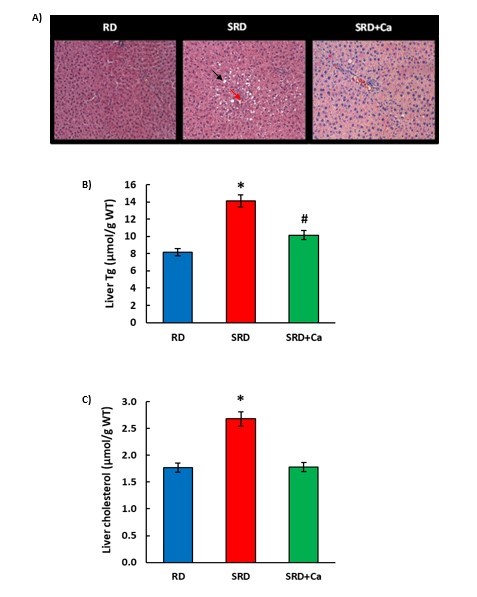
Figure 2: Histological analysis and triglycerides and cholesterol content in liver tissue. A) Representative photomicrograph of histological abnormalities observed in liver sections in female rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis oil (SRD+Ca). The accumulation of lipid droplets (black arrow) and inflammatory foci (red arrow) are observed in the liver section H&E-stained. 400× magnification. B) Liver Tg content, C) Liver cholesterol content. WT: wet tissue. Values are expressed as mean ± SEM, n=6. *P<0.05 SRD vs RD and SRD+Ca, #P<0.05 SRD+Ca vs SRD and RD. when one variable at a time was compared by one-way ANOVA followed by Newman-Keuls´ test.
Histological analysis and triglycerides and cholesterol content in liver tissue
Figure 2 shows an abnormal accumulation of lipid droplets within the cytoplasm of hepatocytes and infiltration of inflammatory cells in histological sections of the SRD group. These results were accompanied by a significant increase (P<0.05) in triglyceride and cholesterol content in the liver tissue. Cannabis oil administration remarkably improved the abnormalities of the histological sections. The triglyceride content decreased significantly (P<0.05), although the values were still higher than those of the RD group, and the cholesterol content was similar to the values of the RD group.
Discussion
Morphological and physiological differences associated with sex are common in animals, and the mechanisms that explain sex differences in response to obesity, MS, and type 2 diabetes mellitus are not well understood. It is essential to clarify the effect of sex on the incidence of these diseases and offer effective strategies for their prevention and treatment [14]. Our previous research using an experimental model induced nutritionally by the administration of a SRD and a sedentary lifestyle in male Wistar rats developed some of the metabolic alterations that mimic many of the aforementioned signs present in human MS [3-5].
Medicinal cannabis therapy has garnered considerable attention in recent years. In this study, we showed data that cannabis oil administration rich in CBD:THC, 2:1 ratio resulted in significant improvement in several parameters of MS in female rats fed a sucrose-rich diet. We observed that cannabis oil administration did not affect body weight and food intake but decreased systolic and diastolic blood pressure in dyslipidemic and hypertensive female rats fed a SRD. Very few studies have investigated the effects of cannabis or cannabinoids on hypertension in female rats. In a clinical study, Valle [15] observed that cannabis use was associated with lower blood pressure levels in both sexes, but in a higher manner in women. In addition, Abuhasira et al. [16] showed that cannabis treatment (with different CBD and THC ratios) for 3 months in hipertensive older adults (women and men) was associated with a reduction in systolic and diastolic blood pressure values. In animal studies, the effects of cannabis oil on blood pressure were inconsistent and varied according to the hypertension model, with the majority being conducted in male rats [17,18]. In addition, we previously reported that cannabis oil administration (CBD:THC, 2:1 ratio) decreased systolic and diastolic blood pressure in male Wister rats fed a SRD [12]. This is the first study evaluating the effect of cannabis oil (CBD:THC, 2:1 ratio) administration on systolic and diastolic blood pressure in SRD-fed hypertensive female rats.
Cannabis plant as a whole has analgesic properties. In our study, we demonstrated that administration of cannabis oil (CBD:THC, 2:1 ratio) significantly increased analgesia in female rats. Moore and Weerts [19] demonstrated that oral administration of THC showed sex differences over the time course of mechanical antinociception. In females the antinociceptive effects were less after 315 minutes, while in males, it was the same at all time points. Antinociceptive effects were observed in both sexes; although males mainly showed slower onset and longer antinociceptive effects compared to females. On the other hand, oral administration of CBD did not detect antinociceptive effects in male or female rats. Despite well-documented sex differences in humans with pain [20-22], few studies have investigated sex differences in cannabinoid analgesia, with diverse results [23-25]. In animal studies, Craft et al. [26] used acute pain tests to show that THC is more potent in female rats than in male rats. We previously demonstrated that male rats showed increased analgesia after cannabis oil administration (CBD:THC, 2:1 ratio) [12]. The present study is the first that evaluated the analgesic effects of cannabis oil, with a CBD:THC, 2:1 ratio cannabinoids ratio, in female rats fed a SRD.
