During the past few years, experiments have been in progress to study the bioavailability of vitamins and minerals provided by nature and chemistry. Vitamins and minerals extracted from plants associated with natural phytonutrients compounds was developed as supplement allowing better compatibility with human physiology. This Natural formula (Arkovital® Pur’Energie) was studied by evaluating the antioxidant activities and comparing them to a formula which contain identical synthetic vitamins and minerals (Synthetic formula). Two clinical studies were conducted respectively on 6 (study 1) and 50 volunteers (study 2) with mean age 45 ± 7 years and 32 ± 12 years, respectively. Volunteers received, for 14 days, 2 pills of Arkovital® Pur’ Energie or 2 capsules of the synthetic formula. Efficacy was evaluated using the PAOT® Skin Technology method (which measures the total antioxidant activity of supplements), classical analysis methods and electrochemical method (able to evaluate of skin oxidative stress status (SOSS) with PAOT Skin® score). Measurements were taken on Day 0 and Day 15 on fasten volunteers. For the study 1, blood samples were taken from volunteers for monitoring of two oxidative stress biomarkers (vitamins C, E) and vitamin B6. The two studies demonstrate that natural vitamins and minerals contained in Arkovital® Pur’Energie have a higher antioxidant power than synthetic vitamins and minerals. After 14 days of supplementation, the antioxidant status of people who received Arkovital® Pur’ Energie is increased significantly (p<0.05) by a factor of 2 compared to the synthetic formula. Oxidative stress biomarkers (vitamin C and E) monitoring in the study 1, did not shown a significant difference (p<0.05) between natural and synthetic formula which could be due to the small volunteers number in each group. The rate of B6 increased 6 times for volunteers who took Arkovital® Pur’Energie and only by 4 times for those who took the synthetic supplement that after 14 days of supplementation.
antioxidants, bioavailability, natural and synthetic vitamins, oxidative stress, PAOT scores
Vitamins originate primarily in plant tissues. Vitamins are organic substances that are essential in small amounts for growth, reproduction, and health maintenance. Vitamins must be included in the diet since they cannot be synthesized at all or in sufficient quantity in the body. Each vitamin performs a specific function; hence one cannot replace another [1].
Organic chemistry allows the synthesis of analogue versions of many biological molecules such as vitamins. These compounds could show some vitamin activity with a different structure then natural version. But the structure/function relationship is fundamental in biology and has an effect on absorption, transport, storage or even the catabolism of a molecule [2].
For example, for vitamin E (tocopherol), the natural form is not identical to the synthetic form. The natural form is composed of 4 RRR-tocopherols (α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol) and 4 tocotrienols. In industrial synthesis, the chemical reaction is not as specific as the nature biosynthesis. For example, instead of selectively obtaining alpha tocopherol RRR alone, a racemic mixture of the 8 chemically possible isomers is obtained (RRR + RRS + RSS ...). A report from the American Institute of Medicine has shown that vitamin E of RRR configuration, the natural form, has a higher biological activity than the other forms, and was therefore retained mainly in plasma and tissues [3]. The vitamins B, except the vitamin B12, which is formed exclusively by bacteria, are synthesized mainly in plants [4,5]. Plant vitamins of the B complex exhibit greater chemical and biochemical diversity than their laboratory analogues (synthetic vitamins) [4,6]. Indeed, the natural vitamins extracted from plants such as: fruits, aromatic herbs, etc. … have a form compatible with human physiology and are associated with other natural compounds present in original matrix due to extraction process such as: co-factors, minerals, polyphenols, etc. … This combination is similar to a food matrix, promoting the synergy of the active ingredients present in the extract.
Vitamins have an antioxidant activity which prevent from oxidative stress phenomena that could happened in human body. Oxidative stress corresponds to an imbalance between the production of free radicals and the ability of antioxidants to inhibit toxic compounds before they cause damage to cells. An imbalance of the antioxidant/oxidant in favor of oxidants induce an oxidative stress status. This oxidative stress is responsible for many ageing and ageing-associated diseases (cardiovascular and neurodegenerative pathologies, cancer, diabetes, etc.)...[7,8]. For this reason, industrialists from different sectors such as cosmetic, nutraceutical and pharmaceutical are constantly looking for new antioxidant molecules to reduce the effects of oxidative stress. This physiological imbalance is regulated by internal natural defense systems composed of enzymes (superoxide dismutases (SOD), catalase, glutathione peroxidases, thioredoxin / thioredoxin reductase pair, etc.). And also, this defense system can be reinforced by the diet which contains antioxidant molecules (vitamins C and E, polyphenols, glutathione, uric acid, bilirubin, ...) and proteins (transferrin, ferritin, ceruleoplasmin) which maintain the transition metals in an inactive state for the formation of Reactive Oxygen Species (ROS). Certain trace elements such as copper, zinc, selenium is essential for the activity of antioxidant enzymes (Cu, Zn-SOD, MnSOD, SeGPx).
Epidemiological studies consistently show that increased consumption of plant-based, antioxidant-rich foods, i.e., fruits, vegetables, whole grains, and nuts, is associated with a reduced risk of developing chronic disease [9,10]. Many naturally occurring compounds with antioxidative action are now known to protect cellular components from oxidative damage and prevent diseases [11,12]. Numerous studies demonstrate that a great number of medicinal and aromatic herbs, as well as fruits and leaves of some berry plants, biosynthesize phytochemicals possessing antioxidant activity and may be used as a natural source of free radical scavenging compounds [10].
Thus, the objectives of the present study were to determine and compare the antioxidant activities of Arkovital® Pur’Energie which represent food supplement containing natural vitamins and minerals, with a synthetic food supplement which contains only synthetic vitamins and minerals. The tested products having the same composition: 9 vitamins (C, B1, B2, B3, B5, B6, B8, B9, E) and 5 minerals (iron, selenium, zinc, manganese, chrome). The antioxidant activity was determined using several methods : Trolox Equivalent Antioxidant Capacity (TEAC) with ABTS•+ Radical cation; Ferric Reducing Antioxidant Power (FRAP); DPPH radical scavenging; Oxygen Radical Absorbance Capacity measurement (ORAC) and PAOT-Liquid® technology which is able to measure the total antioxidant capacity [13,14].
The effect of natural and synthetic supplements on volunteers was monitored using electrochemical method for evaluating skin oxidative stress status (SOSS) with PAOT Skin® score [7]. In addition, we also investigated during 14 days for two groups, the effect of natural and synthetic supplements intake on the level of two oxidative stress biomarkers (vitamin C and vitamin E) and on vitamin B6 in blood.
