The world over artificial respiration is employed as one of the intensive care treatment measures in severe cases of COVID-19 because of the significant respiratory distress patients develop. Nevertheless, the outcome is poor. Lethality varies from country to country and clinic to clinic between 50% and 90%. So, the question arises as to whether the use of oxygen can be a risk factor in the treatment of acute inflammatory diseases in general and of COVID-19 in particular. Oxidative stress is the first and oldest step of cellular defense and starts before the activation of the immune system. This leads to an increase of intracellular oxygen in the mitochondria, followed by an elevated electron flow and the formation of superoxide as well as other reactive oxygen species and reactive nitrogen species. Superoxide reacts with nitric oxide, which is always present in inflammation forming peroxinitrite, the strongest inducer of oxidative stress. This step induces the activation of nuclear factor kB, followed by the production of proinflammatory cytokines. The elevated levels of inducible nitric oxide synthase keep this cycle running. High amounts of superoxide have to be compensated and catabolized by manganese superoxide dismutase 2 into hydrogen peroxide and in a second step by catalase into water. When using artificial respiration, these steps are accelerated considerably in the inflamed tissue of the lung, leading to a significant increase of the electron flow as well as an elevation of superoxide, oxidative stress, and water. As severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) generally induces the production of proteins (and not only those necessary for viral reproduction) the water will remain in the tissue, causing an edema and thus a wet lung syndrome associated with a growing oxygen diffusion distance to red blood cells. Ultimately, patients do not suffocate in spite of, but because of, the presence of high levels of oxygen. The limited number of patients who survive this deleterious treatment describe it as having had a sensation of drowning. The reasons will be discussed.
SARS-CoV-2, COVID-19, artificial respiration, superoxide, oxidative shielding, oxidative stress, oxidative burst, hemofiltration
Energy metabolism and defense mechanisms, developed in parallel in evolution. Their interaction is essential for each of them to function properly. In the beginning, 3.5 billion years ago, the process was anaerobic. In the oldest bacteria known, adenosine triphosphate (ATP) was produced by anoxygenic photosynthesis [1,2]. Oxygen was toxic. But substances and cellular mechanisms of the intermediary metabolism already existed in that past and are still utilized today. These are substances and mechanisms such as glycolysis, glyoxylate and the Krebs cycle, pyruvate dehydrogenase, the pentose phosphate pathway, the synthesis and oxidation of fatty acids, cobalamin, carotenoid and heme synthesis, iron-sulfur cluster synthesis, cytochromes (extremely relevant for drug metabolism), isoprenyl and ubiquinone synthesis and its interaction with the electron transport necessary for the defense mechanisms and proton coupling for ATP synthesis [3].
Almost one billion years later, oxygen became important. Cyanobacteria started producing oxygen in such quantities that the oxygen level in the atmosphere increased to 2-4% which allowed bacteria and viruses to leave the water of the oceans and populate the earth [1]. Mitochondria coordinated both energy production and defense mechanisms. They even organized the innate immune system necessary for the identification of targets for the activation of defense mechanisms [4]. The rising levels of oxygen in the environment facilitated an increased production of energy and promoted oxidative shield mechanisms. More energy production in turn means higher risk due to an increase in the number of electrons which form together with oxygen superoxide anions. At first these oxygen superoxide anions can be catabolized by manganese-superoxide dismutase (Mn-SOD2) to hydrogen peroxide (H2O2), which is still an oxygen radical, and subsequently H2O2 is catabolized to water. In parallel, superoxide reacts with nitric oxide to form peroxynitrite, the most active substance in the development of oxidative stress by reactive ROS and nitrogen species (RNS) [5].
Mitochondria
Proteome of the mitochondrion contains almost 1500 proteins [6]. Two third of these have an enzymatic function, highlighting the enormous metabolic activity of these organelles. Under physiological conditions, the concentration of thousands of nutrients and metabolic substrates is strictly controlled by collective kinetic constants. Consequently, the whole system is mismanaged if even one of these is upregulated or downregulated for a longer period of time. Viral and microbial infections, changes in the natural environment, toxins, nutritional disbalance, and medical treatment can thus cause a mismatch between the optimum and the actually existing concentration of metabolic substrates for the tissue [7]. This point will be of particular interest regarding SARS-CoV-2.