The metabolic effects of cannabis oil have recently been investigated, and its medical usefulness in metabolic diseases continues to be studied. In our study, we observed that cannabis oil administration to SRD-fed female rats significantly improved serum triglyceride and uric acid levels with no change in cholesterol and glucose levels. Serum AST and ALT are used as markers of liver injury. Administration of cannabis oil results in a reduction in serum levels of these enzymes. In the liver, we observed an improvement in the abnormalities of the histological sections observed in the SRD group, and in the triglyceride and cholesterol contents, suggesting a significant decrease in steatosis and liver damage, showing the hepatoprotective effect of cannabis oil used in the present study. We did not find studies evaluating the effect of cannabis oil on dyslipidemia, steatosis and liver damage in animal models of MS in female rats. Other studies in rats suggested that cannabinoids Δ9-THC and/or CBD increased high-density lipoprotein cholesterol concentration, reduced cholesterol and triglycerides serum levels, and decreased hepatic triglyceride accumulation and liver damage in other animal models (streptozotocin induced diabetes rats; induced by chronic alcohol feeding mice, high fat-cholesterol diet) [27-29]. We recently showed that cannabis oil administration (CBD:THC, 2:1 ratio) improves hypertension and dyslipidemia, and mitigates steatosis and liver damage induced by SRD in male rats [12]. Clinical studies have shown that several cannabinoids (CBD, THC, or THCV) have beneficial effects on biochemical parameters, such as blood glucose, total cholesterol, high-density lipoprotein cholesterol, triglycerides, and liver damage, in diabetic patients [30-32].
The results of this study provide new information on the beneficial effects of cannabis oil administration, with CBD:THC 2:1 ratio, useful in the treatment of several parameters included in MS, including hypertension, dyslipidemia, steatosis, and liver damage. However, further research is needed to clarify the mechanisms of action that act in these pathologies.
Conclusion
The present study demonstrated the beneficial effects of cannabis oil on hypertension, dyslipidemia, steatosis, and liver damage induced by SRD in female rats. In addition, the analgesic effect of cannabis oil was observed in SRD-fed female rats. Furthermore, our results suggest that cannabis oil may serve as a natural nutraceutical agent for preventing or ameliorating metabolic disorders related to MS.
Finally, although promising, these results warrant further investigation before extrapolating the use of cannabis oil as a complementary nutraceutical that could help in the treatment of some metabolic disorders present in humans MS.
Acknowledgments
The authors would like to thank Candelaria Mauti for their skilful technical assistance.
Author Contributions
VD: Investigation, Data curation, Formal analysis, Writing the manuscript. MB: Investigation, Data curation, Formal analysis, Writing the manuscript. MVJ: Investigation, Methodology, Data curation, Formal analysis, Writing the manuscript. PI: Investigation, Data curation, Formal analysis, Writing the manuscript. CV: Investigation, Data curation, Formal analysis. DS: Investigation, Data curation, Formal analysis, DA: Investigation, Data curation, Formal analysis. MED: Conceptualization, Methodology, Data curation, Formal analysis, Writing the manuscript. MEO: Funding acquisition, Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Supervision, Writing the manuscript.
Funding Sources
This work was supported by Agencia Santafesina de Ciencia, Tecnología e Innovación from Argentina (PEICID-2021-003).
Conflicts of Interest
None.
Ethical Statement
This study protocol was reviewed and approved by the Institutional Ethics Committee of the Faculty of Biochemistry and Biological Sciences (Universidad Nacional del Litoral, Santa Fe, Argentina), approval number Acta 03/21.
