Background: Since the discovery of BK Virus (BKV) in 1971, it became a growing challenge in the renal transplant field. Many hypotheses over the latest years have been made to justify the increased risk of acquiring BKV infection post-renal transplantation. Excessive immunosuppression remains the primary risk factor. Risk factors such as older recipients, male gender, prolonged cold ischemia time, ureteric stent insertion, degree of HLA mismatching and others have all been linked as additional risks for acquiring BKV infection. Nevertheless, the current literature on risk factors are inconclusive and no single identifiable risk factor can determine recipients who at risk.
Objective: The objective of this review is to delineate and contemplate the potential risk factors published in the literature and leads to BKV nephropathy.
Methodology: For this review, a variety of sources were utilised including EMBASE, Scopus, PubMed/Medline database and Google Scholar for observational studies on probable risk factors predisposing to BK viremia and/or nephropathy.
Results: almost 22 distinctive risk factors were identified.
Discussion: Over immunosuppression remains the major risk factor for acquiring BKV infection post-renal transplant, though it is uncertain whether the occurrence of BKVN (BKV Nephropathy) is owing to quantitative and/or qualitative differences in immune suppressants.
Besides immunosuppression, other probable risk factors for BKV infection were recognized. Whilst some of them were reproducible in many of these studies, they were denied by others. For instance, ureteric stents, recipient's age, race, deceased-donor type and acute rejection episodes, were inconsistently recognised as significant risk factors for BKV infection.
Conclusions: Over immunosuppression remained the reproducible risk factor for BKV infection in all studies, never the less published data on other risks factors varies. This may mirror the patient's geographical area, genetic vulnerability and probably a different BK gene variant with different risk susceptibility, these warrant further investigation.
BK polyomavirus, kidney transplant, Risk factors, BKVN (BK Virus Nephropathy), Polyomavirus Associated Nephropathy.
In 1971, Gardner and colleagues were the first to isolate polyomavirus BK (BKV) from both urine and ureteral epithelial cells of a Sudanese renal transplant recipient, presented with ureteral stenosis and renal failure [1]. They named the virus “BK” after the initials of this patient. Numerous large cells with intra-nuclear inclusions were present in the urine, named later as “decoy cells” because they resembled malignant cells [1-3] Since then, numerous reports on various aspects of BKV in renal transplant recipients have been published [4-8]. Though human polyomaviruses, BK virus (BKV) were discovered in 1971, yet, the understanding of its negative impact was limited till 3 decades later when BKV was identified as a significant reason for interstitial nephritis and allograft failure in renal transplant recipients [9,10]. Factors that precipitated its higher occurrence over the latest years and its pathogenesis remain poorly comprehended. Increased awareness, the ability of clinicians to recognize BK infection at an earlier stage, and the accessibility of better diagnostic tools may all add to the high frequency of BKV infection [11,12].
The human BKV belongs to the Polyomaviridae (PyV) virions, a subgroup of papovaviruses, which includes BKV, JC virus, and simian virus 40 (SV-40). It is a family of small,non-envelopedDNA viruseswith icosahedralcapsidof 40- 45nm in diameter, and can withstand heat up to 50°C for 30 min with little effect on infectivity. BKV has a circular double-stranded DNA genome of approximately 5000 base pairs [2,13-17]. Twelve additional human polyomaviruses have been isolated lately between the years 2007- 2017. These new Polyomaviruses were named based on the site of discovery, the land/territories, the diseases they may cause, or the order of discovery: MWPyV (Malawi), WUPyV (Washington University), and KIPyV (Karolinska Institute) orHuman polyomavirus-3, MCPyV (Merkel cell carcinoma), TSPyV (trichodysplasia spinulosa); HPyV6, HPyV7, HPyV9 and HPyV12 (human polyomaviruses 6,7, 9 and 12), STLPyV (Saint Louis polyomavirus)orHuman polyomavirus-11, New Jersey polyomavirus(NJPyV, also famous aspolyomavirus-13), Lyon IARC polyomavirus (LIPyV or Human polyomavirus-14) [18-22].
BKV can be categorised into four genotypes/subtypes according to the DNA sequence variations in the genomic region of VP1 [23-25]. Genotype I is the predominant subtype of all circulating viruses, accounting for greater than 80% worldwide, followed by genotype IV which is the second most frequent genotype, found approximately in 15% of the normal human population. Alternatively, genotypes II and III are relatively rare and infect only a minority of people [25,26].
Phylogenetic analysis has further identified four subgroups, sub-cloned of subtype I (I/a, I/b-1, I/b-2 and I/c), and six subgroups of subtype IV (IV/a-1, IV/a-2, IV/b-1, IV/b-2, IV/c-1 and IV/c-2). As with subtype-1 subgroups, each of the subtype four subgroups may reflect different geographical and the migration pattern of the human population [14,25-27]. The subgroup of subtype-I(I/b-2)has been noticed mostly in American and European populations, whereas subgroupI/cdominate in Asians. Among subtype IV isolates, subgroupIV/c-2is predominant amongst Americans and Europeans, whereas the other subgroups are more common in Asian populations [15,28]. Apart from the genotypic variations of VP1 region, additional two other forms of BKV present secondary to variations in the NCCR, namely, rearranged (rr) and archetype (ww) variants. Persistent and continuous replication of the viral genome during the reactivation process can result in deletion and duplication in the NCCR sequences with subsequent generation of variant viruses. The clinical and immunological consequences of these genotypes on clinical perspective and the course of the disease are still undefined [26,29]. In a recent study, Korth, et al. 2019 had linked allograft failure with BK genotype II and IV compared to genotype 1 & III (P= 0.007) [30].
Studies revealed as much as 60%-85% of the general population is seropositive for BKV [28,31-40]. Primary infection prevalently happens during early childhood; subsequently, the virus stays dormant throughout life in immune-competent people [2,11,26]. In the setting of immunosuppressive therapy, the virus activates and begins to proliferate inside the interstitium and crosses into the peritubular capillaries, creating a sequence of events that begins with tubular cells lysis and viruria. The outcome relies upon the degree of damage, inflammation and fibrosis [2,3].
In renal allograft recipients, BKV has been correlated with different clinical manifestations, among which are the BKV Nephropathy (BKVN), ureteric stenosis and late-onset haemorrhagic cystitis [2,9,41,42]. Outside renal transplantation, BKV is commonly encountered in patients with Hematopoietic Stem Cell Transplant recipients (HSCT) as haemorrhagic and non-haemorrhagic cystitis [43,44], while in HIV infected patients, BKV may disseminate leading to severe viremia with multi-organ involvement such as meningoencephalitis, Guillain-Barré syndrome, progressive multifocal leukoencephalopathy, vasculopathy, bilateral atypical retinitis and severe interstitial pneumonitis, and eventually lead to death [45-51].
This systematically structured review aims to identify and stratify potential risk factors published in the literature and can predispose to BK nephropathy. Recognizing risk factors can help in laying strategic policy and recommendations for monitoring, screening and initiating early treatment for high-risk patients based on data analysis, which may reflect positively on allograft survival and improve patient’s quality of life.
PICOS
This analysis will use the following PICOS parameters:
- Population: kidney transplant recipients.
- Intervention: identification of statistically significant risk factors, predisposing to BKV reactivation in kidney transplant recipients.
- Comparison: patients who developed BKV viremia/viruria and/or nephropathy post-renal transplant, versus who did not.
- Outcomes: BKV viremia/viruria and/or nephropathy
- Studies: studies based on prospective/ or retrospective analysis with at least 12-24 months of follow-up.
Inclusion and exclusion criteria
Inclusion Criteria: this research intended to define risk factors for BK viremia/nephropathy in adult recipients, hence all analyses accomplished for adults renal transplant recipients with BK viruria/viremia/and nephropathy; published in English were included. However, a few paediatric studies are also pertinent to this research and are also incorporated.
Exclusion Criteria: studies that lack diagnosis/criteria for BK viremia/nephropathy.