Most of the ATP in humans is generated by the combined action of the Krebs cycle and oxidative phosphorylation. Oxidative phosphorylation requires an electron transport chain, molecular oxygen and ATP synthase located within the inner membrane of the mitochondria [8]. The reactions of the Krebs cycle produce reduced nicotinamid adenine dinucleotid (NADH) and reduced flavin adenine dinucleotid (FADH2). Both are electron donors for the electron transport chain that passes them sequentially to O2 to generate energy and to form water. The mitochondrion also contains these enzymes responsible for β-oxidation of fatty acids to NADH and FADH2. Those reductants are located in the center of the mitochondrion and readily react with the electron transport chain. The exergonic reactions of the electron transport chain provide energy for the translocation of protons (H+) from the mitochondrial matrix through the inner mitochondrial membrane to the exterior. Most of the oxygen consumed by humans is metabolized within the mitochondria. The driving force of mitochondrial function is a voltage difference (ΔΕ) between O2 and NADH (Figure 1).
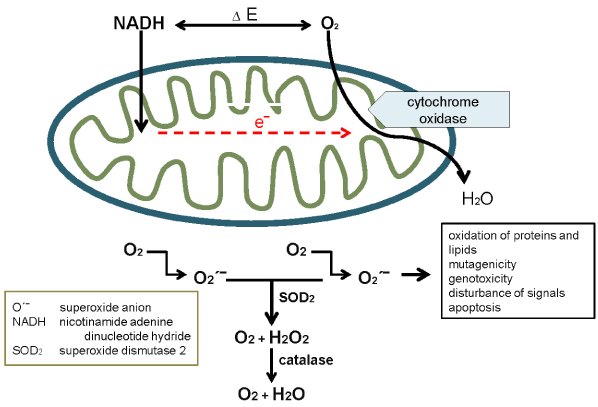
Figure 1. The voltage difference between O2 and NADH is the driving force of the mitochondrion.
The respiratory electron transport chain consists of four independent complexes (I, II, III, and IV). Coenzyme Q (CoQ) transports the electrons from complexes I and II to complex III, cytochrome c from complex III to IV. NADH donates its electrons to complex I. From there electrons are transferred to coenzyme Q to form QH2, which passes its electrons on to complex III. A proton translocation reaction is needed to provide the energy. Complex II (succinate dehydrogenase) transports electrons without providing energy for proton translocation or ATP formation [8-10]. The major sources of physiological oxygen radicals which are natural by-products of oxidative phosphorylation can be found between complex I and CoQ and between CoQ and complex III (Figure 2). They account for about 1% of the total oxygen uptake. This situation changes completely during artificial respiration as an elevated level of electrons and O2 form high amounts and ultimately uncontrollably high amounts of superoxide anions reacting with nitric oxide to form peroxinitrate, which in turn forms free radicals and induces oxidative stress [5]. Mitochondrial DNA is 15 times more sensitive to oxidative damage than nuclear DNA [11].
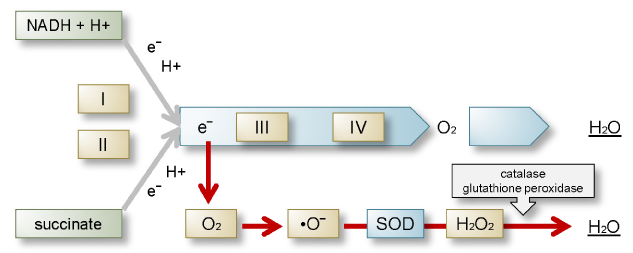
Figure 2. Consequence of artificial respiration (oxygen) in COVID-19: extremely elevated electron flow and superoxide formation (red arrow) followed by excessive oxidative stress and elevated levels of H2O which remain in the tissue because of high protein synthesis caused by SARS-CoV-2.