Materials
Chemicals: Trolox and Gallic acid as antioxidants; 2, 2-diphenyl-1-picrylhydrazyl (DPPH) ; Ethyl alcohol C2H5OH; 2,4,6-tri (2-pyridyl)-1,2,5-triazine (TPTZ-Fe3 +) ; potassium persulfate, ammonium salt of 2,2'-azinobis- acid (3-éthylbenzothiazoline- 6-sulfonic acid) (ABTS); monobasic sodium phosphate 2 H2O; hydrogenophosphate di-sodium 12 H2O; 2,2' Azobis (2 metyl-propionmidine) dihydrochloride)(AAPH); Fluorescein were all purchased from Sigma, (Lezennes, France). Electrocardiogram (ECG) conductive gel was provided by Dermedics (Veauche, France).
Supplements: Food supplements which are: Arkovital® Pur’Energie (containing natural vitamins and minerals), and a synthetic form (vitamins and minerals from chemical synthesis) were supplied by Arkopharma, Carros, France.
Arkovital® Pur’Energie, is a formula without chemical ingredients composed of: Acerola cherry juice powder (Malpighia punicifolia L. or Malpighia glabra L.) - Concentrate of plant extracts titrated in vitamins and minerals [amla fruit (Phyllanthus emblica L.), guava (Psidium guajava L.), holy basil leaf (Ocimum tenuiflorum L.), curry tree leaf (Murraya koenigii (L.) Spreng.), lemon (Citrus limon (L.) Burm. f.)] - sorbitol (from grains) - Anti-caking agent: magnesium stearate (from vegetable oils) (Table 1).
Table 1. Vitamins and minerals composition (mg or µg) of Arkovital® Pur’Energie and the synthetic supplement and Nutrient Reference Value of each compounds (%).
Vitamins and minerals
|
1 capsule Arkovital® Pur’Energie or
synthetic supplement |
Nutrient Reference Value (%) |
B1 (mg) |
0.65 |
59 |
B2 (mg) |
0.83 |
59 |
B3 (mg) |
5.5 |
34 |
B5 (mg) |
5.5 |
92 |
B6 (mg) |
4.2 |
300 |
B8 (µg) |
28 |
55 |
B9(µg) |
110 |
55 |
C (mg) |
72 |
89 |
E (mg) |
15 |
123 |
Iron (mg) |
5 |
35 |
Selenium (µg) |
19 |
35 |
Zinc (mg) |
2.1 |
21 |
Manganese (mg) |
0.52 |
26 |
Chrome(µg) |
32 |
80 |
The synthetic form is a supplement composed of vitamins and minerals from chemical origins: B1 Vitamin - B2 Vitamin - B3 Vitamin - Calcium pantothenate (B5 Vitamin) - B6 Vitamin - B9 Vitamin - C Vitamin - E Vitamin - B8 Vitamin - Iron fumarate - Zinc gluconate - Sodium selenite - Manganese gluconate - Sodium chloride chromium - Bulking agent: cellulose (Table 1).
Methods
Samples preparation
Solutions of each supplement (1 mg/ml) were prepared in Ethyl alcohol C2H5OH and mixed with vortex during 1 min. These solutions were putted under agitation for 30 minutes and stored at 25 ± 1°C until analysis (Maximum storage duration 24 hours).
Physicochemical characterization
Total antioxidant power measurement (PAOT Score)
PAOT (Pouvoir AntiOxydant Total) Liquid® is a patented technology (WO 2020/109736A1) allowing total antioxidant capacity determination in various matrices, such as raw materials and processed food products, cosmetic and medicinal preparations, biological fluids or plant extracts [15]. The measurement was carried out in a reaction medium (1 mL physiological solution at pH ranging from 6.7 to 7.2, temperature 24- 27°C) containing a molecule in a free radical state called mediator (M•). Two microelectrodes, one is the working electrode and the second is the reference electrode, were then immersed in the medium. After addition of 20 μL of pure antioxidants (1 mM final) or studied supplements samples, PAOT-liquid® activity was estimated by registering electrochemical potential modifications in the reaction medium (due to changes in the concentration of oxidized/reduced forms of the mediator M• during reaction with antioxidants as AOX):
(oxidized mediator M• + AOX -→ reduced mediator M + oxidized AOX) [13].
Results were calculated according to the following formula:
Antioxidant activity = ((EPproduct 10 - EPcontrol 0)/ EPcontrol 0)×100%
Where EP control 0 was the electrochemical potential at time 0 and EP product 10 the electrochemical potential obtained after 10 min registration in presence of tested antioxidants or studied supplements samples. Gallic acid was used as a standard and results were expressed as mg Gallic acid equivalents (GAE) L−1 [14].
Anti-radical activity against the radical ABTS • +
The ABTS radical cation scavenging activity was measured according to the method described by Re et al. [16] with slight modifications. In brief, ABTS solution was generated as follows: 6.62 mg of potassium persulfate and 38.4 mg of ABTS reagent were weighed in a glass beaker, 10 mL distilled water was added and then the mixture was perfectly mixed. This solution was kept away from light and let stand for 16 h at 25 ± 2°C to yield a blue-green colored solution containing the ABTS cation radical. Afterward, the ABTS•+ solution was diluted with absolute ethanol until reaching an absorbance of 0.70 ± 0.05 at 734 nm [17]. In the assay, 100 µL sample extract, standard (150-5 µmol trolox/L) and 600 µL ABTS* working solution were vortexed for 30 s in a reaction tube. The absorbance of the lower layer was determined at 734 nm exactly 2 min after starting shaking.
Anti-radical activity against the radical DPPH • +
Antioxidant capacity of supplements was determined by the DPPH (free radical 2,2-diphenyl-1- picryhydrazyl) assay as initially described by Tadolini et al. [18]. All complete details about the protocol were provided in published paper ([19]. Briefly, 2 mL of 0.1 mM DPPH methanolic solution was added to 0.5 mL extract sample at different concentrations (0.025, 0.05, 0.1, 0.5, 1, 5, 10, 100 mg/mL). The mixture was thoroughly stirred and incubated in the dark for 30 min at 25 ± 2°C. After that, absorbance of the mixture was measured at 517 nm by UV/visible spectrophotometry. Trolox was used as standard, and the antioxidant capacity was expressed in “mg Trolox Equivalent (TE) per gram” (mg TE g-1).