Significance of the Research
BKV remains an evolving challenge for renal transplant physicians since it was discovered in 1971. Different theories were generated to explain the increased risk for acquiring BK infection post-transplantation, yet no single clinical risk factor can determine the recipients who will develop BKV nephropathy. Additionally, the results in the current literature are conflicting, and a risk factor may appear as significant in one study while it looks irrelevant in another. We hope from our study to discover out definite risk factors and to identify any possible modifiable factors that favour infection with a view toward BK prevention and to support in laying strategic plan and recommendations for risk factors monitoring, screening and treatment of high-risk patients based on data analysis.
Search strategy
For this analysis, various sources were used through searching PubMed/Medline database, Scopus, EMBASE, EBSCO, Google Scholar, Cochrane Database of Systematic Reviews (CDSR), directory of open access journals (DOAJ) and conference proceedings. The search was conducted, using the following keywords; transplant, kidney transplantation, nephropathy, BK Virus, BKV-associated nephropathy; risk factors, BK viremia and BK viruria. English articles, full articles on BK risk factors, articles in kidney transplant, combined kidney with other organ transplant were then selected. More than 1000 articles were identified. Articles were then analysed for their applicability to clinical practice, a duplicate was removed, and articles in English were selected. Further references were selected from the citation section of individual papers.
Results and data analysis
More than 80 observational studies on risk factors were identified and critically appraised, using PRISMA/CAPS Guidelines. Most of these studies were done retrospectively while few were prospective. The viremic patients were compared to non-viremic to analyse the different potential risk factors. Studies with statistically significant risk factors were analysed and illustrated in the following tables, where Table 1 summarises the predictive risk factors for biopsy-proven BKVN, while Table 2 summarises the most detectable risk factors for BK viremia. Some of the risk factors were statistically significant in some studies while not in others, which will be elaborated further in the discussion. Other risk factors identified by this process will also be reviewed. The significant BK-risk factors can be categorised into three areas; transplant-related, donor-related and recipient-related factors.
Table 1. Potential risk factors for biopsy-proven BKVN (OR: odds ratio; HR: hazard ratio)
Summary of identified risk factors for biopsy-proven BKVN |
Classification |
Risk factors |
BKVN cases/ Overall recipients |
Relative effect (95%CI) |
P-value |
References |
Transplant-related; Immunosuppressive regimen |
Induction with Polyclonal antibody |
25/542 |
OR 11.04 (2.94-41.52) |
0.0003 |
52 |
39/880 |
HR 6.6 (2.3-18.9) |
0.0005 |
53 |
Mycophenolate |
39/880 |
HR=3.5 (1.6-7.5) |
0.0013 |
53 |
Tacrolimus |
39/880 |
HR 3.3 (1.5-7.6) |
0.0038 |
53 |
33/99 |
OR 1.3 (1.02-1.7) |
0.03 |
54 |
Prednisone dose |
33/99 |
OR 1.22 (1.04-1.4) |
0.02 |
54 |
Transplant-related; graft-related factors |
Cold ischemia timing |
9/227 |
HR 1.18 (1.04-1.35) |
0.009 |
55 |
Ureteral Stents |
20/66 |
OR 4.71(1.22- 18.18) |
0.003 |
56 |
Acute rejection |
31/666 |
HR 3.18 (1.42-7.12) |
0.005 |
57 |
9/227 |
HR 4.05 (0.99-16.53) |
0.051 |
55 |
40/404 |
OR 2.1 |
0.0001 |
58 |
39/880 |
HR 5.1 (1.8-14.6) |
0.0021 |
53 |
Donor/recipient-related |
CMV infection |
39/327 |
OR 2.6 (1.29-5.26) |
0.006 |
59 |
31/666 |
HR 2.72 (1.19-6.24) |
<0.001 |
57 |
Donor BKV seropositive |
12/407 |
HR 2.89 (1.33-6.29) |
0.007 |
60 |
ABO-incompatibility |
11/ 62 |
OR 2.32 (NR) |
0.04 |
59 |
Afro-American recipients |
31/666 |
HR 2.89 (1.36-6.14) |
0.006 |
57 |
Recipients age |
31/666 |
HR 1.04 (1-1.07) |
0.048 |
57 |
HLA-DR mismatch |
25/542 |
OR 7.31 (2.58-20.71) |
0.0003 |
52 |
31/666 |
HR 1.45 (1.12–1.88) |
0.006 |
57 |
Donor source (deceased) |
9/226 |
not reported |
0.005 |
55 |
39/327 |
OR 2.2 (1.1-4.43) |
0.024 |
61 |
Table 2. Summary of predictive risk factors for BK viremia (OR: odds ratio; HR: hazards ratio, NR: not recorded)
Classification |
Risk factors |
The occurrence of BKV viremia (viremic patients/total recipients) |
Patients with BK Viremia subjected to risk factor |
Relative effect (95%CI) |
p-value |
Reference |
Transplant-related |
Immunosuppressive regimen (tacrolimus vs cyclosporine) |
102/998 |
64/102 |
HR 0.68 (0.44-1.07) |
0.094 |
62 |
22/265 |
NR (not recorded) |
OR 5.27 (1.2-23.16) |
0.027 |
63 |
38/229 |
32/38 |
HR 2.9 (1.1-8.1) |
0.032 |
64 |
71/251 |
49/71 |
OR 0.99 (0.91-1.25) |
0.19 |
65 |
41/553 |
32/41 |
OR 0.71 (0.24-2.05) |
0.53 |
66 |
111/407 |
84/111 |
HR 0.76 (0.48-1.23) |
0.264 |
60 |
29/183 |
10/29 |
OR 3.65 (1.42-9.39) |
<0.0-01 |
67 |
History of previous transplant |
48/352 |
9/48 |
OR 2.74 (1.05-7.15) |
0.039 |
68 |
71/251 |
20/71 |
OR 1.53 (0.62-4.42) |
0.07 |
65 |
ureteral stent |
23/198 |
10/ 23 |
HR 3 (NR) |
0.018 |
69 |
93/600 |
NR |
OR 1.65 (1.05-2.6) |
0.0315 |
70 |
171/1318 |
76/171 |
HR 1.36 (1.05-2.6) |
0.024 |
71 |
89/403 |
44/89 |
OR 1.92 (1.04-3.74) |
0.044 |
72 |
71/251 |
24/71 |
OR 0.737 (0.49- 1.09) |
0.129 |
65 |
Acute rejection |
163/609 |
21/163 |
HR 2.01 (1.096- 3.685) |
0.02 |
73 |
48/352 |
11/ 48 |
OR 3.79 (1.5-9.58) |
0.005 |
68 |
102/998 |
44/102 |
HR 0.96 (0.58- 1.56) |
0.89 |
62 |
71/251 |
50/71 |
OR 0.92 (0.85-1.02) |
0.02 |
65 |
80/412 |
36/80 |
NR |
<0.001 |
57 |
16/30 |
5/16 |
NR |
0.021 |
74 |
Cold ischemic time (min) |
102/998 |
NR |
NR |
0.30 |
62 |
warm ischemic time (min) |
57/194 |
53/57 |
Not recorded |
0.019 |
75 |
|
Donor and recipient characteristics |
HLA mismatch (4–6) |
102/998 |
|
HR 0.77 (0.49–1.24) |
0.28 |
62 |
HLA-A2 match |
102/998 |
21/102 |
HR 0.51 (0.28–0.8) |
0.011 |
62 |
B44 match |
102/998 |
3/102 |
HR 0.31 (0.076–0.85) |
0.019 |
62 |
C6 match |
102/998 |
0/102 |
HR 0.24 (0.013–1.12) |
0.075 |
62 |
