Abstract
Scientists at universities across Iraq are actively working to report actual incidents and accidents occurring in their laboratories, as well as structural improvements made to improve safety and security, to raise awareness and encourage openness, leading to widespread adoption of robust Chemical Safety and Security (CSS) practices. This manuscript is the seventh in a series of seven case studies describing laboratory incidents, accidents, and laboratory improvements. In this study, we summarize unsafe practices involving the improper installation of placed ionic liquid sample with bad filling in split tube furnace using quartz crucible. Furnace was instilled to reach 1000 oC within four hours. Vapors of sample crystallization water confined inside the quartz tube and spilled out toward the furnace flanges due to high temperature. Pressure explosion occurs causes the ionic liquid to spill out and contaminated the quartz tube inner surfaces. Ionic liquid used in this study has a potential acute health effects: Hazardous in case of eye contact (irritant), of ingestion, of inhalation. Slightly hazardous in case of skin contact (irritant). Repeated or prolonged exposure to the substance can produce target organs damage. This requires placing the oven in a well-ventilated place.
Key words
Split tube furnace, Ionic liquid, Pressure explosion
Introduction
OTF-1200X-UL furnace is configured to meet UL/CSA certificate standard. The furnace can adopt a 60, 70, 80 or 100mm O.D quartz tube and a pair of stainless steel vacuum sealing flanges with needle valves & gauge allow heating samples under vacuum or gas flow conditions. The temperature of this tube furnace is controlled by a high precision digital controller which provides 30 programmable segments with +/- 1°C accuracy. The max. working temperature is 1200°C [1] Figure1.
The early history of ionic liquids began in 1888, when ethanol ammonium nitrate (mp 52-55 °C) was reported by Gabriel [2]. Later in 1914, one of the earlier known room temperature ionic liquids was [C2H5NH3][NO3], excogitated by Walden [3]. He viewed the physical properties of ethyl ammonium nitrate, [C2H5NH3][NO3], which had a melting point of 12 oC produced from the reaction of concentrated nitric acid with ethylamine.
In 1951 Hurly and Weir declared that a room temperature ionic liquid could be prepared by mixing and warming 1-ethylpyridinium chloride with aluminum chloride [4]. In 1970s and 1980s Osteryoung et al. and Hussy et al. carried extensive studies on organic chloride- aluminum chloride ambient temperature ionic liquid and the first major review of room temperature ionic liquids was published [5].
The use of ionic liquids as thermal fluids was first suggested by Rogers et al. in 2001 [6]. Characterization of some room temperature ionic liquids (RTILs) as candidates for thermal storage media and heat transfer fluids in thermal applications were investigated. Several ionic liquids samples was prepared from ammonium alum [NH4Al(SO4)2.12H2O], as inorganic salt with urea [NH2CONH2], or acetamide [CH3CONH2], as organic compounds, and aluminum nitrate [Al(NO3)3.9H2O] with urea or acetamide compounds in different mole ratios were investigated alone and with addition of some improving materials to study their synergetic effect [7].
Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal"[8] that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain water of crystallization or water of hydration.
The notation "hydrated compound⋅nH2O", where n is the number of water molecules per formula unit of the salt, is commonly used to show that a salt is hydrated [9]. This research spans a series of research specialized in safety aspects to lay the groundwork for work in a secure workplace environment [10-20]. Working safely with hazardous chemicals requires proper use of laboratory equipment. Maintenance and regular inspection of laboratory equipment are essential parts of this activity. Many of the accidents that occur in the laboratory can be attributed to improper use or maintenance of laboratory equipment.Tube furnaces are often used for high-temperature reactions under reduced or normal pressure. The proper choice of glassware or metal tubes and joints is required, and the procedures should conform to safe practice with electrical equipment and evacuated apparatus [21].
We emphasize the importance of having all necessary information of the chemical that we wish to deal with, including the safety data sheet (SDS) and based on that we can make the risk assessment form. Also, we would like to recommend adding good and functional smoke detectors as well as some oxygen sensors to ensure safe working area.
Experimental work
On December 2014 MSc. student placed ionic liquid sample (aluminum ammonium sulfate dodecahydrate), as inorganic salt with urea) in split tube furnace using boat type quartz crucible to study the thermal properties for the prepared ionic liquid. Split tube furnace was controlled by a high precision digital controller; the program was instilled to reach 1000 oC within four hours.
As the thermal treatment for the sample must be done at atmospheric condition, no need to support the furnace with vacuum or any gas, just to be sure that the two side valves were opened Figure 1.
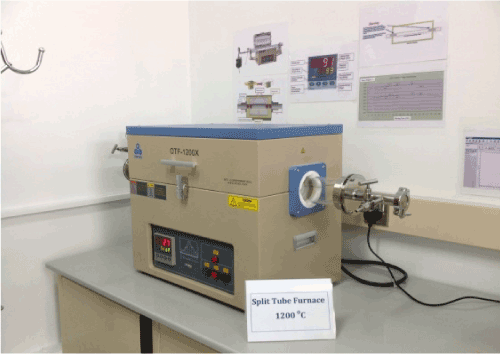
Figure 1. Split tube furnace
Sample in progress
As the temperature in the furnace increased water of hydration for the sample begin to evaporate from sample surface which is the easiest part of the sample to eviscerated its water caused the sample surface to solidified. Water vapors confined inside the quartz tube and spilled out toward the furnace flanges .Water condensation occurs at outside part of the tube due to low temperature Figure 2.