Ferric reducing ability of plasma measurement (FRAP)
One assay used to assess the antioxidant capacity was the ferric reducing antioxidant power (FRAP) assay, in order to determine the ferric reducing activity of supplements. In a reaction tube, 100 µL of samples solution, standard, blank and 600 µL of FRAP reagent, consisting of ferric chloride and TPTZ in acetate buffer (pH 3.6), were shaken on a thermoshaker (25 ± 1°C, 1400 rpm). After 6 min of shaking, the mixtures were transferred into half micro-cuvettes (1.5 ml) and centrifuged for 30 s at 1000 g to separate the layers. Finally, the absorbances of the lower layer of samples standards and blank at 595 nm were measured exactly 8 min after starting shaking [17]. Trolox was used as standard, and the antioxidant capacity was expressed in “mg Trolox equivalent per gram” (mg TE g-1).
Oxygen radical absorbance capacity measurement (ORAC)
In the oxygen radical antioxidant capacity (ORAC) assay, AAPH (2,2-azobis [2-amidinopropane] dihydrochloride) was used as a peroxyl radical generator and fluorescein as a fluorescent probe. Filters were used to select an excitation wavelength of 485 nm and an emission wavelength of 535 nm. A total of 175 µL of a mixture containing fluorescein (3 µM), and AAPH (221 mM) was injected into each well of the microplate. 25 µL of diluted sample, blank, or Trolox calibration solution (50-200 µM) were added. The fluorescence at 37°C was recorded every 2 min for 1 hour. The final ORAC value was calculated from the net area under the fluorescence decay curve [20]. Trolox was used as standard, and the antioxidant capacity was expressed in “mg Trolox equivalent per gram” (mg TE. g-1).
Clinical characterization
Panel selection criteria and patients’s consent
For each study presented in this paper, the trial objectives, study design, risks, and benefits were explained and written informed consent was obtained from all participants. As blood samples were needed for analysis of vitamin C, E and B6, the full study protocol was approved by the institutional ethics committee of the Liège University Hospitals (reference 2017/342) and conducted in accordance with the 1964 Declaration of Helsinki and the European guidelines for Good Clinical Practice.
A first study (Study 1) was conducted on 6 volunteers who meet the inclusion criteria. The panel was composed of six males (45 ± 7 years), fasted for 12 h, smoker or occasional smoker which have vitamin C deficiency and fatigue signs. All subjects were not exposed to sun before experimentation and did not apply products with an antiwrinkle/anti-ageing/antioxidant action on the forearms during two weeks before the study started. They were also not allowed to consume fruits or fruit juices, or alcohol, or medication (i.e., analgesic, anti-inflammatory, antibiotic), or nutritional supplements or to practice sport during the 72-h preceding the experimentation.
A second study (Study 2) was conducted on 50 volunteers (32 ± 12 years), in the same condition as Study 1.
Supplements taking protocol
In the two studies, volunteers were divided in two groups: two groups of 3 volunteers in the Study 1 and two groups of 25 volunteers in the Study 2. In each study Arkovital® Pur’Energie (Arkopharma, Carros, France) was taken by the first group (Group 1, Study 1 n=3; Group 1, Study 2, n=25) and the synthetic supplement was taken by the second group (Group 2, Study 1, n=3; Group 2, Study 2, n=25). For each study duration (14 Days), 2 capsules or pills per day was taken by each volunteer in each study (each product provides 144 mg of vitamin C per day: two capsules or pills of 72 mg).
Skin antioxidant activity (PAOT Skin Score®)
PAOT (Pouvoir AntiOxydant Total)-Skin Score®, reflecting the redox equilibrium between skin antioxidants and oxidants, was measured using the equipment described in Figure 1 (PAOTSCAN Analyzer) (patent number: WO 2020/109736 A1). For the determination of antioxidant and pro-oxidant activities, the same patch, containing ECG conductive gel, mediator M, and microelectrodes as described by Pincemail et al., was directly put in contact with skin volunteers [7].
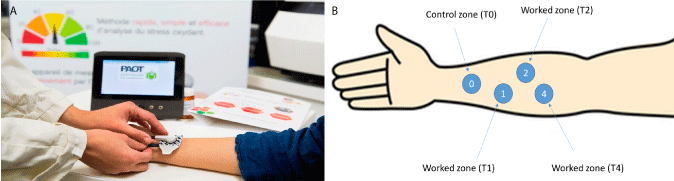
Figure 1. (A). Photography of the electrochemical equipment (PAOTSCAN Analyzer) for measurement of SOSS in a non-invasive way. For details, see the Material and Methods section [7]. (B). Illustration of control and worked area for a measurement of skin Oxidative Stress Status.
In Study 1, a group of 6 males volunteers (45 ± 7 years), fasted for 12 hours, as described in selection criteria, were recruited for determining of PAOT-Skin Score® values for individuals with vitamin C deficiency. Measurements before supplements intake were done for the determination of the basal PAOT-Skin Score® (T0).
The PAOT-Skin Score® measurements were made at the forearm participants before supplements intake and also 1, 2, 4 hours and 14 days after supplements intakes.
In study 2, a group of 50 volunteers (32 ± 12 years) were selected with study 1 selection criteria and with the same measurements protocols. Volunteers participated for the measurement of PAOT-Skin Score® at 2 times. The firsts measurements were made before any supplements intakes (day 0) and the seconds measurements were made after 14 days of supplements intakes (day 14).
In the two studies, to calculate the antioxidant efficiency, we took into consideration the evolution of the oxidative stress status of the skin (without supplement intake), which is due to the external contribution (dinner breakfast, lunch, smoking ...) and this according to the following equation:
Antioxidant Efficiency (%) = ((PAOT product th -PAOT Product 0h) / PAOT product 0h) × 100
With
PAOT Product th: Total antioxidant Power of skin at th of supplements intakes,
PAOT Product 0h: Total antioxidant Power of skin at 0h (before supplements intakes),
Measurement of vitamin C, E and B6: in blood
In study 1, blood samples were taken before supplements intakes and 1, 2, 4 hours and 14 days of supplementation. These samples were collected on heparinized lithium tubes for the determination of vitamins C, E and B6 in volunteer’s blood.
Measurements of vitamin C and E
For all participants, vitamins C and E were assessed in plasma or whole blood according to analytical protocols as described in detail in Passerieux et al., and Pincemail et al., [8,21]. For vitamin C, 0.5 mL plasma (ethylenediaminetetracetic acid (EDTA) blood) was immediately transferred to ice-cold tubes containing 0.5 mL of 10% metaphosphoric acid. The whole mixture was frozen on dry ice. Analyses were performed on the day of blood collection by a spectrophotometric method using the reduction of 2,6-dichlorophenolindophenol (Perkin Elmer Lambda 40, Norwalk, USA)[7,8,22]. Plasma vitamin E (α- and γ-tocopherols) was assayed by HPLC procedure (Alliance,Waters, USA) coupled with a diode array detector (PDA 2996, Waters, USA) using Chromsytemskits (32 000, 34 000, and 68 000) [7,8,23,24].