DQ7 match |
102/998 |
18/102 |
HR 1.63 (0.91–2.78) |
0.097 |
62 |
DR7 match |
102/998 |
3/102 |
HR 0.49 (0.12–1.36) |
0.19 |
62 |
DR15 match |
102/998 |
3/102 |
HR 0.35 (0.084–0.93) |
0.034 |
62 |
Mean Lymphocyte percentage (%) |
16/52 |
Not recorded/16 |
HR 0.878 (0.783–0.984) |
0.026 |
76 |
16/268 |
16/16 |
AUC 0.77 (0.589–0.951) |
0.012 |
77 |
57/194 |
44/57 |
Not mentioned |
0.006 |
75 |
GCSF use |
80/666 |
42/80 |
HR 1.76 (0.87–3.57) |
0.0006 |
57 |
CMV infection |
80/666 |
17/80 |
|
0.001 |
57 |
Panel-reactive antibody test > 50% |
60/629 |
18/60 |
OR 3.352 (1.737-6.338) |
< 0.001 |
78 |
Afro-Caribbean ethnicity |
60/629 |
20/60 |
OR 2.882 (1.549-5.259) |
0.0024 |
78 |
Male Recipient |
102/998 |
78/102 |
HR 2.38 (1.46-4.09) |
<0.001 |
62 |
42/487 |
35/42 |
OR 2.49 (1.05-5.89) |
0.038 |
79 |
71/251 |
64/71 |
OR 1.42 (0.99-1.98) |
0.033 |
65 |
41/553 |
30/41 |
OR 1.77 (0.79-3.85) |
0.24 |
66 |
111/407 |
73/111 |
HR 1.04 (0.68-1.6) |
0.842 |
62 |
20/174 |
15/20 |
OR 3.47 (1.11-10.86) |
0.03 |
67 |
Risk factors
Several risk factors might attribute to the occurrence of BKVN. Generally, all published risk factors can be organised into three categories (Table 3):
Table 3. Potential Risk factors associated with an increased risk of post-transplant BKV re-activation
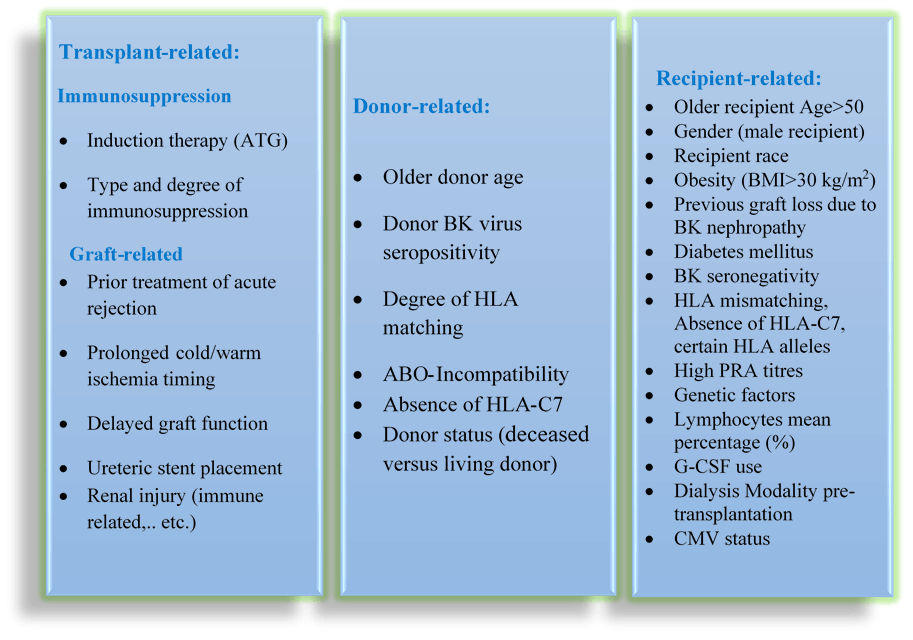
- Transplant-related risk factors
- Donor-related risk factors
- Recipient-related risk factors
Immunosuppression
Induction, as well as maintenance immunosuppression, appears to impact the BKVN risk.
BK Association with induction therapy: BK nephropathy was identified before the era of induction therapy [80]. However, potent therapeutics used for induction was thought to add further risk in activating BKV. Polyclonal anti-thymocyte globulin induction therapy had significantly increased the risk of BKVN in 2 different retrospective analyses (P-value of 0.0003 and 0.0005) [52,53]. This was further supported in a broader analysis of USA Organ Procurement and Transplantation Network (OPTN) of 48,000 kidney transplants performed between 2003 to 2006 [81].
Further international studies had confirmed this finding [82-84]. This is not surprising given the immunosuppressive effects of this agent as a lymphocyte depleting drug, leading to impaired cell-mediated immunity against BKV infection. However, others did not find any association of BK with anti-thymocyte therapy [54,55,57,61]. On the other hand, induction with a humanised monoclonal antibody alemtuzumab and Basiliximab failed to demonstrate the increased incidence of BKVN in different retrospective cohort studies, though it induces pan-T cell depletion, leading to impairment in the cellular immune system [57,85-88].
BK Association with maintenance immunosuppressant therapy: Immunosuppression appears as the primary risk for BKV infection. There is some supportive evidence to propose an increased risk of specific immunosuppressive regimens, such as tacrolimus compared withcyclosporine; and mycophenolate-mofetil (MMF) compared to azathioprine, though prospective data are necessary to confirm these findings.83,88-92 Tacrolimus, compared tosirolimusor cyclosporine, activates BKV proliferation through a mechanism involving FK-binding protein (BP-12) [93]. Furthermore, Thölking, et al. suggested a fast Tacrolimus metabolic rate (a low C/D ratio of <1.05 ng/mL*1/mg) to be an independent risk for BKVN and CNI nephrotoxicity, however, patients with BKVN in that analysis were low in number, limiting the efficacy of this test [94]. Brennan [69] prospectively assessed the changes in viruria and viremia with the three immunosuppressive combination therapies. He reported higher incidences of viruria with the tacrolimus-MMF therapy (46%) compared with the cyclosporine-MMF combination (13%, P=0.002). However, he did not find any difference in viremia or BK-nephritis related to either of calcineurin inhibitors or other adjuvant immunosuppression.
On the other hand, tacrolimus therapy was only classified as a risk factor for BK viremia in 7 multivariate analyses, [62-68] (Table 2) and a risk factor for BKVN in other two multivariate analyses [53,54]. The rest of studies which correlated tacrolimus with BK viremia and showed clearance of viremia upon reducing or withdrawing tacrolimus had small sample size (11 to 60 patients) and did not have control groups [95-98]. Besides being retrospective analyses, the protocol of lessening immunosuppression was heterogeneous in these studies and includes either drawing of one agent (MMF or the calcineurin inhibitor), or reducing the dose of both agents without removing any drug, which creates a few confounders in their data analysis. Further retrospective analyses found that BKV nephropathy is correlated with a combination of tacrolimus in higher doses (levels >8 ng/ml) and MMF (dosages 1.5-2g/d)/or mycophenolic-acid (> 1g/d) [54,83,99,100] mTOR-inhibitors (sirolimus or everolimus) on the other hand, may have an inhibitory effect on BKV replication in the urothelial cells [101,102]. Sirolimus can inhibit the expression of BKV LT-antigen in both primary human renal tubular cells and immortalised human renal cells [103]. Moreover, mTOR inhibitors control the differentiation of memory CD8 T-cells. Thus, it improves the immune reaction against BKV infection [102].
Of particular interest, BKVN has been reported in recipients getting almost every immunosuppressive drug or combination of drugs, including calcineurin-free triple-drugs [104,105], dual therapy with a calcineurin inhibitor and sirolimus [106-108], mycophenolic acid [61] and tacrolimus monotherapy [109]. Additionally, the analysis of USA Organ Procurement registry data has suggested an influence of maintenance steroids on the development of BKV, although the hazard ratio with steroid therapy was modest (HR 1.16, 95% CI 1.02-1.31) [110]. Taken altogether, it appears that the overall status of immunosuppression is responsible for the development of BKV infection rather than a particular drug. Indeed, some considered BKV replication to be an indicator of excess immunosuppression [111].
