Diabetic foot syndrome demonstrates wound chronicity due to impaired tissue perfusion in lower limbs. Previous studies showed interferon-gamma (IFN-γ), a central inflammatory mediator in diabetic foot syndrome, to induce the truncated form of tryptophanyl-tRNA synthetase (mini-TrpRS) that has strong angiostatic properties. Recently we reported that mini-TrpRS signalling could be blocked in the presence of IFN-γ with D-tryptophan in vitro. Here we discuss the IFN-γ/mini-TrpRS axis in the pathology of diabetic foot syndrome and emerging therapeutic options.
Mini tryptophanyl-tRNA synthetase, interferon-gamma, diabetic foot syndrome
Approximately 25% of patients with diabetes will develop diabetic foot syndrome [1]. This severe complication of progressing diabetes is responsible for approximately 85% of lower limb amputations associated with a dramatic decrease in quality of life and increased mortality [2]. The aetiology of diabetes can be traced to well-recognized risk factors such as genetic predisposition, ageing, male sex, and lifestyle factors, including unhealthy diet and low physical activity. Most of these traditional risk factors are either non-modifiable (genetics, ageing, sex) or hard to modify in diabetic foot syndrome patients such as exercise or even diet. The substantial evidence, however, suggests that inflammatory pathways are the principal and common pathogenic mediators in the course of diabetes and its complications under stimuli of traditional risk factors [3,4]. Among them, interferon-gamma (IFN-γ), an important pro-inflammatory cytokine primarily involved in the host defence against acute infections, is also chronically elevated in patients with diabetes and considered as a crucial risk factor for diabetic foot syndrome [5,6]. In particular, IFN-γ is a critical cytokine in angiostatic responses that impairs normal tissue perfusion and microvascular reaction to injury [7]. Despite the importance of the anti-angiogenic properties of IFN-γ, the molecular mechanisms by which IFN‐γ elicits its actions on vasculature remained poorly understood. Our recent findings support novel thinking to how IFN-γ may only indirectly contribute to the pathology of diabetic foot syndrome by up-regulating angiostatic pathways and hold promising treatment implications for patients with this debilitating complication of diabetes.
Impaired tissue perfusion in lower limbs is a leading cause of wound chronicity in patients with progressing diabetes. Innovations in enhancing wound revascularization would lead to a significant improvement in patient care and their quality of life.
The WARS1 gene-encoded human tryptophanyl-tRNA synthetase (TrpRS) exists as two forms; a full-length protein and a truncated variant known as ‘mini-TrpRS’ in which most of the N-terminal domain is deleted during alternative splicing of the WARS1 precursor mRNA [8]. IFN-γ strongly stimulates the production of mini-TrpRS, and it is the only human tRNA-ligase whose expression is induced by IFN-γ [9]. The significance of this phenomenon was recognized following the discovery that mini-TrpRS, unlike its full-length variant, is also a potent negative regulator of angiogenesis outside its primary role in aminoacylation [10-12]. Angiostatic activities of human mini-TrpRS were extensively studied using several human and animal models, including human umbilical vein endothelial cells (HUVEC) migration and proliferation, chick chorioallantoic membrane, mouse matrigel, and mouse retinal angiogenesis assays [10]. Importantly, our recent discovery shows that mini-TrpRS signalling in the presence of IFN-γ in vitro can be effectively blocked with D-tryptophan, a cognate amino acid acting as a decoy substrate for mini-TrpRS [13]. It has become evident that mini-TrpRS signalling inevitably occurs in the context of more complex regulation mediated by IFN-γ to execute specific outcomes depending on the pathological environment and the cell type involved [13,14]. An important question, whether a direct neutralization of mini-TrpRS would limit the progression of diabetic foot syndrome, is posed. In this regard, D-tryptophan would appear an ideal intervention to be investigated in patients with diabetic foot syndrome. Chemically, D-tryptophan is an enantiomer of L-tryptophan, an essential amino acid found in most human proteins, and a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 [15]. In experimental models, D-tryptophan is readily cleared from plasma, and there is no appreciable conversion of D-tryptophan to metabolically active L-tryptophan [16,17]. As such, side effects such as unintended stimulation of serotonin production in the brain [16] or other metabolic functions of the L-tryptophan [17] would be expected to be negligible in treated patients with a diabetic foot ulcer. Indeed, D-tryptophan, also known as NLG8189 and Indoximod (methylated D-tryptophan), has not been trialled exactly in patients with diabetic foot syndrome but oncological patients; the drug was investigated at a dose up to 2,000 mg twice daily, was well tolerated, and no patients discontinued treatment due to toxicity [18]. A dose up to 800 mg showed good oral bioavailability with a linear dose-response of the area under the curve (AUC) and the maximum serum concentration (Cmax) [18]. Within this frame of reference, studies investigating diabetic foot syndrome therapy based on D-tryptophan are planned for future work.