The physiological process of ATP production is also the dominant source of reactive oxygen species (ROS) and consequently of oxidative stress [10]. Oxygen is an element urgently needed but also an element causing risks when activated to form singlet oxygen (1O2) by means of energy transfer or by electron transfer forming superoxide anion radicals (•O2‾) [12]. As long as ROS remain compensated in a homeostatic and physiological reaction or are used for controlled defense for a limited period of time, deleterious effects can be avoided. The most important enzymes to act on •O2‾ are superoxide dismutases (SODs) which exist in three forms: manganese superoxide dismutase (Mn-SOD2) in the mitochondria as well as copper-zinc superoxide dismutase (Cu/Zn-SOD2) in the cytosol and membranes.
Superoxide
There are several metabolic steps leading to the production of superoxide. The most important in this context is the electron transport chain in the mitochondrion during ATP generation. Superoxide exists in two interconvertible forms. The more important one has an unpaired electron and a negative charge (•O2‾), the other one is an acid (•OH). The negative charge keeps superoxide in the cells where it serves as an intracellular signalling molecule. Higher levels are damaging to cells. Therefore enzymes (SODs) are needed to degrade superoxide. They can be found in all organisms dependent on the presence of oxygen in the air [10]. There are three forms: the first form is involved in the energy metabolism of the mitochondria, the second one can be found in the cytoplasm of cells and the third one outside of cells. Each SOD is encoded by its own gene and has a distinct structure. Superoxide is metabolized by SOD to H2O2. Both •O2‾ and H2O2 have low reactivity. In the presence of transition metals however, they develop high amounts of ROS.
Reactive nitrogen species
RNS include nitric oxide (NO•) and nitrogen dioxide (•NO‾2) both of which are free radicals and peroxynitrite (ONOO‾), a nonradical. They all have high oxidizing potential. The toxic effect of these molecules is referred to as “nitrosative stress” [13,14]. They are also classified as ROS and summarized as reactive oxygen and nitrosative species (RONS). NO• is a relatively stable free radical with a half-life of 1s. It is produced by oxidation of L-arginine to L-citrulline by the enzyme nitric oxide synthase (NOS) which takes three forms: inducible NOS (iNOS), endothelial NOS (eNOS) and neuronal NOS (nNOS) [15,16]. NO• is a soluble lipid that can diffuse through membranes and is thus critically important in neurogenic membranes containing a different structural correlation of lipids and proteins [17].
NO• has both physiological and pathophysiological properties [18]. Because of its regulatory function it is considered a cytokine, in spite of the fact that it is structurally not identical to cytokines [19]. It causes vasodilation to regulate blood flow and consequently directs immune cells towards the site of inflammation. It also activates natural killer (NK) cells. This positive effect is blocked when high levels of superoxide activate NO• and •O2‾ to form ONOO‾, a highly potent inducer of oxidative stress. This is the strongest inducer of oxidative stress and switch for the activation of nuclear factor-κB (NF-κB) which in turn triggers the production of proinflammatory cytokines (Figure 3). The blocking effect of OONO‾ acts more strongly on NK cells than the promoting effect of NO• [20,21]. ONOO‾ can cause DNA fragmentation, lipid and protein oxidation, as well as nitration [22,23].

Figure 3. The compounds nitric oxide, superoxide, and peroxinitrite as regulatory substances of the innate immune system and the role of nitric oxide synthase inducing a vicious cycle.
All coronaviruses have the highest affinity for the respiratory tract among all organs and functional systems. Generally, the inflammation involves the pharynx, trachea, bronchi, and bronchioli. However, in COVID-19 the inflammation often extends to the entire pulmonary tissue corresponding to an enormous expansion of the inflamed area. This provokes a strong antioxidative defense reaction by the activation of the NO/•O2‾/ONOO‾ cascade followed by the upregulation of proinflammatory cytokines such as IFN-γ, IL-1, IL-6, IL-8, TNF-α and others by activating NF-κB and the sympathetic nervous system, which also interacts with the immune cells. This cascade is controlled by SODs, glutathione peroxidase (GPx), catalase, Treg cells, IL-10, cortisol and the parasympathetic nervous system [24]. The mitochondria are the regulators of those mechanisms. As shown before the voltage difference between O2 and NADH (ΔΕ) is the driving force. The uptake of even physiological amounts of oxygen by artificial respiration with compressed air in particular will deregulate and escalate the whole process of inflammation. The more oxygen is used, the higher the production of electrons and consequently of superoxide will be. It is less reactive in physiological concentrations. It becomes very reactive however, if a high quantity is formed, producing ONOO‾ by reacting with NO•. The oxidative and nitrosative stress may become so acute that it results in an oxidative burst which in turn leads to damage of granulocytes, especially neutrophils, and macrophages breaking down cellular defense mechanisms (Figure 4) [10].