Allograft Rejection
Acute rejection was an independent risk factor for BK virus activation in several studies [57,58,74,112,113]. Dharnidharka, et al. [110] showed that acute rejection in the initial 6-months post-transplantation might increase the risk of BKV replication. Moreover, in 5 univariate analyses, higher incidences of viremia were demonstrated in those who had at least one episode of acute rejection during the follow-up period (Table 2 and 3) [57,62,65,68,73]. The effect of allograft rejection might be related to the intense treatment of rejection with immunosuppressive medication rather than the rejection itself [74,114]. Theodoropoulos, et al [57] had reported that recipients with acute rejection episodes had 17.7% higher rate of BK viruria (P< 0.001), 22.7% higher rate of BK viremia (P< 0.001) and 39% higher rate of BKVN (P< 0.001) and the rejection episodes had preceded the onset of clinical BKVN in 6/19 (32%) of the cases at a median time of 11 weeks. Furthermore, he noticed that cellular rejections were associated significantly with BKVN, while the humoral rejections were only correlated with viruria; an interesting finding which he could not explain [57].
Prolonged warm ischemia timing
In a single report, Steubl, et al. had reported a statistically significant BK viremia in patients with prolonged warm ischemia timing (P=0.019) [75]. A probable explanation for this is that prolonged warm ischemia can induce renal injury and leads to a pro-inflammatory status, which may contribute to viral reactivation [115]. Nevertheless, Warm ischemia timing was not a significant factor in different studies [42,74,116].
Prolonged cold ischemia timing (CIT)
Allograft damage resulted from Ischemia-reperfusion lesions; appear experimentally to facilitate BKV replication in ischemic adult mouse kidneys [101]. Prolonged CIT was an independent risk factor for the development of viremia and BKVN [55]. However, this was not a constant finding in other studies [57,68,74,78].
Delayed graft function (DGF)
DGF was independently associated with BKV replication and development of BKVN [55,117,118], though this was not confirmed in a broader cohort analysis [58,78,114].
Ureteric stent placement
5-multivariate analyses have demonstrated an increased risk of BKV in recipients who had a ureteric stent placement with significant risk ratios ranging from 1.36 to 3 [56,69-72], proposing that these patients might benefit from an early BKV screening. Wingate, et al. in 2017, reported only a prolonged stent duration of >3weeks is associated significantly with increased risk of BK Viremia compared with short stent duration <3weeks or no stenting (P=0.044) [72]. This data needs further validation before we can recommend using stents for less than three weeks based on a single centre report. Furthermore, other data had failed to show any correlation between stenting and BKV infections [18] while others did not include ureteric stenting among their data analysis [55,68,87,110], possibly because they are using stents routinely as part of their post-operative clinical practice.
Donor age
Favi, et al. [78] had identified the elderly donors (≥ 60 years) as a significant predictive risk factor for BKVN in multivariate analysis (P=0.048). Nevertheless, this was insignificant findings in other studies [58,68].
ABO-incompatibility
In a single study from John Hopkins Hospital, Sharif, et al. had demonstrated a higher BKVN incidence amongst ABO-incompatible recipients than in HLA-incompatible recipients (17.7% vs 5.9%) even after adjusting the number of rejection episodes [59]. Nonetheless, it is not clear whether the ABO-incompatibility in itself expands the risk of BKVN or BK disease is a consequence of intensified desensitisation regimens received by ABO-incompatible individuals. T-cell depletion caused by desensitisation regimens might increase the risk of T-cell-dependent infectious diseases such as CMV, BKV infections and even severe sepsis.119 A study which included 26 ABO-incompatible renal transplant recipients and continued follow-up for one year post-transplantation revealed a higher risk of BKV in patients that received intensified desensitisation with r-ATG [120].
Donor source
The corresponding analyses revealed strong associations between BK-viremia/BKVN and deceased donor [55, 63-65,67,121], however, this was not observed in other analyses [57,58,78,114].
BK Donor/recipients serostatus
Bohl,et al. [122], had recognised BK virus antibody-positive donors and recipients (BKV D+/R+) as a predictive risk for developing post-transplant BK viremia (46% BKV D+ versus 15% BKV D-). Interestingly, all of the pairs in which both donor and recipient were BK antibody-positive showed the same BK-subtypes and sequences, proposing donor origin of BK virus [122]. However, Shah recognised the seronegative recipients to have an increased risk of acquiring BKV if they got a transplant from a seropositive donor (BKV D+/R−) [123].
Recipient age, gender and weight
Plenty of studies had identified the importance of recipient male gender [62,65,67,68,87,124] and older recipient age [57,68,87,113] in the development of BKVN, though such findings have not been consistently observed in all analyses [55,76,114]. Similarly, Obesity (BMI over 30 kg/m2) was an independent predictor for BK viremia in a single study [125], but not in others [68,78]. In a separate analysis, presented by Mehta, et al. as a poster abstract, the advanced age was a risk factor for BK viremia among Non-Asian patients but not among East Asian or Pacific Islander descent patients [126].
Recipient race
Recipient race might be a strong predictive risk for developing BK viremia/nephropathy. African-American race has a higher rate of BKVN independent of other confounding risk factors in many analyses [57,71,87,124,127]. Similarly, Afro-Caribbean was a significant risk in a recent analysis with P-value of 0.0024 [78]. Alternatively, others had found Caucasian ethnicity to be at increased risk of BKVN rather than African American ethnicity [81,128,129]. Till date, the majority of publications which investigated ethnicity as a risk factor for BKV disease in adult's renal recipients are for USA population and only a single article was found for New Zealand [55] and an additional one for the Royal London Hospital (London, UK), where the author included Afro-Caribbean race [78]. Other than those, there are no comments on ethnicity in Asian, nor in European publications, which we feel should be addressed in future publications where a mixture of a population is present.
HLA mismatching
Presences of 4 or more mismatches are strong independent predictors of BK viremia [112,122,124]. Some had attributed this to the increase in rejection episodes, higher occurrence of steroid-resistant rejections that necessities treatment with lymphocyte depleting agent such as anti-thymocyte therapy [10,58]. Additionally, a relationship has been reported inconsistently between particular HLA alleles and the development of BKVN in kidney recipients [ 9,82]. In a retrospective cohort analysis, donor-recipient matching of HLA-A2, HLA-B44, and HLA-DR15 had a low rate of BKV infection [62], while recipient HLA-Cw4, HLA-B35 and HLA-A1were significantly associated with post-transplant BKV infection in another study, whereas recipient HLA-Cw6 and HLA-Cw7 did not increase the risk [18]. BK risk was further increased in recipients who had both HLA B35 and Cw4 (B35/Cw4 +/+) [18]. Interestingly, in a single-centre data, HLA-C7 seemed to give a protective/defensive role against BKV, and lack of HLA-C7 allele in a donor or a recipient had raised the risk of BK-viremia by at least 3-folds [122].
Previous transplant
Previous transplantation was considered as a statistically significant predictive risk for BK viremia in 2 separate studies [68,125]. However, this factor was insignificant in different analyses [57,60,68]. Perhaps the intense immunosuppression and desensitisation therapy at pre-transplant play a role in that.
High PRA titre
Higher pre-transplant PRA level of > 50% increases the risk of BK infection. Similarly, it may owe to the desensitisation and over-immunosuppression received before transplantation [78], yet this was an insignificant factor in other analyses [58,68].
Diabetes mellitus
Pre-transplant diabetes duration was an independent factor for BKV positivity in 2 multivariate analyses; in SPK (Simultaneous pancreas and kidney transplant)recipients (P= 0.028) and renal transplant recipients (P=0.008) [61,117]. Other than these two studies, the presence of diabetes mellitus as a risk factor for BKVN/BK-viremia was suggested in isolated case reports [131-133], while unidentified in more extensive cohort studies [56,57,78,130].
Conversely, Thangaraju, et al. [113] reported a lower incidence of BK viremia in patients with diabetes as a cause of their ESRD, and he related this to lower levels of immunosuppressant used for these patients, however, we disagree with this thought since most centres do not lower immunosuppressant for diabetic patients.
Recipient BK-seronegativity
Recipient BK-seronegativity might be a potential risk of BKV infection, particularly if they received a transplant from a seropositive donor (BKV D+/R−) [123].
