Abstract
Natural and Synthetic medications, including polyphenols, flavonoids, and vitamins, have critical roles in lowering oxidative stress while also functioning as antioxidants and immunomodulators. Scientific evidence regarding the effectiveness of vitamin and mineral supplements in healthy individuals remains scarce. Iron and zinc are currently the trace minerals of most concern when it comes to vegetarian diets' nutritional value. The aim of this study evaluates the bioavailability, antioxidant, and immunomodulatory properties of Storg-I (organic Murraya koenigii leaf extract) and Storg Zn (Psidium guajava leaf extract) with synthetic vitamins in order to assess their therapeutic potential in modulating oxidative stress and immune function. The DPPH assay was used to determine antioxidant activity, and immunomodulation was investigated in rats utilizing humoral antibody titers and hematological analysis. In addition bioavailability was investigated using atomic absorption spectrometry (AAS). Storg-Zn and Storg-I showed antioxidant activity with IC50 values of 113.96 µg/ml and 127.95 µg/ml, respectively, and synthetic-Zn and synthetic-Iron showed antioxidant activity with IC50 values of 113.97 µg/ml and 111.05 µg/ml respectively, in the DPPH experiment. Both test compounds dramatically boosted antibody levels and improved hematological markers in rats. Bioavailability tests demonstrated that Storg-I and Storg-Zn were well absorbed, with each having distinct pharmacokinetic characteristics. This study shows that Storg-Zn, produced from Psidium guajava, improves immunological function and works as a powerful antioxidant, whilst Storg-I has great bioavailability and promise as a natural iron supplement with fewer adverse effects.
Keywords
antioxidant, atomic absorption spectrometry, immunomodulatory,murraya koenigii, psidium guajava
Introduction
Natural and synthetic drugs are widely used as therapeutic tools for the prevention or treatment of many diseases. These drugs mainly include polyphenols, chalcones (precursors of flavonoids), vitamins, carotenoids, and proteins. Experimental and epidemiological studies have shown that many natural and synthetic drugs are involved in the reduction of oxidative stress caused by free radicals and act as antioxidants [1]. Recently, the nutraceutical and pharmaceutical industries have increasingly engaged in finding natural alternative compounds as potential antioxidants and immunomodulators. The use of phytochemicals has been introduced as a good source of natural antioxidants and immunomodulators. This effort aims to increase understanding and awareness of immune system modulation and to obtain new phytochemicals with distinct targets and processes [2].
Immunomodulation is a complex process through which immune responses are either enhanced or suppressed to influence the progression of disease. Immunomodulators, a broad class of drugs, include both immune suppressants and immune stimulants. These medications have been used to combat diseases such as rheumatoid arthritis, ulcerative colitis, and malignancies as well as the dysregulated immune responses [3].Free radicals can damage cells through oxidative stress, but this process may be prevented or slowed by antioxidants. These molecules help protect cells either by enhancing the body’s natural antioxidant defenses or by directly neutralizing reactive oxygen species (ROS) [4]. Many plants used in traditional medicine are reported to have immune-modulating activities. The Murraya koenigii extract and Psidium guajava has also been previously reported as a promising immunomodulatory agent, which acts by stimulating humoral immunity and the phagocytic function [5,6].
Murraya koenigii (Curry Leaves/KadhiPatta/MithaNimba/GiriNimba) and Psidium guajava L (guava) are treasures in Indian medicinal herbs that are widely used as spices, fruits, condiments, and for treating various diseases in India [6,7]. Curry leaves contain many essential ingredients such as carbohydrates, proteins, fibers , calcium, phosphorus, iron, magnesium, copper, minerals, and vitamins like nicotinic acid, vitamin B, C, A, and E, as well as antioxidants, plant sterols, glycosides, and flavonoids [8]. In contrast, guava leaves primarily consist of rutin, naringenin, gallic acid, catechin, epicatechin, kaempferol, isoflavonoids, and flavonoids like quercetin and guaijaverin [9]. Psidium guajava and Murraya koenigii leaves have been studied for their excellent antioxidant capacity; however, they remain understudied herbs [10,11]. The carbazole alkaloids present in the leaves exhibit various biological activities, including anti- tumor, anti-oxidative, anti-mutagenic, and anti-inflammatory effects. The application of curry leaves paste aids in treating bruises, burns, rashes, and insect bites [12]. Since guava leaves contain flavonoids, they are often utilized for their antibacterial and antidiarrheal properties. The flavonoid quercetin helps relax the intestinal muscular lining, while polysaccharides enhance antioxidant activity. Additionally, guava has an immune-stimulating effect [9]. Guava leaves contain zinc, while Murraya koenigii (curry leaves) are rich in iron.