Figure 4. (1) Normal mitochondrial function (2) Upregulation of mitochondrial function by uncontrollable oxidative stress and immune activation due to oxygen.
This may damage the sensitive mitochondrial DNA and immediately influence mitochondrial function. It has been demonstrated that the therapeutic treatment of various diseases with oxygen increased mortality without improving other patient-relevant outcomes [25]. The authors published a meta-analysis of 25 randomized studies with 26,000 patients treated with low and high-rate artificial respiration for various indications. Mortality was higher in all cases treated with high O2 flow than in the groups suffering from the same disease but treated with low levels of oxygen. In artificial treatment of COVID-19 patients, the oxygen reaches the inflamed tissue directly and is not distributed or attenuated through the blood stream. Therefore, its effect in COVID-19 is far more pronounced than in the cases referred to in the studies. A leakage of superoxide develops between complex I and CoQ and complex III, depending on the quantity and properties of CoQ. The overload of superoxide leads to an even stronger activation of inflammation by the vicious NO/ONOO‾ cascade increasing the production of H2O2 and ultimately H2O by catalase and SOD. Latest findings demonstrate that SARS-CoV-2 in contrast to other coronaviruses does not only lead to elevated protein synthesis for viral reduplication but higher levels of protein synthesis in general [26]. This increased production of proteins is an important factor for H2O accumulation in the tissue causing a wet lung syndrome associated with the sensation of drowning. The same group showed that cholesterol levels are lowered, which inevitably leads to a reduced synthesis of sex hormones and steroid hormones. As a result, the anti-inflammatory effect of cortisol is blocked. The effect can be even more pronounced in patients who had been treated with statins. Additionally, statins can also lead to an increased NO level. Consequently, insulin resistance can be expected. It always develops in acute inflammation due to an increase in circulating glucose and free fatty acids which are no longer absorbed by the adipose tissue, liver, or muscles in order to provide energy for the central nervous system and the immune system [24,27].
Environmental factors influence the extent of oxidative stress. Transition metals with atomic numbers 21-30, 39-48, 57-80, and 89-112 are electron donors and catalyze the formation of superoxide and free radicals. It is also one of the reasons why COVID-19 takes a more severe course in people in polluted regions like China, Lombardy, and large cities all over the world. It also is one the contributing factors for the severe clinical course in elderly patients as their total body burden is generally higher. Metals are also inducers of cell adhesion molecules on the surface of the endothelium [28]. They are catalyzers of superoxide but may as well increase the risk of thromboembolic processes often seen in COVID-19 patients. It has been shown just recently that nitrogen dioxide levels are a contributing factor to COVID-19 fatality [29]. The antioxidative capacity depends on the quality and quantity of micronutrients. The presence of CoQ might be particularly important. Although there is no current data on this aspect for COVID-19 yet, it is a well-known fact that a mismatch of micronutrients causes mitochondrial dysfunction [7].
Oxygen is a promoter of oxidative stress as a defense reaction and as an activator of the innate immune system. As long as these reactions stay under control, the required shielding effect is guaranteed, but high levels of oxygen render them uncontrollable. Artificial respiration increases this risk considerably within a short period of time. So, what could be the solution? In patients showing signs of oxidative and nitrosative stress spiraling out of control, high dosages of substances of the cellular antioxidant defense system should be administered, such as vitamin C and E, reduced glutathione, taurine, cysteine, methionine, s-adenosyl-L-methionine, melatonin, selenium, and polyamines [30]. They should be combined with anti-inflammatory substances and drugs. In severe cases, corticosteroids should be taken into consideration in spite of the viremia. In case of symptoms of wet lung disease, hemofiltration should be started immediately to extract the fluid from the blood and consequently from the lung via osmotic pressure. This should alleviate the sensation of drowning within 2 to 3 hours. Additionally, a therapeutic concept to avoid pulmonary fibrosis is required until the radiograph has normalized.