Genetic factors and hereditary diseases
Small, preliminary studies have implicated a possible genetic predisposition to BKVN, including a lower number of recipient natural killer (NK) cell-activating receptors anddonor/recipientmismatch at the MHC class I polypeptide-related sequence A (MICA) locus [111,134].
Moreover, in a single Korean centre, a higher incidence of BKVN was observed among paediatrics kidney allograft recipients with Alport syndrome, despite Alport's comprised only 6% of the study population. Nevertheless, every patient with BK viremia and Alport syndrome developed BKVN, while only 11.1% of patients with BK viremia in the absence of Alport syndrome had progressed to BKVN in a multivariate analysis (hazard ratio 13.2, P = 0.002) [135].
Lymphocyte mean percentage (%)
Renal recipients with lower total lymphocytes percentage were noted in 3 multivariate analyses to increase the BKV activity (with a P-value of 0.026, 0.012, and 0.006 respectively); suggesting a reduced total lymphocyte count can be an independent predictor for preceding BK viremia [75-77]. According to Comolli [136], lymphopenia may associate with alterations in the total of BKV-specific CD4+ and/or CD8+ T cells. Additionally, lymphopenia can result from elevated MMF levels or elevated tacrolimus levels, which may elevate the MMF–AUC level via the enterohepatic circulation [75].
Granulocyte-colony stimulating factor (G-CSF) use
Theodoropoulos, et al. had identified the granulocyte-colony stimulating factor (G-CSF) as a risk factor for BKVN (P-value of 0.036) and BK viremia (P=0.006) in univariate analysis [57]. Unlike recipients of Hematopoietic Stem Cell Transplant, the use of G-CSF is limited in routine clinical practice for post-renal recipients unless the patient has severe neutropenia which again might reflect the adverse effects of immunosuppressant's or severe viral infection making the interpretation of this factor difficult.
Modality of RRT pre-transplant
Hosseini-Moghaddam [18] had reported a higher incidence of BK viremia in recipients who were on haemodialysis pre-transplantation compared to PD in the univariable prospective analysis (P=006). Though the mechanism was unclear, a postulation of a chronic inflammatory response accompanying the haemodialysis process leading to chronic activation of the immune system and hypercytokinemia were anticipated 18 Further studies had confirmed this [68,137], while others found an insignificant association of BK reactivation with the pre-transplant dialysis modalities [78].
CMV
The studies regarding CMV serostatus as a risk factor for BK reactivation is contradictory, while some had reported CMV-viremia to protect against consequent BK-viremia, possibly due to a decrease in the intensity of immunosuppression following CMV diagnosis [73], others had identified CMV infection as a potential factor for BKV replication and development of BKVN [57,68,78,124,138,139].
Nearly three decades of research have prompted just a minimal comprehension of BKV and its pathophysiology. This truth proves to the elusive nature of the virus and the challenge they pose to their investigators. The main objective of this review is to determine the potential risk factors for BKV infection. We believe that over immunosuppression remains the leading risk factor for acquiring BK infection post-transplant. However, it remains unclear whether the development of BKVN is attributable to quantitative and/or qualitative differences in immune suppression. In addition to immunosuppression, earlier studies showed many probable risk factors of BKV infection, including donor characteristics risk factors, recipient characteristics and transplant-related risks. Some of these factors appeared constant in many multivariate analyses with statistical significance such as ureteric stents placement, recipient's age, recipient's race, deceased donor and acute rejections episodes; however, this was not established in other larger cohort analyses. Moreover, the diversity in published data may reflect patient's geographical area, their genetic susceptibility and probably different BK variant gene with different risk susceptibility which may explain the wide variety of risk factors that appear strongly significant in some study and irrelevant in others. With a view to prevention, it is crucial to distinguish the potentially modifiable factors that favour infection. Hence a prospective randomised cohort study with larger sample size is warranted, preferably involving many countries to eliminate any sample bias and to overcome any geographical or ethnical boundaries that may create confounding results. Additionally, it will help to readdress the immunosuppression protocol to reduce the BKV and to define the appropriate follow up period for screening those patients into consideration to reduce acute or chronic rejection episodes.
The authors declare that they have no potential conflict of interest and no funding relevant to this article to disclose.
- Gardner S, Field A, Coleman D, Hulme B (1971) New human papovavirus (BK) isolated from urine after renal transplantation. The Lancet 1: 1253-1257. [Crossref]
- Reploeg MD, Storch GA, Clifford DB (2001) BK virus: a clinical review. Clin Infect Dis 33: 191-202. [Crossref]
- Drachenberg CB, Papadimitriou JC, Ramos E (2006) Histologic versus molecular diagnosis of BK polyomavirus–associated nephropathy: a shifting paradigm? Clinical Journal of the American Society of Nephrology 1: 374-379.
- Lecatsas GA, Prozesky OW, Van Wyk J, Els HJ (1973) Biological Sciences: Papova Virus in Urine after Renal Transplantation. Nature 241: 343.
- Coleman DV, Mackenzie EF, Gardner SD, Poulding JM, Amer B, et al. (1978) Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol 31: 338-347. [Crossref]
- Mackenzie EFD, Poulding JM, Harrison PR, Am B (1978) Human polyoma virus (HPV)—A significant pathogen in renal transplantation. Proc Eur Dial Transplant Assoc 15: 352-360 [Crossref]
- Hogan TF, Border EC, Mcbain JA, Padgett BL, Walker DL (1980) Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med 92: 373-378. [Crossref]
- Gardner SD, MacKenzie EF, Smith C, Porter AA (1984) Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol 37: 578-86. [Crossref]
- Van Aalderen MC, Heutinck KM, Huisman C, Ten Berge IJ (2012) BK virus infection in transplant recipients: clinical manifestations, treatment options and the immune response. Neth J Med 70: 172-83. [Crossref]
- Bohl DL, Brennan DC (2007) BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol 1: S36-S46. [Crossref]
- Dall A, Hariharan S (2008) BK virus nephritis after renal transplantation. Clin J Am Soc Nephrol 3: S68-S75. [Crossref]
- Hariharan S (2006) BK virus nephritis after renal transplantation. Kidney Int 69: 655-62. [Crossref]
- Sawinski D, Goral S (2015) BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant 30: 209-217. [Crossref]
- Tremolada S, Akan S, Otte J, Khalili K, Ferrante P, et al. (2010) Rare subtypes of BK virus are viable and frequently detected in renal transplant recipients with BK virus-associated nephropathy. Virology 404: 312-318. [Crossref]
- Hirsch HH (2019) Virology, epidemiology, and pathogenesis of JC polyomavirus, BK polyomavirus and other human polyomaviruses. UpToDate.
- Shah KV (1990) Polyomaviruses. In: Fields BN, Knipe DM (Eds) (2d Edn) Raven Press, New York Virology pp: 1609-1623.
- Imperiale MJ, Major EO (2007) Polyomaviruses. In: Fields (5th Edn) Knipe DM, Howley PM (Eds), Virology 2: 2263.
- Hosseini-Moghaddam SM (2007) Risk Factors for BK Virus Infection after Kidney Transplantation, London, Ontario 2016. Electronic Thesis and Dissertation Repository.