Iron and zinc are important trace elements that deserve particular consideration when assessing nutritional adequacy. Meat, poultry, and fish provide some iron in the highly accessible heme form, while animal products supply the majority of the zinc in U.S. diets [13], although plant foods are often significant sources of trace elements like copper, manganese, and iron [14]. Zinc is essential for the growth of every living organism, and the rapid proliferation and specialization of immune cells depend on a steady supply of this trace mineral in the right amounts [15]. It has been suggested that zinc functions as a co-factor or pro-antioxidant agent via three different methods: preserving free sulfhydryl groups in proteins, competing with redox-active metals, and particularly triggering the antioxidant system response. Zinc is necessary for the metabolism of DNA and RNA and is needed for various specific enzymes, metalloproteins, and immune system stability. Growth and development retardation, as well as morbidity, are due to zinc deficiency [16]. Iron plays a crucial role in essential functions like oxygen and electron transport and is a vital component of many proteins and enzymes [17]. Iron deficiency is the most common form of malnutrition. It is a general term for suboptimal levels of iron in the body for health. Factors responsible for this include poor diet, increased micronutrient needs, and health issues such as diseases and infections. Body iron status can be improved by the intake of dietary supplements and fortified foods [18]. Scientific research is currently being conducted on the potential therapeutic benefits of employing medicinal plant components to modulate immune response. The present study aims to explore the comparative Bioavailability, antioxidant, and immunomodulatory activities of Storg-I and Strog-Zn with Synthetic vitamins.
Materials and methods
Plant materials
Storg-I is a certified organic curry leaf extract standardized to contain 3.6% organic plant-based iron, along with essential cofactors and co-nutrients. Storg-Zn is an organic guava leaf extract standardized at 4%. Both Storg-I and Storg-Zn are manufactured, patented (Mother patent No. 437203, Storg I - IND Appln No: 202043054115, Storg Zn - IND Appln No: 202043054116, US Appln No: 17024731, EU Appln No: EP21193681.0, PCT Appln No: PCT/IB2020/062000) and registered by Star Hi Herbs Pvt. Ltd., Jigani, Bangalore, Karnataka, India.
Bio-fortification method
Bio-fortified dried curry and guava leaves, free of foreign material, are crushed to a size of 8–10 mm using a hammer mill. Extraction is performed with 5% lemon juice and raw materials at a 1:3 ratio, heated to 70–80°C for 3 hours. After filtration, the extract passes through membrane filtration at 15–60 psi, followed by column elution with cation exchange resins. The column is eluted with 5% lemon juice in water, and the eluate is concentrated at 70–80°C to achieve 30%–40% TDS. The concentrated solution is spray-dried at an inlet temperature of 180–190°C and an outlet temperature of 80–100°C. The dried powder is then collected and packed in an airtight drum.
In Vitro evaluation of the antioxidant activity of Storg-Zn and Storg-I compared to synthetic zinc and iron
The Storg-Zn and Strog-I ability to scavenge free radicals was assessed using the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging assay [19]. A solution of 0.1 mM DPPH in methanol was prepared, and 2.4 mL of this solution was mixed with 1.6 mL of test samples in methanol at different concentrations (50, 100, 150, 200, and 250 μg/mL). The sample was vortexed thoroughly and left in the dark at room temperature for 30 minutes. The absorbance of the samples was measured spectrophotometrically at 517 nm. Butylatedhydroxytoluene (BHT) was used as a reference drug at different concentrations (20, 40, 60, 80, and 100 μg/mL). The percentage of DPPH radical scavenging activity was calculated using the following equation:
Where, A0 is the absorbance of the control and A1 is the absorbance of the samples. Then the percent of inhibition was plotted against concentration, and the IC50 was calculated from the graph. The experiment was repeated three times at each concentration.