References
- Ahmed M, Kumari N, Mirgani Z, Saeed A, Ramadan A, et al. (2022) Metabolic syndrome; Definition, Pathogenesis, Elements, and the Effects of medicinal plants on it’s elements. J Diabetes Metab Disord 21: 1011–1022. [Crossref]
- Lemieux I, Després J (2020) Metabolic Syndrome: Past, Present and Future. Nutrients 12: 3501-3508. [Crossref]
- Lombardo YB, Drago S, Chicco A, Fainstein-Day P, Gutman R, et al. (1996) Long-term administration of a sucrose-rich diet to normal rats: relationship between metabolic and hormonal profiles and morphological changes in the endocrine pancreas. Metab 45: 1527-1532. [Crossref]
- Chicco AG, D’Alessandro ME, Hein GJ, Oliva ME, Lombardo YB (2009) Dietary chia seed (Salvia hispanica L.) rich in a-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br J Nutr 101: 41–50. [Crossref]
- D’Alessandro ME, Selenscig D, Illesca P, Chicco A, Lombardo BY (2015) Time course of adipose tissue dysfunction associated with antioxidant defense, inflammatory cytokines and oxidative stress in dyslipemic insulin resistant rats. Food Funct 6: 1299-1309. [Crossref]
- Odieka AE, Obuzor GU, Oyedeji OO, Gondwe M, Sunday Hosu Y (2022) The Medicinal Natural Products of Cannabis sativa Linn: A Review. Molecules 27: 1689. [Crossref]
- Bonini A, Premoli M, Tambaro S, Kumar A, Maccarinelli G, et al. (2018) Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol 227: 300–315. [Crossref]
- Bridgeman MB, Abazia DT (2017) Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. P T 42: 180-188. [Crossref]
- Bielawiec P, Harasim-Symbor E, Chabowski A (2020) Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front Endocrinol 11: 114. [Crossref]
- Gruden G, Barutta F, Kunos G, Pacher P (2016) Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol 173: 1116–1127. [Crossref]
- Horváth B, Mukhopadhyay P, Haskó G, Pacher P (2012) The Endocannabinoid System and Plant-Derived Cannabinoids in Diabetes and Diabetic Complications. Am J Pathol 180: 432-442. [Crossref]
- Degrave V, Vega Joubert MB, Vaccarini C, Sedan D, Andrinolo D, et al. (2023) Effects of Cannabis Oil on Cannabinoid-Induced Tetrad, Blood Pressure, and Metabolic Parameters in an Experimental Model of Metabolic Syndrome. J Food Nutr Metabol 6: 1-9.
- Creus A, Benmelej A, Villafañe N, Lombardo YB (2017) Dietary Salba (Salvia hispanica L) improves the altered metabolic fate of glucose and reduces increased collagen deposition in the heart of insulin-resistant rats. Prostaglandins Leukot Essent Fatty Acids 121: 30–39. [Crossref]
- Velasco M, Ortiz-Huidobro RI, Larqué C, Sánchez-Zamora YI, Romo-Yáñez J, et al. (2020) Sexual dimorphism in insulin resistance in a metabolic syndrome rat model. Endocr Connect 9: 890–902. [Crossref]
- Valle A (2023) Association between cannabis use and blood pressure levels according to comorbidities and socioeconomic status. Sci Rep 13: 2069-2072.
- Abuhasira R, Azar S, Nemirovski A, Tam J, Novack V (2022) Herbal Cannabis Use Is Not Associated with Changes in Levels of Endocannabinoids and Metabolic Profile Alterations among Older Adults. Life 12: 1539-1550. [Crossref]
- Remiszewski P, Jarocka-Karpowicz I, Biernacki M, Jastrząb A, Schlicker E, et al. (2020) Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int J Mol Sci 21: 1295-1319. [Crossref]
- Kicman A, Toczek M (2020) The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int J Mol Sci 21: 6740-6789. [Crossref]
- Moore CF, Weerts EM (2022) Cannabinoid tetrad effects of oral Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in male and female rats: sex, dose-effects and time course evaluations. Psychopharmacology 239: 1397–1408. [Crossref]
- Unruh AM (1996) Gender variations in clinical pain experience. Pain 65: 123-167. [Crossref]
- Bartley EJ, Fillingim RB (2013) Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111: 52–58. [Crossref]
- Osborne RN, Davis KD (2022) Sex and gender differences in pain. Int Rev Neurobiol 164: 277-307. [Crossref]
- Redmond WJ, Goffaux P, Potvin S, Marchand S (2008) Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin 24: 1017–1024. [Crossref]
- Cooper ZD, Haney M (2014) Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 136: 85–91. [Crossref]
- Cooper ZD, Haney M (2016) Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend 167: 112–120. [Crossref]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD (2012) Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther 340: 787–800. [Crossref]
- Comelli F, Bettoni I, Colleoni M, Giagnoni G, Costa B (2009) Beneficial effects of a Cannabis sativa extract treatment on diabetes-induced neuropathy and oxidative stress. Phytother Res 23: 1678-1684. [Crossref]
- Assa-Glazer T, Gorelick J, Sela N, Nyska A, Bernstein N, et al. (2020) Cannabis extracts affected Metabolic Syndrome parameters in mice fed high-fat/cholesterol diet. Cannabis Cannabinoid Res 5: 202-214. [Crossref]
- Wang Y, Mukhopadhyay P, Cao Z, Wang H, Feng D, et al. (2017) Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci Rep 7: 12064. [Crossref]
- Farokhnia M, Portelli J, Lee MR, McDiarmid GR, Munjal V, et al. (2020) Effects of exogenous ghrelin administration and ghrelin receptor blockade, in combination with alcohol, on peripheral inflammatory markers in heavy-drinking individuals: Results from two human laboratory studies. Brain Res 1740: 146851.
- Adejumo AC, Alliu S, Ajayi TO, Adejumo KL, Adegbala OM, et al. (2017) Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLos One 12: e0176416. [Crossref]
- Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, et al. (2016) Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 39: 1777-1786.