We plan a double-blind, placebo-controlled clinical trial to evaluate the safety, tolerability, Pharmacokinetic (PK), Pharmacodynamics (PD), and explore the effect of D-tryptophan combined with standard wound care compared to placebo in patients with diabetic foot syndrome. In phase 1a, we will assess a single ascending dose (SAD) followed by a multiple ascending dose (MAD) study over 12 weeks in phase 1b (Figure 1). The development of a novel pharmacological treatment based on D-tryptophan is directed at combining with the current standard of wound care to aid with the clinical management of this debilitating complication of diabetes.
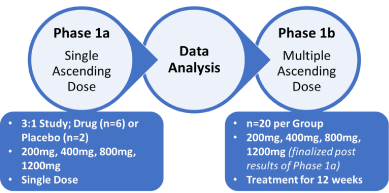
Figure 1. Schematic of Phase 1a/b clinical trial of D-tryptophan for treating patients with diabetic foot syndrome
The primary objective is to assess the safety and pharmacokinetic tolerability of D-tryptophan in treated individuals (Phase 1a), with the secondary objective to assess the effect of D-tryptophan on biological markers of wound healing.
Conceptualization, EB, VV, UHM, and CSM; Writing – Original Draft Preparation, EB and VV; Writing – Review & Editing, UHM, and CSM; Visualization, VV and CSM.
This work was supported by the College of Medicine and Dentistry, James Cook University.
None.
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ (2003) Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care 26: 1435-1438. [Crossref]
- Singh N, Armstrong DG, Lipsky BA (2005) Preventing foot ulcers in patients with diabetes. JAMA 293: 217-28. [Crossref]
- Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793-1801. [Crossref]
- Tuttolomondo A, Maida C, Pinto A (2015) Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop 6: 62-76. [Crossref]
- Nosratabadi R, Arababadi MK, Hassanshahi G, Yaghini N, Pooladvand V, et al. (2009) Evaluation of IFN-gamma serum level in nephropatic type 2 diabetic patients. Pak J Biol Sci 12: 746-749. [Crossref]
- Weigelt C, Rose B, Poschen U, Ziegler D, Friese G, et al. (2009) Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care 32: 1491-1496. [Crossref]
- Laato M, Heino J, Gerdin B, Kahari VM, Niinikoski J (2001) Interferon-gamma-induced inhibition of wound healing in vivo and in vitro. Ann Chir Gynaecol 215: 19-23. [Crossref]
- Frolova LY, Grigorieva AY, Sudomoina MA, Kisselev LL (1993) The human gene encoding tryptophanyl-tRNA synthetase: interferon-response elements and exon-intron organization. Gene 128: 237-245. [Crossref]
- Wakasugi K, Schimmel P (1999) Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284: 147-151. [Crossref]
- Wakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, et al. (2002) A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. P Natl Acad Sci USA 99: 173-177. [Crossref]
- Kise Y, Lee SW, Park SG, Fukai S, Sengoku T, et al. (2004) A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase. Nat Struct Mol Biol 11: 149-156. [Crossref]
- Nakamoto T, Miyanokoshi M, Tanaka T, Wakasugi K (2016) Identification of a residue crucial for the angiostatic activity of human mini tryptophanyl-tRNA synthetase by focusing on its molecular evolution. Sci Rep 6: 24750.
- Biros E, Moran CS (2020) Mini tryptophanyl-tRNA synthetase is required for a synthetic phenotype in vascular smooth muscle cells induced by IFN-gamma-mediated beta2-adrenoceptor signaling. Cytokine 127: 154940.
- Fleckner J, Martensen PM, Tolstrup AB, Kjeldgaard NO, Justesen J (1995) Differential Regulation of the Human, Interferon-Inducible Tryptophanyl-Transfer-Rna Synthetase by Various Cytokines in Cell-Lines. Cytokine 7: 70-77. [Crossref]
- Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, et al. (2002) Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett 511: 102-106. [Crossref]
- Budny J, Dow RC, Eccleston D, Hill AG, Ritchie IM (1976) A comparison of D and L-tryptophan on the cerebral metabolism of 5-hydroxytryptamine and dopamine in the dog, Br J Pharmacol 58: 3-7. [Crossref]
- Kepert I, Fonseca J, Muller C, Milger K, Hochwind K, et al. (2017) D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol 139: 1525-1535. [Crossref]
- Soliman HH, Minton SE, Han HS, Ismail-Khan R, Neuger A, et al. (2016) A phase I study of indoximod in patients with advanced malignancies. Oncotarget 7: 22928-22938. [Crossref]