Figure 2. Water condensation outside of quartz tube
The Major Problem
Water of crystallization starts to boil inside the crucible as the temperature still increased to reach the programmable set temperature (1000 oC). The surface of the sample almost blocked caused the pressure to accumulate inside the sample Figure 3.

Figure 3. Ionic liquid surface blocked
Pressure explosion occurs causes the ionic liquid to spill out and contaminated the quartz tube inner surfaces Figures 4,5.

Figure 4. Quartz tube partially contaminated

Figure 5. Quartz tube all inner surfaces contaminated
Conclusion
Completely filled crucible with ionic liquid (10ml) treated with high temperature (1000oC) causes sample surface to solidify. Water of crystallization starts to boil inside the crucible leads to sudden pressure explosion for the sample. The opened side valves protected the quartz tube from breakage Figure 6.
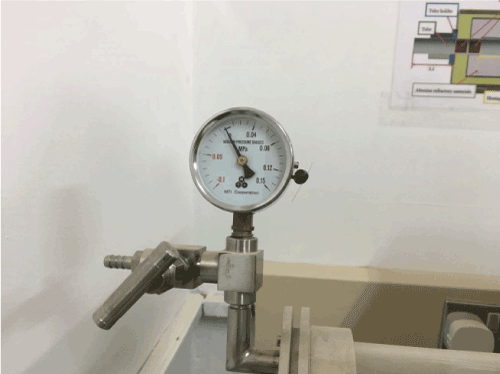
Figure 6. The opened side valve
Assuming that furnace valves were closed in case of vacuum or under certain gases, serious hazard will occur due to glass breakage.
Lesson Learned
In case of such salts (ionic liquids) thermal treatment, sample preparation must be done in appropriate amount to ensure uniform heat distribution to all parts of the sample which prevents drying some parts without the other as stated in the case provided.
A successful experiment was done keeping in mind the lessons derived from past experience Figure 7.