- Rasmussen B, Fletscher IR, Brocks JJ, Kilbun MR (2008) Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455: 1101-1104. [Crossref]
- Hohmann-Marriott M, Blankenship RE (2011) Evolution of photosynthesis. Ann Rev Plant Biol 62: 515-548. [Crossref]
- Tang KH, Blankenship RE (2010) Both forward and reverse TCA cycles operate in green sulfur bacteria. J Biol Chem 285: 35848-35854. [Crossref]
- West AP, Shadel GS, Gosh S (2011) Mitochondria in innate immune response. Nat Rev Immunol 11: 389-402. [Crossref]
- Beckman J, Koppenol W (1996) Nitric oxide, superoxide and peroxinitrite: the good, the bad and the ugly. Am J Physiol 271: C1424-C1437. [Crossref]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, et al. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112-123. [Crossref]
- Naviaux RK (2012) Oxidative Shielding or Oxidative Stress? J Phamacol Exp Ther 342: 608-616. [Crossref]
- Hatefi Y (1985) The mitochondrial electron transport chain and oxidative phosphorylation system. Annu Rev Biochem 54: 1015-1069. [Crossref]
- Hinkle PC, McCarty RE (1978) How Cells Make ATP. Sci Am 238: 104-123. [Crossref]
- Littarru GP (1994) Energy and defense. Casa Editricata Scientifica Internationale.
- Roskoski R Jr. (1996) Biochemistry. W.B. Saunders Company, Philadelphia. pp: 124.
- Boveris A (1984) Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol 105: 429-439. [Crossref]
- Klatt P, Lamas S (2000) Regulation of protein function glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: 4928-4944. [Crossref]
- Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, et al. (2004) The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxidspecies. Putting perspective on stressful biological situation. Biol Chem 385: 1-10. [Crossref]
- Nathan C, Xie QW (1994) Nitric oxide synthase: roles, tolls and controls. Cell 78: 915-918. [Crossref]
- Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26: 190-195. [Crossref]
- Devlin TM (2002) Biological Membranes: Structure and Membrane Transport. (5th edn), Textbook of Biochemistry. Wiley-Liss, pp: 493-534.
- Bredt DS, Hwang P, Glatt C, Lowenstein C, Reed R, et al. (1994) Nitric oxide: a physiological messenger molecule. Ann Rev Biochem 63: 175-195. [Crossref]
- Roitt I, Brostoff J, Male D (2001) Immunology. (6th edn), Mosby.
- Nathan C (1992) Nitric oxide as a secretory product of mammalian cells. FASEB J 6: 3051.3064. [Crossref]
- Pall M (2007) Explaining Unexplained Illnesses. Harrington Park Press, New York.
- Carr A, Mecall MR, Frei B (2000) Oxidation of LDL by myeloperoxidase and reactive nitrogen species reaction pathways and antioxidant protection. Atherioscler Thromb Vasc Biol 20: 1716-1723. [Crossref]
- Valko M, Leibfritz D, Moncol J, Mark TD, Cronin C, et al. (2007) Free radicals and antioxidants in normal physiological function and human disease. Int J Biol 39: 44-84. [Crossref]
- Straub RH (2015) The Origin of Chronic Inflammatory Systemic Diseases and Their Sequelae. Elsevier.
- Chu DK, Kim LH-Y, Young PY, Zamiri N, Almenawer SA, et al. (2018) Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA). A systematic review and meta-analysis. Lancet 391: 1693-1705. [Crossref]
- Bojkova D, Klann K, Koch B, Widera M, Krauese D, et al. (2020) Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 583: 469-472. [Crossref]
- Fisher-Wellmann K.H., Neufer P.D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab 23: 142-153. [Crossref]
- Klein CL, Nieder P, Wagner M, Köhler H, Bittinger F, et al. (1994) The role of metal corrosion in inflammatory processes: Induction of adhesion molecules by heavy metal ions. J Mat Sci 5: 798-807.
- Ogen Y (2020) Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci Total Environ 726: 138605. [Crossref]
- Bansal M, Kaushal N (2014) Oxidative Stress, Mechanisms and their Modulation. Springer India, New Delhi, India.