- Lim ES, Reyes A, Antonio M, Saha D, Ikumapayi UN, et al. (2013) Discovery of STL polyomavirus, a polyomavirus of ancestral recombinant origin that encodes a unique T antigen by alternative splicing. Virology 436: 295-303. [Crossref]
- Gheit T, Dutta S, Oliver J, Robitaille A, Hampras S, et al. (2017) Isolation and characterization of a novel putative human polyomavirus. Virology 506: 45-54. [Crossref]
- Korup S, Rietscher J, Calvignac-Spencer S, Trusch F, Hofmann J, et al. Identification of a novel human polyomavirus in organs of the gastrointestinal tract. PloS one 8: e58021. [Crossref]
- Mishra N, Pereira M, Rhodes RH, An P, Pipas JM, et al. (2015) Identification of a novel polyomavirus in a pancreatic transplant recipient with retinal blindness and vasculitic myopathy. J Infect Dis 210: 1595-1599. [Crossref]
- Jin L, Gibson PE, Booth JC, Clewley JP (1993) Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol 41: 11-17. [Crossref]
- Krumbholz A, Bininda-Emonds OR, Wutzler P, Zell R (2008) Evolution of four BK virus subtypes. Infect Genet Evol 8: 632-643. [Crossref]
- Takasaka T, Goya N, Tokumoto T, Tanabe K, Toma H, et al. (2004) Subtypes of BK virus prevalent in Japan and variation in their transcriptional control region. J Gen Virol 85: 2821-2827. [Crossref]
- Ambalathingal GR, Francis RS, Smyth MJ, Smith C, Khanna R (2017) BK polyomavirus: clinical aspects, immune regulation, and emerging therapies. Clin Microbiol Rev 30: 503-528. [Crossref]
- Zhong S, Randhawa PS, Ikegaya H, Chen Q, Zheng HY, et al. (2009) Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J Gen Virol 90: 144-152. [Crossref]
- Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, et al. (2009) Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 199: 837-846. [Crossref]
- Olsen GH, Hirsch HH, Rinaldo CH (2009) Functional analysis of polyomavirus BK non‐coding control region quasispecies from kidney transplant recipients. Journal of medical virology 81: 1959-1967.
- Korth J, Anastasiou OE, Bräsen JH, Brinkhoff A, Lehmann U, et al. (2019) The detection of BKPyV genotypes II and IV after renal transplantation as a simple tool for risk assessment for PyVAN and transplant outcome already at early stages of BKPyV reactivation. J Clin Virol 113: 14-19.
- Kaydani GA, Makvandi M, Samarbafzadeh A, Shahbazian H, Fard MH (2015) Prevalence and distribution of BK virus subtypes in renal transplant recipients referred to Golestan Hospital in Ahvaz, Iran. Jundishapur J Microbiol 8: e16738. [Crossref]
- Gardner SD. Prevalence in England of antibody to human polyomavirus (BK). Br Med J 1: 77-78. [Crossref]
- Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, et al. (2003) Population‐based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 71: 115-123. [Crossref]
- Mäntyjärvi RA, Meurman OH, Vihma L, Berglund B (1973) A human papovavirus (BK), biological properties and seroepidemiology. Annals of clinical research 5: 283-287.
- Portolani M, Marzocchi A, Barbanti-Brodano G, La Placa M (1974) Prevalence in Italy of antibodies to a new human papovavirus (BK virus). J Med Microbiol 7: 543-546. [Crossref]
- Rziha HJ, Bornkamm GW, zur Hausen H (1978) BK virus: I. Seroepidemiologic studies and serologic response to viral infection. Medical microbiology and immunology 165: 73-81.
- Sroller V, Hamšíková E, Ludvíková V, Vochozková P, Kojzarová M, et al. (2014) Seroprevalence rates of BKV, JCV, and MCPyV polyomaviruses in the general Czech Republic population. J Med Virol 86: 1560-1568. [Crossref]
- Shah KV, Daniel RW, Warszawski RM (1973) High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis 128: 784-787. [Crossref]
- Antonsson A, Green AC, Mallitt KA, O'Rourke PK, Pawlita M, et al. (2010) Prevalence and stability of antibodies to the BK and JC polyomaviruses: a long-term longitudinal study of Australians. J Gen Virol 91: 1849-1853. [Crossref]
- Yi-ling SI, Qi LI, Xue LI, Hai-jing SO, Hao-jie HA, et al. (2004) Prevalence of BK Virus in Peripheral Blood Leukocytes from Healthy Individuals in China [J]. Chinese Journal of Nosoconmiology p. 3.
- Smith RD, Galla JH, Skahan K, Anderson P, Linnemann CC, et al. (1998) Tubulointerstitial nephritis due to a mutant polyomavirus BK virus strain, BKV (Cin), causing end-stage renal disease. J Clin Microbiol 36: 1660-1665. [Crossref]
- Saundh BK, Baker R, Harris M, Welberry Smith MP, Cherukuri A, et al. (2012) Early BK polyomavirus (BKV) reactivation in donor kidney is a risk factor for development of BKV-associated nephropathy. The Journal of infectious diseases 207: 137-141.
- Chan PK, Ip KW, Shiu SY, Chiu EK, Wong MP, et al. (1994) Association between polyomaviruria and microscopic haematuria in bone marrow transplant recipients. Journal of Infection 29: 139-146.
- Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R (1986) Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med 315: 230-234. [Crossref]
- Bratt G, Hammarin AL, Grandien M, Hedquist BG, Nennesmo I, et al. (1999) BK virus as the cause of meningoencephalitis, retinitis and nephritis in a patient with AIDS. Aids 13: 1071-1075. [Crossref]
- Vidal JE, Fink MC, Cedeno-Laurent F, Delbue S, Ferrante P, et al. (2007) BK virus associated meningoencephalitis in an AIDS patient treated with HAART. AIDS research and therapy 4: 13.
- Daveson KL, Ong CW, Bowden S, Koina ME, Hallam LA (2013) BK virus-associated progressive multifocal leukoencephalopathy. The Medical Journal of Australia 198: 216-218.
- Bakri FG, Bahou YG, Al-Sammarrai FA, Hadidy A, Gharaibeh A, et al. (2013) Fatal encephalitis due to BK virus in a patient with common variable immunodeficiency: a case report. J Clin Virol 57: 363-369. [Crossref]
- Chittick P, Williamson JC, Ohl CA (2013) BK virus encephalitis: case report, review of the literature, and description of a novel treatment modality. Ann Pharmacother 47: 1229-1233. [Crossref]
- Cubukcu-Dimopulo O, Greco A, Kumar A, Karluk D, Mittal K, et al. (2000) BK virus infection in AIDS. Am J Surg Pathol 24: 145. [Crossref]
- Hedquist BG, Bratt G, Hammarin AL, Grandien M, Nennesmo I, et al. (1999) Identification of BK virus in a patient with acquired immune deficiency syndrome and bilateral atypical retinitis. Ophthalmology 106: 129-132. [Crossref]
- Smith JM, Dharnidharka VR, Talley L, Martz K, McDonald RA (2007) BK virus nephropathy in pediatric renal transplant recipients: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry. Clin J Am Soc Nephrol 2: 1037-1042. [Crossref]
- Prince O, Savic S, Dickenmann M, Steiger J, Bubendorf L, et al. (2008) Risk factors for polyoma virus nephropathy. Nephrol Dial Transplant 24: 1024-1033. [Crossref]
- Manitpisitkul W, Wilson NS, Haririan A (2010) Immunosuppressive agents as risk factors for BK virus nephropathy: an overview and update. Expert Opin Drug Saf 9: 959-969. [Crossref]
- Hsiao CY, Pilmore HL, Zhou L, de Zoysa JR (2016) Outcomes of renal transplant recipients with BK virus infection and BK virus surveillance in the Auckland region from 2006 to 2012. World J Nephrol 5: 497. [Crossref]
- Thomas A, Dropulic LK, Rahman MH, Geetha D (2007) Ureteral stents: a novel risk factor for polyomavirus nephropathy. Transplantation 84: 433-436. [Crossref]
- Theodoropoulos N, Wang E, Penugonda S, Ladner DP, Stosor V, et al. (2013) BK virus replication and nephropathy after alemtuzumab‐induced kidney transplantation. Am J Transplant 13: 197-206. [Crossref]
- Awadalla Y, Randhawa P, Ruppert K, Zeevi A, Duquesnoy RJ (2004) HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am J Transplant 4: 1691-1696. [Crossref]
- Sharif A, Alachkar N, Bagnasco S, Geetha D, Gupta G, et al. (2012) Incidence and outcomes of BK virus allograft nephropathy among ABO-and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol 7: 1320-1327. [Crossref]
- Brochot E, Descamps V, Handala L, Faucher J, Choukroun G, et al. (2019) BK polyomavirus in the urine for follow-up of kidney transplant recipients. Clinical Microbiology and Infection 25: 112-e1.