In-vivo immunomodulatory activity of Storg- Zn and Storg-I with synthetic zinc and iron
Animals and grouping
The studies were conducted in accordance of ethical clearance was obtained from Institutional Animal Ethics Committee (IAEC) before the experiment (BCP/IAEC/06/2022). Sprague-Dawley (SD) rats weighing 150-200 g were used in this study. They were supplied by the animal house, Bharathi College of pharmacy Mandya, Karnataka, India. The rats were acclimatized and housed in polypropylene cages with provisions for a water bottle holder and a feed hopper, using corn cobs as bedding material. The animals were kept under conventional laboratory conditions (maximum temperature 24°C, minimum temperature 23°C, and relative humidity ranging from a maximum of 63% to a minimum of 48%) with a 12/12 hour dark/light cycle. Before the trial, all the animals were acclimatized to laboratory conditions for a week.
Animals were splitted into 9 groups i.e.
Group I served as control group,
Group II served as negative control (20 mg/kg cyclophosphamide),
Group III served as positive control (50 mg/kg levamisole),
Group IV and V treated as 50 and 100 mg/kg Storg-I treatment groups
Group VI and VII treated as 50 and 100 mg/kg Storg-Zn treatment groups and
Group VIII and IX treated as 100 mg/kg Synthetic Iron and100 mg/kg Synthetic Zn respectively.
Antigen preparation
Sheep red blood cell (SRBC) preparation and standardization
Sheep blood was withdrawn from the external jugular vein in a 1:1 proportion of freshly prepared Alsever's solution. SRBCs were separated from the collected blood by centrifuging at 2500 rpm for 10min and washed with pyrogen-free normal saline (0.9% w/v). 20% SRBC in 0.1 ml is considered as the final concentration [20].
Humoral antibody titre assay
Every group, with the exception of the control group, received 0.1 ml of 20% SRBC on days 7 and 14. Cyclophosphamide was administered to all groups (apart from the control group) on days 9 and 16 in order to decrease immunity. Every antigenically sensitized and challenged rat was given moderate ether anesthesia on the 14th and 21st days to allow blood to be extracted from the retro-orbital plexus. Serum was then separated for antibody titer testing. A microtiter plate was loaded with 20 µL of serially diluted serum and 20 µL of saline. After adding 20 µL of SRBC to each of these dilutions, the plates were incubated for an hour at 37°C, and the presence of hemagglutination and antibody responses was monitored [21].
Hematological analysis
The blood was collected from each group via retro-orbital plexus into heparinized collection tubes. To estimate hematological parameters, 0.08 ml of blood was mixed with 0.02 ml of EDTA acid (33.33 mg /ml). At the end of the experiment, blood was collected and subjected to the analysis of complete blood cell counts, including RBC count, platelet count, total WBC count, and differential WBC counts [22].
In-vivo bioavailability study of Storg- Zn and Storg-I in comparison with synthetic zinc and iron
Animals
The study was conducted using Sprague -Dawley rats (n=6) weighing 150-200 g. The animals were obtained from the institutional animal laboratory. The experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC), [BCP/IAEC/06/2022]. Animals were acclimated for 7 days with standard laboratory pellets and water ad libitum. The variation in body weight among the animals did not exceed 20% of the mean body weight. The room was environmentally controlled at a temperature of 23°C with a relative humidity of 60%. The photoperiod consisted of 12 hours of light and 12 hours of darkness.
Experimental design
This study aimed to compare the bioavailability of Storg-I, Storg-Zn, Synthetic Iron, and Zinc. SD rats (8-12 weeks, weighing 150- 200 g) were selected as the animal model and were divided into 4 groups, each containing 6 animals. The rats underwent a 2-week medication washout period, and food was withheld for 12 hours prior to dosing, although water was available throughout. A single oral dose of 200 mg/kg of either Storg-I or Synthetic Iron was administered via gavage, while a separate group received Storg-Zn and Synthetic Zn using the same dosing protocol. Blood samples (0. 6 ml) were collected from the retro-orbital plexus at intervals of 0.5, 1, 2, 4, 8, and 24 hours post-dosing. These samples were centrifuged for plasma separation and analyzed using Atomic Absorption Spectrometry (AAS) for Storg-I, Storg-Zn, Synthetic Zn, and Iron. Plasma was separated by centrifugation at 6000 rpm for 10 minutes, and the resulting plasma sample from each blood sample was divided into two aliquots and stored in suitably labeled heparin collecting tubes at -20°C until used. Estimations of Cmax, Tmax and t1/2 of all test samples were carried out using the Atomic Absorption Spectrometry (AAS) method [23].