Figure 7.
Acknowledgements
The authors acknowledge the Al-Nahrain University and Babylon University for their encouragement.
References
- http://www.mtixtl.com/1200CSplitTubeFurnace-OTF-1200X-UL.aspx
- Gabriel S, Weiner J (1988) On some Derivatives of Propylamines. J. American Chemical Society 21: 2669-2679.
- Walden P (1914) Ueber die Molekulargrösse und elektrische Leitfähigkeit einiger geschmolzenen salze. J. Bull. Acad. Imper. Sci. St. Petersburg 8: 405-422.
- Mohammed A, Inamuddin (2012) Properties and Applications of Ionic Liquids, Green Solvent 2nd edition. Springer Science and Business Media Dordrecht 3-21.
- Wilkes J (2002) A Short History of Ionic Liquids- from Molten Salts to Neoteric Solvents. J. Green Chemistry 4: 73-80.
- Wu B, Reddy R, Rogers R (2001) Novel Ionic Liquid Thermal Storage for Solar Thermal Electric Power Systems. Proceedings of Solar Forum
- Huda S (2015) Investigation of Thermal Properties for some Ionic Liquids containing Urea and Acetamide. A Thesis submitted to the College of Science/Al-Nahrain University as a partial fulfillment of the requirements for the Degree of Master of Science in Chemistry.
- Farlex (2009) Hydrate. TheFreeDictionary.com 07-08.
- Nomenclature of Inorganic Chemistry IUPAC Recommendations (2005). Table IV Multiplicative Prefixes, p.258.
- Al-Zuhairi A, Al-Dahhan W, Hussein, Rodda K, Yousif E (2016) Teaching Laboratory Renovation. Oriental Journal of Physical Sciences 1: 31-35.
- Ali A, Shaalan N, Al-Dahhan W, Yousif E (2016) For a Safer Working Environment with Hydrofluoric Acid in Iraqi Industrial Plants. Open Journal of Safety Science and Technology 6: 77-80.
- Shireen R, Al-Dahhan, W, Al-Zuhairi A, Hussein F, Rodda K et al. (2016) Fire and Explosion Hazards Expected in a Laboratory. Journal of Laboratory Chemical Education 4: 35-37.
- Al-Dahhan W, Al-Zuhairi A, Hussein F, Rodda K, Yousif E (2016) Laboratory biological safety cabinet (BSC) explosion. Karbala International Journal of Modern 2: 276-279.
- Al-Zuhairi A, Al-Dahhan W, Hussein F, Rodda K, Yousif E (2017) A Vision to Promote the Forensic DNA Facility at Al-Nahrain University in Terms of Safety Measures. Oriental Journal of Physical Sciences 2: 37-41.
- Ali A, Shaalan N, Al-Dahhan W, Hairunisa N, Yousif E (2017) A Technical Evaluation of a Chemistry Laboratory: A Step Forward for Maintaining Safety Measures. Oriental Journal of Physical Sciences 2: 68-71.
- Hussein F, Al-Dahhan W, Al-Zuhairi A, Rodda E, Yousif E (2017) Maintenance and Testing of Fume Cupboard. Open Journal of Safety Science and Technology 7: 69-75.
- Ibrahim A, Yousif E, ALShukry A, Al-Zuhairi A (2016) Hazard Analysis and Critical Control Point HACCP System. Iraqi National Journal of Chemistry 16: 172-185.
- Yousif E, Al-Dahhan W, Abed R, Al-Zuhairi A, Hussein F (2016) Improvement of A Chemical Storage Room Ventilation System. Journal of Progressive Research in Chemistry 4: 206-210.
- Yousif E, Al-Dahhan W, Ali A, Rashad A, Akram E (2017) Mind What You Put in a Furnace: A Case Study for a Laboratory Incident. J Environ Sci Public Health 1: 56-61.
- Yousif E, Al-Dahhan W, Ali AH, Jber NA, Rashad AR (2017) A Glimpse into Establishing and Developing Safety Measures in the Department of Chemistry, College of Science, Al-Nahrain University in 2016. Orient J Phys Sciences 2(2).
- Prudent practices in the laboratory (2001) handling and management of chemical hazards / Committee on Prudent Practices in the Laboratory, Board on Chemical Sciences and Technology, Division on Earth and Life Studies.