- Wong Lok, Yan Ivy (2019) BK virus infection in renal transplant recipients: single centre experience.
- Masutani K, Ninomiya T, Randhawa P (2013) HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol Dial Transplant 28: 3119-3126. [Crossref]
- Hasegawa M, Ito T, Saigo K, Akutsu N, Maruyama M, et al. (2014) Association of DNA amplification with progress of BK polyomavirus infection and nephropathy in renal transplant recipients. Transplant Proc 46: 556-559. [Crossref]
- Huang G, Zhang L, Liang X, Qiu J, Deng R, et al. (2014) Risk factors for BK virus infection and BK virus–Associated nephropathy under the impact of intensive monitoring and pre-emptive immunosuppression reduction. Transplant Proc 46: 3448-54. [Crossref]
- Vu D, Sakharkar P, Shah T, Naraghi R, Yasir Q, et al. (2014) Association of interferon gamma gene polymorphisms with BK virus infection among Hispanic renal allograft recipients. Transplantation 97: 660-667. [Crossref]
- Moura EB, Petzhold SV, Amaral AR, Deboni LM, FRANÇA PH (2017) Evaluation of the predisposition and clinical impact of BK virus replication in kidney transplant patients. Anais da Academia Brasileira de Ciências 89: 675-684.
- Dogan SE, Celebi ZK, Akturk S, Kutlay S, Tuzuner A, et al. (2017) Prevalence and risk factors of BK viremia in patients with kidney transplantation: a single-center experience from Turkey. Transplant Proc 49: 532-536. [Crossref]
- Jacobi J, Prignitz A, Büttner M, Korn K, Weidemann A, et al. (2013) BK viremia and polyomavirus nephropathy in 352 kidney transplants; risk factors and potential role of mTOR inhibition. BMC nephrol 14: 207. [Crossref]
- Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, et al. (2005) Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 5: 582-594. [Crossref]
- Kayler L, Zendejas I, Schain D, Magliocca J (2013) Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis 15: 202-207. [Crossref]
- Maliakkal JG, Brennan DC, Goss C, Horwedel TA, Chen H, et al. (2017) Ureteral stent placement and immediate graft function are associated with increased risk of BK viremia in the first year after kidney transplantation. Transpl Int 30: 153-161. [Crossref]
- Wingate JT, Brandenberger J, Weiss A, Scovel LG, Kuhr CS (2017) Ureteral stent duration and the risk of BK polyomavirus viremia or bacteriuria after kidney transplantation. Transpl Infect Dis 19: e12644. [Crossref]
- Elfadawy N, Flechner SM, Liu X, Schold J, Srinivas TR, et al. (2013) CMV Viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation 96: 1097-1103. [Crossref]
- Ertugrul G, Yanaral T (2019) BK virus in kidney transplantation: A single center experiences. Arch Organ Transplant 4: 010-013.
- Steubl D, Baumann M, Schuster T, Fischereder M, Krämer BK, et al. (2012) Risk factors and interventional strategies for BK polyomavirus infection after renal transplantation. Scandinavian journal of urology and nephrology 46: 466-474.
- Li P, Cheng D, Wen J, Xie K, Li X, et al. (2018) Risk factors for BK virus infection in living-donor renal transplant recipients: a single-center study from China. Ren Fail 40: 442-446. [Crossref]
- Velioglu A, Aksu B, Asicioglu E, Arıkan H, Tinay I, et al. (2015) Association of BK virus titers with lymphocyte count in renal transplant patients. Transplantation proceedings 47: 1421-1424.
- Favi E, Puliatti C, Sivaprakasam R, Ferraresso M, Ambrogi F, et al. (2019) Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J Clin Cases 7: 270. [Crossref]
- Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, et al. (2013) Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant 13: 136-145. [Crossref]
- Ramos E, Drachenberg CB, Papadimitriou JC, Hamze O, Fink JC, et al. (2002) Clinical course of polyoma virus nephropathy in 67 renal transplant patients. Journal of the American Society of Nephrology 13: 2145-2151.
- Buehrig CK, Lager DJ, Stegall MD, Kreps MA, Kremers WK, et al. (2003) Influence of surveillance renal allograft biopsy on diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney int 64: 665-673. [Crossref]
- Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, et al. (2002) Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347: 488-496 [Crossref]
- Mengel M, Marwedel M, Radermacher J (2003) Incidence of polyomavirus nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant 18: 1190-1196. [Crossref]
- Namba Y, Moriyama T, Kyo M (2005) Prevalence, characteristics, and outcome of BK virus nephropathy in Japanese renal transplant patients: analysis in protocol and episode biopsies. Clin Transplant 19: 97-101. [Crossref]
- Dadhania D, Snopkowski C, Ding R, Muthukumar T, Chang C, et al. (2008) Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation 86: 521-528. [Crossref]
- Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, et al. (2005) Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79: 1277-1286. [Crossref]
- Cannon RM, Brock G, Marvin MR, Eng M, Buell JF (2012) Analysis of BK viral infection after alemtuzumab induction for renal transplant. Transpl Infect Dis 14: 374-379. [Crossref]
- Schold JD, Rehman S, Kayler LK, Magliocca J, Srinivas TR, et al. (2009) Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transplant Int 22: 626-634. [Crossref]
- Ison MG, Parker M, Stosor V, Kaufman DB (2009) Development of BK nephropathy in recipients of simultaneous pancreas-kidney transplantation. Transplantation 87: 525-530. [Crossref]
- Radtke J, Dietze N, Fischer L, Achilles EG, Li J, et al. (2016) Incidence of BK polyomavirus infection after kidney transplantation is independent of type of immunosuppressive therapy. Transplant Infectious Disease 18: 850-855.
- Fink JC, Wiland AM, Rochussen JR, Drachenberg CB, Papadimitriou JP (2000) The increased incidence of BK polyomavirus in renal transplant recipients treated with Prograf. Transplantation 69: S385.
- Nickeleit V, Hirsch HH, BINET IF, Gudat F, PRINCE O, et al. (1999) Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol 10: 1080-1089. [Crossref]
- Hirsch HH, Yakhontova K, Lu M, Manzetti J (2016) BK polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP‐12. Am J Transplant 16: 821-832. [Crossref]
- Thölking G, Schmidt C, Koch R, Schuette-Nuetgen K, Pabst D, et al. (2016) Influence of tacrolimus metabolism rate on BKV infection after kidney transplantation. Scientific reports 6: 32273.
- Alméras C, Foulongne V, Garrigue V, Szwarc I, Vetromile F, et al. (2008) Does reduction in immunosuppression in viremic patients prevent BK virus nephropathy in de novo renal transplant recipients? A prospective study. Transplantation 85: 1099-1104. [Crossref]
- Halim MA, Al-Otaibi T, Gheith O, Zkaria Z, Mosaad A, et al. (2014) Active management versus minimization of immunosuppressives of BK virus-associated nephropathy after a kidney transplant. Exp Clin Transplant 12: 528-533. [Crossref]
- Weiss AS, Gralla J, Chan L, Klem P, Wiseman AC (2008) Aggressive immunosuppression minimization reduces graft loss following diagnosis of BK virus-associated nephropathy: a comparison of two reduction strategies. Clin J Am Soc Nephrol 3: 1812-1819. [Crossref]
- Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, et al. (2010) Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus‐associated nephropathy. Am J Transplant 10: 2615-2623. [Crossref]
- Rocha PN, Plumb TJ, Miller SE, Howell DN, Smith SR (2004) Risk factors for BK polyomavirus nephritis in renal allograft recipients. Clin Transplant 18: 456-462. [Crossref]
- Skulratanasak P, Mahamongkhonsawata J, Chayakulkeereeb M, Larpparisutha N, Premasathiana N, et al. (2018) BK Virus Infection in Thai Kidney Transplant Recipients: A Single-Center Experience. Transplant Proc 50: 1077-1079. [Crossref]
- Atencio IA, Shadan FF, Zhou XJ, Vaziri ND, Villarreal LP (1993) Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol 67: 1424-1432. [Crossref]
- Jouve T, Rostaing L, Malvezzi P (2016) Place of mTOR inhibitors in management of BKV infection after kidney transplantation. J Nephropathol 5: 1-7. [Crossref]
- Barraclough KA, Isbel NM, Staatz CE, Johnson DW (2011) BK virus in kidney transplant recipients: the influence of immunosuppression. J Transplant 2011: 750836. [Crossref]
- Lipshutz GS, Flechner SM, Govani MV, Vincenti F (2004) BK nephropathy in kidney transplant recipients treated with a calcineurin inhibitor‐free immunosuppression regimen. American Journal of Transplantation 4: 2132-2134.