Measurement of vitamin B6
Measurement of plasma vitamin B6 concentration [using measurement of plasma PyridoxaL (PL)] was performed according to the method of Ubbink et al. [25]. Briefly, the method was based on a precolumn pyridoxal phosphate semicarbozone derivatization, followed by isocratic HPLC as described by Ubbink et al. [25,26].
Statistical analysis
A statistical comparison test was made between the values obtained at day 0 T1 h, T2 h, T4 h and T14 days and those obtained at T0 (Day 0) to assess the efficacy of the products. Shapiro-Wilk test, threshold at 1% was used for verification of the normality of distributions. The statistical analysis of measured parameters evolution during the study was performed with ANOVA test (Using Tukey Method). The significance threshold was fixed at 5% (p < 0.05). The statistical analysis was performed using Minitab software.
PAOT liquid scores of supplements
Table 2 shows total antioxidant activity expressed with PAOT Score® for Arkovital® Pur’Energie and synthetic supplement. Results are expressed for 1 gram of each tested samples. Arkovital® Pur’Energie had the best score (mean value 104.67 ± 8.61) in compared to synthetic supplement (mean value 80.63 ± 2.10). These results shown that Arkovital® Pur’Energie had a highest antioxidant activity then Synthetic supplement.
Table 2. The total antioxidant power (PAOT) expressed with PAOT-LIQUID® SCORES / gram of supplement
|
PAOT-Liquid® Scores / (g) |
PAOT-Liquid® mg (GAE) g−1) * |
Synthetic supplement |
80.63 ± 2.10 |
0.034 ± 0.0009 |
Arkovital® Pur’Energie |
104.67 ± 8.61 |
0.045 ± 0.0037 |
|
|
0.544 ± 0.016 [14] * |
Naringin |
|
0.053 ± 0.0003 [14] * |
HesperIdin methyl chalcone |
|
0.052 ± 0.0006 [14] * |
Per gram of 1mM reference solution *
For comparison, Trolox tested at 1 mM solution which is the antioxidant reference used in most in vitro assays, had a value of 544.16 mg (GAE) L−1. At least, both naringin 1 mM solution (53.28 mg (GAE) L−1) and hesperidin methyl chalcone 1 mM solution (51.85 mg (GAE) L−1) from the flavanone group presented a score which was below those of tested supplements [14].
As shown in Table 2, the PAOT-Liquid® Scores were also expressed as equivalence references with Gallic acid. The total antioxidant powers (PAOT Score®) of 1 gram of Arkovital® Pur’Energie and synthetic supplement were equivalent to 0.034 ± 0.0009 mg (mean value) and to 0.045 ± 0.0037 mg (mean value) of Gallic acid respectively.
The antioxidant activities of supplements were also evaluated using FRAP, TEAC, DPPH and ORAC assay. The results varied according to the assay used (Table 3). For these four methods, Trolox (T) was used as a standard and the antioxidant capacity was calculated as mg Trolox equivalents (TE) per g of supplement.
Table 3. Antioxidant capacity of synthetic supplement and Arkovital® Pur’Energie measured with several methods: DPPH, TEAC, FRAP and ORAC expressed with mg Trolox equivalent / gram of supplement (mg (TE)/g)
Samples |
DPPH Assay (mg (TE) g-1) |
TEAC (ABTS) Assay (mg (TE) g-1) |
FRAP Assay (mg (TE) g-1) |
ORAC Assay (mg (TE) g-1) |
Synthetic supplement |
18.79 ± 1.38 |
28.82 ± 2.87 |
31.22 ± 2.24 |
134.73 ± 10.85 |
|
70.07 ± 19.31 |
36.57 ± 0.26 |
180.07 ± 16.38 |
185.94 ± 13.88 |
Trolox equivalent antioxidant capacity (TEAC) using radical ABTS•+
Trolox Equivalent Antioxidant Capacity (TEAC) assay is based on the scavenging of the 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical (ABTS(*)) converting it into a colorless product. The addition of antioxidants will reduce this radical and cause discoloration of the mixture. Using the TEAC assay (Table 3), Arkovital® Pur’Energie showed higher antioxidant activity (36.57 ± 0.26 mg (TE) g-1) than synthetic supplement (28.82 ± 2.87 mg (TE) g-1) (Table 3) (1.3 times higher than synthetic supplement). The antiradical power of 1-gram supplement Arkovital® Pur’Energie against anti-radical ABTS+• radical is equivalent to 36.57 mg of Trolox. More the value is high, more the supplement is efficient.
It is already known that vitamins have antioxidant activity, and according to Gliszczyńska-Świgło et al. pyridoxine (active form of vitamin B6) and ascorbic acid were able to scavenge ABTS•+ radical cation although pyridoxine antioxidant activities expressed as the TEAC value were relatively low as compared to vitamin C [27]. TEAC values of thiamine and folic acid are comparable to such natural antioxidants as some carotenoids [28] and polyphenols, e.g., naringin and monosubstituted benzoic acid derivatives [29,30]. Altogether, in Gliszczyńska-Świgło study, the results obtained indicate that some water-soluble vitamins, may act as potent antioxidants although they scavenge free radicals such as ABTS•+ radical cation relatively slowly. However, antioxidant activities of water-soluble vitamins could become important in view of nutritional supplementation and fortification of food especially with thiamine and folic acid [27].
Anti-radical activity against the radical DPPH • +
The two complements (Arkovital® Pur’Energie and synthetic supplement) reacted in this assay and scavenged the DPPH• radical (Table 3). Supplement samples lead to a decrease in the DPPH• absorbance caused by the presence of antioxidant vitamins. DPPH• is a free radical (with purple color) reduced to a yellow compound in the presence of anti-free radical compounds (antioxidants). The intensity of the color, measured in a spectrophotometer, is inversely proportional to the anti-radical activity of the compounds which it is desired to determine the activity. More the value is high, more the supplement is efficient. The average of 70.07 ± 19.31 was registered for Arkovital® Pur’Energie compared to 18.79 ± 1.38 for synthetic supplement. The anti-radical activity of 1 gram Arkovital® Pur’Energie against DPPH• radical is equivalent to 70.07 mg of Trolox.