Determination of Iron by atomic absorption spectrometry (AAS)
Iron is determined by atomic absorption spectrophotometry by means of an oxidizing air-acetylene flame, using an iron hollow-cathode lamp, at a wavelength of 248.3 nm, slit: 0.5 nm, and lamp intensity: 5 mA.
Stock and standard solutions
Place 10 ml of stock solution (1 g/l of iron) in a 100-ml flask, fill to volume with demineralized water. Calibration range is 2, 4, 6, 8 mg/1 of iron. Place successively 1.0, 2.0, 3.0, 4.0 ml of the solution at 100 mg/1 of iron in four 50 ml vials, fill to volume with demineralized water.
Sample preparation
Each sample is diluted with demineralized water in order to have a concentration of iron between 0 and 8 mg/l. Pass successively the calibration solutions and the blank which will be demineralized water or a water-acid solution with concentrations used for samples. Successively present the calibration solutions, and samples; note the corresponding absorbance.
Determination of zinc by atomic absorption spectrometry (AAS)
Zinc is determined by atomic absorption spectrometry by direct aspiration of the sample into an air-acetylene flame, using a zinc hollow-cathode lamp, at a wavelength of 213.8 nm, slit: 0.5 nm and lamp intensity: 3.5 mA.
Stock and standard solution
Prepare Zinc standard solution having concentration of 6000 ppm/100ml with demineralized water containing 0.001 per ml of concentrated HNO3. From above stock solution prepare series of at least six working containing from 1000 ppm to 6000 ppm per 100 ml with demineralized water containing 0.001 per ml of concentrated HNO3.
Sample preparation
Prepare sample solution having concentration of 4000 ppm/100ml with demineralized water containing 0.001 per ml of concentrated HNO3. Acidified water is used to dilute each sample in order to determine its zinc concentration. The calibration solutions and the blank (acidified water) should be passed progressively. The corresponding absorbance was noted after presenting the samples and calibration solutions.
Statistical analysis
Average of all results were compiled and mean ± standard error of mean (SEM) was determined. One-way ANOVA followed by Dunnett’s–Tukey’s multiple comparison tests were used to compile all the data. P values<0.05 were considered indicative of statistical significance. These analyses were done using Graphpad prism version. 10.0.3(273).
Results
In-vitro antioxidant activity
The antioxidant activity of Storg-I and Storg-Zn, along with synthetic iron and zinc, is shown in Figure 1. The antioxidant activity of Storg-I and Storg-Zn was compared with that of synthetic iron, zinc, and standard BHT. The free radical antioxidant scavenging activity of standard BHT, Storg-I, and synthetic iron was found to have IC50 values of 60.52, 127.95, and 111.05 µg/ml, respectively. Additionally, standard BHT, Storg-Zn, and synthetic zinc were found to have IC50 values of 60.52 µg/ml, 113.96 µg/ml, and 113.97 µg/ml. The present study indicates that Storg-I exhibits a substantial effect, while Storg-Zn shows significant free radical scavenging activity compared to synthetic iron and zinc. The findings suggest that Storg-I and Storg-Zn could be potential sources of natural antioxidants that may be important as therapeutic agents in preventing and slowing the progression of aging and age-associated oxidative stress-related degenerative diseases.