- Lipshutz GS, Mahanty H, Feng S, Hirose R, Stock PG, et al. (2004) Polyomavirus-associated nephropathy in simultaneous kidney-pancreas transplant recipients: a single-center experience. Transplant Proc 36: 1097-1098 [Crossref]
- Hirsch HH, Mohaupt M, Klimkait T (2001) Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J Infect Dis 184: 1494-1495. [Crossref]
- Josephson MA, Gillen D, Javaid B, Kadambi P, Meehan S, et al. (2006) Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation 81: 704-710. [Crossref]
- Mannon RB, Hoffmann SC, Kampen RL, Cheng OC, Kleiner DE, et al. (2005) Molecular evaluation of BK polyomavirus nephropathy. Am J Transplant 5: 2883-2893. [Crossref]
- Randhawa PS, Gupta G, Vats A, Shapiro R, Viscidi RP (2006) Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin Vaccine Immunol 13: 1057-1063. [Crossref]
- Dharnidharka V, Cherikh W, Abbott K (2009) An OPTN Analysis of National Registry Data on Treatment of BK Virus Allograft Nephropathy in the United States. Transplantation 87: 1019-1026. [Crossref]
- Ajit P Limaye, Daniel C Brennan (2018) BK virus-induced nephropathy in kidney transplantation: Clinical manifestations and diagnosis. Up-To-Date.
- Hässig A, Roos M, Etter A, Bossart W, Müller N, et al. (2014) Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl Infect Dis 16: 44-54. [Crossref]
- Thangaraju S, Gill J, Wright A, Dong J, Rose C, et al. (2016) Risk Factors for BK Polyoma Virus Treatment and Association of Treatment with Kidney Transplant Failure: Insights from a Paired Kidney Analysis. Transplantation 100: 854-861 [Crossref]
- Pai D, Mann DM, Malik A, Hoover DR, Fyfe B, et al. (2015) Risk factors for the development of BK virus nephropathy in renal transplant recipients. Transplant Proc 47: 2465-2469. [Crossref]
- Fishman JA (2002) BK virus nephropathy–polyomavirus adding insult to injury. N Engl J Med 247: 527-530. [Crossref]
- Li X, Sun Q, Chen J, Ji S, Wen J, et al. (2013) Immunophenotyping in BK virus allograft nephropathy distinct from acute rejection. Clin Dev Immunol 2013: 412902. [Crossref]
- Mindlova M, Boucek P, Saudek F, Skibova J, Jedinakova T, et al. (2012) Prevalence and risk factors of polyomavirus BK replication in simultaneous pancreas/kidney transplant recipients from a single transplant center. Clinical transplantation 26: 267-274.
- Bressollette-Bodin C, Coste-Burel M, Hourmant M, Sebille V, Andre-Garnier E, et al. (2005) A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am J Transplant 5: 1926-1933. [Crossref]
- Schachtner T, Stein M, Reinke P (2015) ABO desensitization affects cellular immunity and infection control after renal transplantation. Transpl Int 28: 1179-1194. [Crossref]
- Kauke T, Klimaschewski S, Schoenermarck U, Fischereder M, Dick A, et al. (2016) Outcome after Desensitization in HLA or ABO-Incompatible Kidney Transplant Recipients: A Single Center Experience. PloS one 11: e0146075. [Crossref]
- Huang G, Wang C, Zhang L, Fei J, Deng S, et al. (2015) Monitoring of polyomavirus BK replication and impact of preemptive immunosuppression reduction in renal-transplant recipients in China: a 5-year single-center analysis. Diagn Microbiol Infect Dis 81: 21-26.
- Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault‐Keener M, Schnitzler MA, et al. (2005) Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant 5: 2213-2221. [Crossref]
- Shah KV (2000) Human polyomavirus BKV and renal disease. Nephrology Dialysis Transplantation 15: 754-755.
- Patel H, Agarwal K, Pawar A, Leeaphorn N, Agrawal N, et al. (2020) Incidence and risk factors of kidney allograft loss due to BK virus: UNOS data set. Data presented at the live virtual 2020 National Kidney Foundation Spring Clinical Meetings held March 25 to 28. ePoster p. 465.
- Ziedina I, Folkmane I, Chapenko S, Murovska M, Sultanova A, et al. (2009) Reactivation of BK Virus in the Early Period After Kidney Transplantation. Transplant Proc 41: 766-768. [Crossref]
- Mehta S, Ali N, Lonze B, Stachel A (2018) Impact of Age and Race on BK Viremia among Kidney Transplant Recipients. Am J Transplant 18: 837-837.
- Sood P, Senanayake S, Sujeet K, Medipalli R, Zhu YR, et al. (2012) Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation 94: 814-821. [Crossref]
- Vasudev B, Hariharan S, Hussain SA, Zhu YR, Bresnahan BA, et al. (2005) BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney int 68: 1834-1839. [Crossref]
- Hirsch HH, Randhawa P (2013) AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation. Am J Transplant 13: 179-188. [Crossref]
- Koukoulaki M, Grispou E, Pistolas D, Balaska K, Apostolou T, et al. (2009) Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis 11: 1-0. [Crossref]
- Barreto P, Almeida M, Dias L, Vieira P, Pedroso S, et al. (2016) BK virus nephropathy in kidney transplantation: A literature review following a clinical case. Portuguese Journal of Nephrology & Hypertension 30: 259-268.
- McGilvray ID, Lajoie G, Humar A, Cattral MS (2003) Polyomavirus infection and acute vascular rejection in a kidney allograft: coincidence or mimicry? Am J Transplant 3: 501-504. [Crossref]
- Pappo O, Demetris AJ, Raikow RB, Randhawa PS (1996) Human polyoma virus infection of renal allografts: histopathologic diagnosis, clinical significance, and literature review. Mod Pathol 9: 105-109. [Crossref]
- Trydzenskaya H, Juerchott K, Lachmann N, Kotsch K, Kunert K, et al. (2013) The genetic predisposition of natural killer cell to BK virus-associated nephropathy in renal transplant patients. Kidney int 84: 359-265. [Crossref]
- Cho YH, Hyun HS, Park E, Moon KC, Min SI, Ha, et al. (2019) Higher Incidence of BK Virus Nephropathy in Pediatric Kidney Allograft Recipients with Alport Syndrome. J Clin Med 8: 491 [Crossref]
- Comoli P, Binggeli S, Ginevri F, Hirsch HH (2006) Polyomavirus‐associated nephropathy: update on BK virus‐specific immunity. Transplant Infectious Disease 8: 86-94. [Crossref]
- Mitterhofer AP, Umbro I, Pietropaolo V, Meçule A, Russo GE, et al. (2012) Polyomavirus BK infection in end-stage renal disease: analysis of viral replication in patients on hemodialysis or peritoneal dialysis. Transplant Proc 44: 1869-1872. [Crossref]
- Borni-Duval C, Caillard S, Olagne J, Perrin P, Braun-Parvez L, et al. (2013) Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation 95: 1498-505. [Crossref]
- Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, Bauer C, Wolk K, et al. (2018) BKV, CMV, and EBV Interactions and their Effect on Graft Function One Year Post-Renal Transplantation: Results from a Large Multi-Centre Study. EBioMedicine 34: 113-121. [Crossref]