Ferric reducing ability of plasma measurement (FRAP)
The two complements (Arkovital® Pur’Energie and synthetic supplement) reduced the ferric di-TPTZ complex used in the FRAP assay (Table 3). FRAP method is based on the reduction of ferric ion (Fe3 +) to ferrous ion (Fe2 +) by an antioxidant compound present on the tested supplement. More the value is high, more the supplement is efficient. The two supplements showed a noteworthy ferric reducing activity. Arkovital® Pur’Energie showed significantly higher antioxidant activity, up to maximum five times higher (180.07 ± 16.38 mg TE g-1) than synthetic supplement (31.22 ± 2.24 mg TE g-1). The reducing power of 1 gram Arkovital® Pur’Energie is equivalent to 180.07 mg of Trolox. The ferric reducing activity is mainly influenced by the size of the conjugated double bond (CDB) system [17]. According to Gliszczyńska-Świgło, with FRAP assay, Peridoxine did not revealed the ability to reduce Fe3+ to Fe2+, unlike vitamin C used as antioxidant reference [27].
Oxygen radical absorbance capacity measurement (ORAC)
Antioxidant activity measured by ORAC assay in Table 3 showed the same attendance as the previous assays. Using ORAC assay, synthetic supplement gave lower antioxidant activity than Arkovital® Pur’Energie. The protective power of 1 gram of Arkovital® Pur’Energie is equivalent to 185.94 ± 13.88 mg TE compared to 134.73 ± 10.85 mg TE for the synthetic supplement. Measurement of protective power of the fluorescein by the ORAC method is based on the detection of fluorescence drop of fluorescein (FL), due to its reaction with peroxyl radical ROO*, in a food matrix containing antioxidant compounds. More antioxidant activity is high, more the fluorescence decrease.
As mentioned above, the assessment of the antioxidant capacity of food samples requires the use of various methods [19,31]. In the present study, we have applied the FRAP, TEAC, DPPH and ORAC assay to supplements samples. Studied supplements (9 vitamins and 5 minerals) gave highest antioxidant activity with ORAC methods which is due to the presence of a large fraction of hydrophilic vitamin (vitamin C principally) known for its high antioxidant activity. Arkovital® Pur’Energie showed the highest antioxidant capacity regardless of the method used. The presence of active forms of vitamins from plants extract and their higher bioavailability recognized in the literature [1,2,32-34] can explain the important antioxidant capacity of Arkovital® Pur’Energie. According to Muller et al., hydrophobic compounds were responsible for the high FRAP, and TEAC values of samples such as vitamin E and apolar polyphenols. So low values of FRAP, and TEAC in our study can be explain by small fraction of hydrophobic antioxidants compared to hydrophilic antioxidants in studied supplements.
Study of oxidative stress status (PAOT SCORE) evolution in volunteers
As shown in Figure 2, the basal PAOT-Skin Score®, for the study 1, of the two groups (Arkovital® Pur’Energie and synthetic supplement) did not shown a significant difference (p<0.05) (mean: 51.06 and 48,81 respectively for Arkovital® Pur’Energie and synthetic supplement groups).
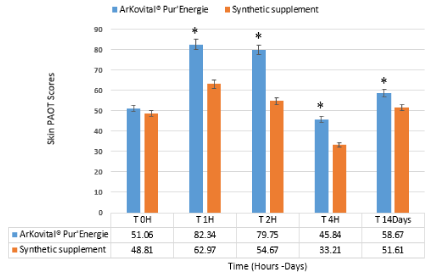
Figure 2. Evolution of PAOT-Skin Score® in volunteers before and after 0, 1, 2, 4 hours and 14 days of Arkovital® Pur’Energie and synthetic supplement intake, study 1. Skin PAOT scores were calculated from n=3 for Arkovital® Pur’Energie and n=3 for Synthetic supplement. *significant difference (p<0.05) between synthetic and natural supplements at the same time. Products supplied by Arkopharma, Carros, France
The basal PAOT-Skin Score® rised up to 82.34 and 79.75, respectively, 1 and 2 hours after intake of natural vitamins and minerals supplement containing 144 mg of vitamin C and 30 mg of vitamin E (n = 3). However, statistical analysis revealed that such increases were not significant. The PAOT-Skin Score® returned to its basal value after four hours. Similar findings were found but to a lesser extent when using synthetic vitamins and minerals as a supplement (Figure 2). These results have been published previously in the study of Pincemail et al. [7]. After 14 days of supplement intake, the PAOT-Skin Score® remained higher than the basal level. The PAOT-Skin Scores® were 58.67 and 51.61 after 14 days of supplement intake for Arkovital® Pur’Energie and the synthetic supplement, respectively. However, the results show a significant difference (p<0.05) in oxidative status (PAOT Score) between the two products (Natural and Synthetic). The activity of the products is observed from the first measurements at 1 hour. A peak of total antioxidant power (PAOT Score®) is observed between 1 and 2 hours for both products (Natural and Synthetic) due to the action of antioxidants provided by the supplements.
As showed in Figure 3 (A, B), both natural and synthetic supplements increased the antioxidant efficiency, but, clearly, the natural vitamins (Arkovital® Pur’Energie) shown the higher efficiency compared with synthetic vitamins. A peak of antioxidant effectiveness is observed about 1 h and 30 minutes after products taking. One hour after taking the supplements, the Arkovital® Pur’Energie product was twice as effective as the synthetic supplement. The same trends were registered at 2 and 4 hours after the supplementation.
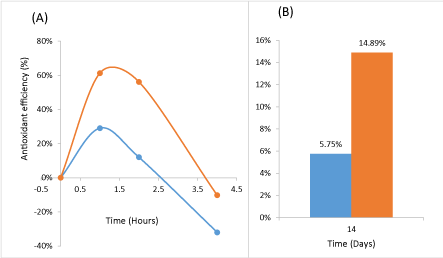
Figure 3. Antioxidant efficiency evolution of product during 4 hours after Arkovital® Pur’Energie and synthetic supplement intake (A) and after 14 days of Arkovital® Pur’Energie and synthetic supplement intake (B) for two groups: Arkovital® Pur’Energie group: n=3 and synthetic supplement group: n=3, study 1.
The supplements shown a significant antioxidant efficiency with an increase of PAOT skin scores (n=6) due to antioxidant vitamins contained in supplements. The analysis showed a significant difference in skin antioxidant efficiency between natural and synthetic products. The antioxidant efficiency of Arkovital® Pur’Energie increased by more than 60% between 1 and 2 hours after taking, while at the same time, the efficiency was 30% for the synthetic supplement. On day 15, the basal antioxidant state in volunteers supplemented with Arkovital® Pur’Energie increased by 2.6 times compared to volunteers who take the synthetic supplement.
As shown in Figure 4 corresponding to the study 2, the activity of supplements was observed from the first measurements at 0 day and at days 15 after 14 days of supplements intake. A peak of total antioxidant power (PAOT Score®) was observed after 2 weeks for both supplements (Arkovital® Pur’Energie and synthetic supplement), this is due to the action of antioxidants provided by products. However, the results show a significant difference in oxidative status between the two products. The difference between control and treated panel for Natural Arkovital® Pur’Energie represented more than 7 PAOT score units compared to 3 PAOT Score units for synthetic supplement.
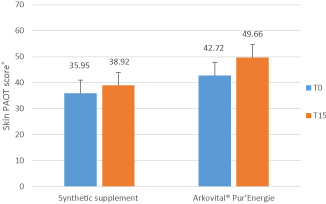
Figure 4. Evolution of the antioxidant / oxidant activity (Oxidative Stress Status) of volunteer’s skin before (T0) and at days 15 after 14 days of supplements intake, study 2. Arkovital® Pur’Energie group: n=25 and synthetic supplement group: n=25
Ascorbic acid concentration measurement in human blood: Study 1
Figure 5 shows the mean values observed for vitamin C in blood, which is an oxidative stress biomarker's.
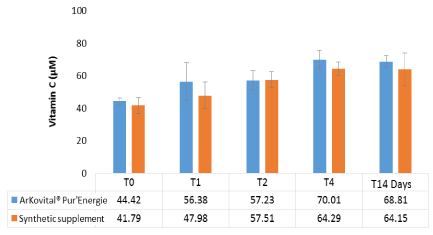
Figure 5. Kinetic of vitamin C in volunteers during 4 hours (0,1,2,4 hours) and after14 days of supplements intake in Arkovital® Pur’Energie group: n=3 and in Synthetic supplement group: n=3
As shown on figure 5, the initial values (T0) were: 44.42 and 41.79 µM respectively for Arkovital® Pur’Energie and synthetic supplement groups before supplements intake. The reference values of vitamin C in human blood are between 35 and 106 µM [8,21]. When compared to reference values defined as previously described, the concentration of all antioxidants was within the normal range. The Vitamin C kinetics in Figure 5 shown slow increase until 4 hours where a maximum of vitamin C levels was registered at 70.01 and 64.29 µM respectively for Arkovital® Pur’Energie and synthetic supplement groups. After 14 days, the vitamin C levels of the two volunteers’ groups were improved by more than 50%. However, no significant difference was observed between the two supplements (p<0.05). Ascorbic acid (AA) is not a synonym for vitamin C, though it certainly has vitamin C (antiscorbutic) properties (dehydroascorbic acid (DHAA) is the other biologically active form). Foods generally contain both biologically active forms of vitamin C [1,35,36], yet most synthetic vitamin C only contains isolated ascorbic acid [1,37]. Jacob has written, ‘The bioavailability of vitamin C in food and “natural form” supplements is not significantly different from that of pure synthetic AA’. According to Mangels et al. since serum ascorbic acid levels were at similar levels after various vitamin C containing foods and synthetic ascorbic acid were consumed, the bioavailability is similar [1,35,37]. The study itself appears to be an excellent one, but its conclusion ignore that fact that it may be possible that DHAA or other food constituents associated with natural vitamin C may have positive effects other than raising serum ascorbate levels [1]. The same results were obtained in our study because there is no significant effect between concentration of vitamin C in blood for Arkovital® Pur’Energie and synthetic supplement groups but it is possible that the combination of natural vitamins and minerals with co-factors present in the original matrix may have positive effects by improving bioavailability and also an increase in antioxidant activity as seen in the results presented before.
Vitamin E concentration measurement in human blood: Study 1
Figure 6 shows the mean values observed for vitamin E in blood which is the second oxidative stress biomarkers studied in this paper. As shown on figure 6, the vitamin E values at the different measurement times were between 22 and 25 µM for the two supplements groups. The reference values of vitamin E in human blood are between 19.9 µM-44.6 µM [8,21]. When compared to these reference values, the concentration of vitamin E was within the normal range.
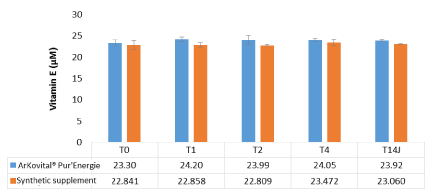
Figure 6. Kinetic of vitamin E in blood volunteers for 4 hours (0,1,2,4 hours) and after 14 days of supplements intake: Arkovital® Pur’Energie group: n=3 and Synthetic supplement group: n=3
Natural vitamin E as found in foods is [d]-alpha tocopherol, whereas chemical synthesis produces a mixture of eight epimers [1,38]. Vitamin E is a group of eight compounds (α, γ, β and δ tocopherols and α, β, δ, γ and tocotrienols), which differ in their methyl substitution and saturation. The predominant form in the body, is α-tocopherol, comprising over 90% of vitamin E. This form has been widely researched owing to its antioxidant and non-antioxidant functions [39]. Also, the peculiarity of natural vitamin E is the presence of tocotrienols which is not found in synthetic vitamin E, i.e., in the majority of food supplements. Natural vitamin E (RRR-α-tocopherol) found in feeds is not identical to synthetic vitamin E (all-rac-α-tocopheryl acetate), and has higher biological activity than synthetic vitamin E [40-43]. Our kinetic study shown no significant variation of vitamin E in blood and no significant difference (p<0.05) between natural and synthetic supplement intake, but these results did not demonstrate that the synthetic and natural forms had the same effect in human body. Most studies on vitamin E attempt to demonstrate that although synthetic vitamins have some of the benefits of natural vitamins, they really do not replace all the benefits of natural ones [1].
Vitamin B6 concentration measurement in human blood: Study 1
Figure 7 shows the mean values observed for vitamin B6 in blood volunteers which is involved in production of hemoglobin and in supporting the immune system. The reference values of vitamin B6 in human blood are between 10-140 nM.
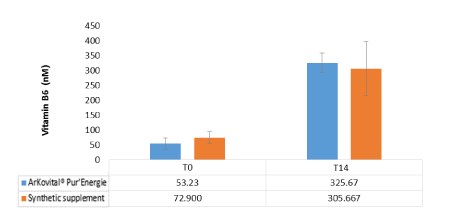
Figure 7. Blood Vitamin B6 (Pyridoxal phosphate) values of volunteers before and after 14 days of supplements intake for the two groups: Arkovital® Pur’Energie group: n=3 and Synthetic supplement group: n=3
When compared to these reference values, the concentration of vitamin B6 was within the normal range. After 14 days of taking, the rate of B6 increased 6 times for volunteers who took the Arkovital® Pur’Energie (mean from 53.27 nM at T0 hours to 325.67 nM at T14 days) product and only by 4 times for those of synthetic supplement (means from 72.90 nM at T0 hours to 305.66 nM at T14 days). Vitamin B6 intake in synthetic supplement and Arkovital® Pur’Energie for 2 capsules per day was 4.2 mg/capsule and is represent 300% of Nutrient Reference Value which explain the big increase of vitamin B6 in volunteer’s blood. According to Vinson et al., an animal study found that natural food complex vitamin B6 was absorbed 2.54 times more into the blood and was retained 1.56 times more in the liver than an isolated vitamin [44] which is consistent with our results. An understanding of the various forms and quantities of these forms in foods is important in the evaluation of the bioavailability and metabolism of vitamin B6. One of the forms in which vitamin B-6 exists is ‘5′0-(beta-D-glycopyransosyl) pyridoxine [1]. Only plant foods have been found to contain this interesting form of vitamin B6’ [38]. The most common form in vitamin pills is pyridoxine hydrochloride which is not naturally found in food [45]. At least one synthetic vitamin B6 analogue has been found to inhibit natural vitamin B6 action [46].
According to Lindschinger et al., a study which aimed to understand the mode of action of natural vitamin B complexes compared to synthetic vitamin B complexes in healthy volunteers, shown that unlike synthetic vitamin B group, the natural vitamin group showed a significant increase in blood vitamin B12 levels compared to the baseline, both at the end of 6 weeks of supplementation and after the elimination phase that followed, indicating a sustained effect of natural vitamin B12.
The fact that natural B vitamins lowered homocysteine levels even more significantly than synthetic analogs may be due to the even greater complexity of natural vitamin complexes of plant origin and their interactions. This is an aspect that should not be underestimated, since the B vitamins synthesized by plants have a much wider spectrum of bound and chemically heterogeneous organic compounds than their chemically degenerate laboratory analogues [4].
In the present work, we have applied the FRAP, TEAC, DPPH and ORAC assays to supplements samples. Arkovital® Pur’Energie showed the highest antioxidant activities for all tested methods. Also, both supplements containing 9 vitamins and 5 minerals gave highest antioxidant activity with ORAC methods due to the presence of a large fraction of hydrophilic vitamins (vitamin C principally) known for their high antioxidant activity. Similarly, the electrochemical PAOT measurement shown that Arkovital® Pur’Energie had the best score compared to the synthetic supplement which means that natural supplement had a highest antioxidant activity. The PAOT-Skin Scores® results show a significant difference in oxidative status (PAOT Score) between the Natural and Synthetic formula. For the study 1, the antioxidant activities of supplements were observed from the first measurements at 1 hour after intake. Peak of total antioxidant powers (PAOT Score®) were observed between 1 and 2 hours for both supplements due to the action of antioxidants provided by those supplements. After 14 days, the basal state of volunteers with Arkovital® Pur’Energie increased to 2.6 times compare to synthetic supplements group. This result was confirmed in the second study in which a peak of total antioxidant power was also observed after 2 weeks with a significant difference in oxidative status between the two products.
In the study 1, Vitamins C and E kinetics didn’t show significant variation in blood (p<0.05) between Arkovital® Pur’Energie and synthetic supplement due to the small groups (n=3 each group). But for vitamin B6 the rate at day 15 increased 6 times for volunteers who took Arkovital® Pur’Energie and only by 4 times for those of synthetic supplement.
It’s also important to remember that in natural foods, other constituents could be find such as DHAA or phytonutrients, and associated to natural form of vitamins, could improve the bioavailability of those vitamins and increased antioxidants in human bodies improving their self defaces against oxidative stress. The most of studies about Vitamin E help demonstrate that although synthetic vitamins have some of the benefits of natural vitamins, they really do not replace all the benefits of natural ones [1].
As perspectives, it would be very useful to measure biomolecules (vitamins C, E and B6) in urine and to validate the obtained results on bigger groups to better understand the notion of bioavailability of biomolecules (mainly vitamins E and C).
This work was carried out in collaboration between the authors. S. Meziane, M. Kaci and J. Magand designed the study, wrote the protocol, helped in the statistical analysis and wrote the first the manuscript. They performed the experimental work, managed the analyses of the study. M. Mathé, S. Raimond and JL. Yvon made the tested products and ensured their quality. The authors read and approved the final manuscript, and they have taken due care to ensure the integrity of the work.
We do not have any grant money and this work was not supported by grant funding.
All data and materials are fully available and are shown within the manuscript.
The necessary approval to conduct this study was obtained from the institutional ethics committee of the Liège University Hospitals and conducted in accordance with the 1964 Declaration of Helsinki and the European guidelines for Good Clinical Practice. Informed consent was obtained from all participants. A full explanation about the purpose of the study were given.
The authors give their consent for the publication to be published in the Journal.
The authors declare that they have no competing interests.
- Thiel R (2000) Natural vitamins may be superior to synthetic ones. Medical Hypotheses 55: 461-469.
- Kiyose C, Muramatsu R, Kameyama Y, Ueda T, Igarashi O (1997) Biodiscrimination of alpha-tocopherol stereoisomers in humans after oral administration. The American Journal of Clinical Nutrition 65: 785-789.
- Institute of Medicine (2000) 6 Vitamin E. In: Dietary reference intakes, pp: 186-283.
- Lindschinger M, Tatzber F, Schimetta W, Schmid I, Lindschinger B, et al. (2020) Bioverfügbarkeit eines natürlichen versus eines synthetischen Vitamin-B-Komplexes und deren Auswirkungen auf metabolische Prozesse. MMW-Fortschritte der Medizin 162: 17-27.
- Kennedy DO (2016) B vitamins and the brain: mechanisms dose and efficacy—a review. Nutrients 8: 68.
- Roje S (2007) Vitamin B biosynthesis in plants. Phytochemistry 68: 1904-1921.
- Pincemail J, Kaci M, Cheramy-Bien Defraigne JO, Meziane S (2019) Electrochemical Methodology for Evaluating Skin Oxidative Stress Status (SOSS) Diseases 7: 40.
- Pincemail J, Defraigne JO, Cheramy-Bien JP, Dardenne N, Donneau AF, et al. (2012) On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm. Redox Report 17: 139-144.
- Griel AE, Kris-Etherton PM (2006) Tree nuts and the lipid profile: a review of clinical studies. British Journal of Nutrition 96: S68-S78.
- Turan A, Celik I (2016) Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity in rats. International Journal of Biological Macromolecules 91: 554-559.
- Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, et al. (2009) Ficus carica L.: Metabolic and biological screening. Food and Chemical Toxicology 47: 2841-2846.
- Gülçin I, Bursal E, Şehitoğlu MH, Bilsel M, Gören AC (2010) Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum Turkey. Food and Chemical Toxicology 48-9: 2227-2238.
- Kaci M, Belhaffef A, Meziane S, Dostert G, Menu P, et al. (2018) Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. Colloids and Surfaces B: Biointerfaces 167: 165-175.
- Pincemail J, Kaci M, Kevers C, Tabart J, Ebabe ER, et al. (2019) PAOT-Liquid® Technology: An easy electrochemical method for evaluating antioxidant capacity of wines. Diseases 7: 10.
- Poutaraud A, Guilloteau L, Gros C, Lobstein A, Meziani S, et al. (2018) Lavender essential oil decreases stress response of horses. Environmental Chemistry Letters 16: 539-544.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26-10: 1231-1237.
- Müller L, Fröhlich K, Böhm V (2011) Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP: ABTS bleaching assay (αTEAC: DPPH assay and peroxyl radical scavenging assay. Food Chemistry 129: 139-148.
- Tadolini B, Juliano C, Piu L, Franconi F, Cabrini L (2000) Resveratrol inhibition of lipid peroxidation. Free Radical Research 33: 105-114.
- Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J (2009) Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chemistry 113: 1226-1233.
- Tabart J, Auger C, Kevers C, Dommes J, Pollet B, et al. (2018) The potency of commercial blackcurrant juices to induce relaxation in porcine coronary artery rings is not correlated to their antioxidant capacity but to their anthocyanin content. Nutrition (Burbank Los Angeles County Calif.
- Passerieux E, Hayot M, Jaussent A, Carnac G, Gouzi F, et al. (2015) Effects of vitamin C, vitamin E, zinc gluconate and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: a double-blind randomized controlled clinical trial. Free Radical Biology and Medicine 81: 158-169.
- Omaye ST, Turnbull JD, Sauberlich HE (1979) [1] Selected methods for the determination of ascorbic acid in animal cells tissues and fluids. Methods in Enzymology 62: 3-11.
- Zhao B, Tham SY, Lu J, Lai MH, Lee LK, et al. (2004) Simultaneous determination of vitamins C, E and β-carotene in human plasma by high-performance liquid chromatography with photodiode-array detection. J Pharm Pharm Sci 7: 200-204.
- Maury J, Gouzi F, De Rigal P, Heraud N, Pincemail J, et al. (2015) Heterogeneity of systemic oxidative stress profiles in COPD: A potential role of gender. Oxidative Medicine and Cellular Longevity 2015: 201843.
- Ubbink JB, Serfontein WJ, De Villiers LS (1985) Stability of pyridoxal-5-phosphate semicarbazone: Applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. Journal of Chromatography B: Biomedical Sciences and Applications 342: 277-284.
- Mackraj I, Thirumala G, Gathiram P (2009) Vitamin B6 deficiency alters tissue iron concentrations in the Wistar rat. Journal of Trace Elements in Medicine and Biology 23: 43-49.
- Gliszczyńska-Świgło A (2006) Antioxidant activity of water-soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chemistry 96: 131-136.
- Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA (1996) Antioxidant activities of carotenes and xanthophylls. FEBS letters 384: 240-242.
- Rice-evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Research 22: 375-383.
- Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine 20: 933-956.
- Frankel EN, Meyer AS (2000) The problems of using one‐dimensional methods to evaluate multifunctional food and biological antioxidants. Journal of the Science of Food and Agriculture 80: 1925-1941.
- Acuff RV, Dunworth RG, Webb LW, Lane JR (1998) Transport of deuterium-labeled tocopherols during pregnancy. The American Journal of Clinical Nutrition 67: 459-464.
- Vinson JA, Bose P (1988) Comparative bioavailability to humans of ascorbic acid alone or in a citrus extract. The American Journal of Clinical Nutrition 48: 601-604.
- Uchida E, Kondo Y, Amano A, Aizawa S, Hanamura T, et al. (2011) Absorption and excretion of ascorbic acid alone and in acerola (Malpighia emarginata) juice: comparison in healthy Japanese subjects. Biological and Pharmaceutical Bulletin 34: 1744-1747.
- Jacob R (1999) Vitamin C in modern nutrition. Health and Disease Shilis ME Olson JA Shike M, Ross AC eds 9th edition Philadelphia Lippincott Williams & Wiikins.
- Vanderslice JT, Higgs DJ (1991) Vitamin C content of foods: sample variability. The American Journal of Clinical Nutrition 54: 1323S-1327S.
- Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, et al. (1993) The bioavailability to humans of ascorbic acid from oranges, orange juice and cooked broccoli is similar to that of synthetic ascorbic acid. The Journal of Nutrition 123: 1054-1061.
- Shils ME, Olson JA, Shike M (1994) Modern nutrition in health and disease.
- Lodge JK (2005) Vitamin E bioavailability in humans. Journal of Plant Physiology 162: 790-796.
- Johansson B, Persson Waller K, Jensen SK, Lindqvist H, Nadeau E (2014) Status of vitamins E and A and β-carotene and health in organic dairy cows fed a diet without synthetic vitamins. Journal of Dairy Science 97: 1682-1692.
- Meglia GE, Jensen SK, Lauridsen C, Waller KP (2006) α-Tocopherol concentration and stereoisomer composition in plasma and milk from dairy cows fed natural or synthetic vitamin E around calving. Journal of Dairy Research 73: 227-234.
- Dersjant‐Li Y, Peisker M (2010) Utilization of stereoisomers from alpha‐tocopherol in livestock animals. Journal of Animal Physiology and Animal Nutrition 94: 413-421.
- Vagni S, Saccone F, Pinotti L, Baldi A (2011) Vitamin E bioavailability: Past and present insights.
- Vinson J (1989) Relative bioavailability of trace elements and vitamins found in commercial supplements. Nutrient Availability: Chemical and Biological Aspects 125-127.
- Leklem J, Shils M, Olson J, Shike M, Ross A (2010) Vitamin B6. Modern nutrition in health and disease.
- Nakano H, McMahon LG, Gregory III JF (1997) Pyridoxine-5′-β-d-glucoside exhibits incomplete bioavailability as a source of vitamin B-6 and partially inhibits the utilization of co-ingested pyridoxine in humans. The Journal of Nutrition 127: 1508-1513.