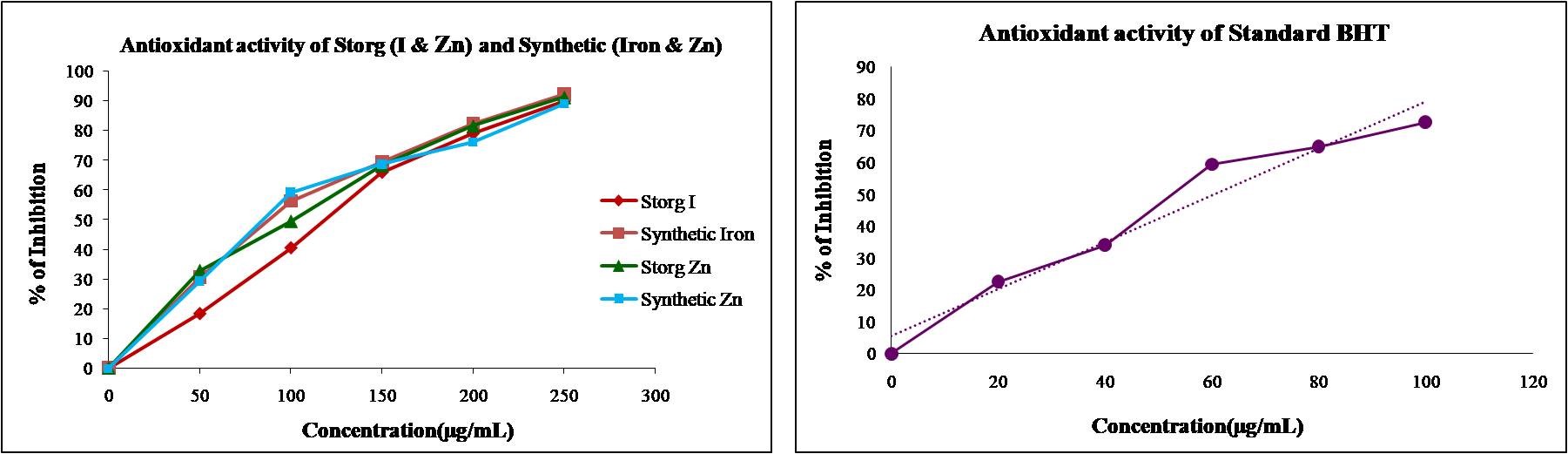
Figure 1. Antioxidant activity and IC¬50 value of storg I, Storg Zn, synthetic Iron and zinc compared with standard BHT
In-vivo Immunomodulatory activity
Hemagglutination assay
The humoral immune response was evaluated using the antibody titer. The levels of primary and secondary antibody titer in rat serum increased, which is evidence that Storg I and Synthetic Iron enhanced the humoral immune response to SRBC (Table 1). The study found that Storg-I, at doses of 50 and 100 mg/kg, resulted in a considerable rise in humoral antibody titer, while Synthetic Iron, at a dose of 100 mg/kg, further enhanced the humoral immune response to SRBC. Table 1 illustrates how Storg-Zn affects the primary and secondary antibody responses on the HA titer. Comparing the cyclophosphamide-treated group to the control group, a notable drop in the antibody titer was observed. The antibody titer increased significantly after administering Storg-Zn (100 mg/kg/p.o.) in comparison to the positive group and synthetic zinc. The treatment with Storg-Zn dramatically increased antibody titers, as shown by the results, suggesting that the serum of rats was producing more IgG and IgM antibodies.
Hematological analysis
The hematological count was significantly reduced by cyclophosphamide at a dose of 20 mg/kg. In comparison to the control group, animals treated with cyclophosphamide (negative control group) had significantly lower RBC, WBC, and platelet counts. Blood counts were restored after treatment with Storg-I and Storg-Zn in both low and high doses. Administration of a 100 mg/kg dose of Synthetic Iron and Synthetic Zinc also showed an increase in blood parameters when compared to the cyclophosphamide-treated group. A dose-dependent increase in blood parameters was observed in the treatment groups (Table 2).
Bioavailability study
The pharmacokinetic investigations of the oral administration of Storg-I, Storg-Zn, and synthetic drugs at a single dosage of 200 mg/kg to rats were effectively conducted in this work using the AAS method. The average plasma concentration-time curves for the two formulations and their synthetic drugs are shown in Table 3 and Figure 2. The results show that Storg-I, at a dose of 200 mg/kg of body weight, was found to take 2 hours to reach the maximum concentration of 197.3 µg/mL, while Synthetic Iron took 2 hours to reach the maximum concentration of 172 µg/mL in blood serum. Storg-Zn and Synthetic Zn took 2 hours to reach the maximum concentrations of 298 µg/mL and 70 µg/mL, respectively. Therefore, Storg-I and Storg-Zn possess efficient absorption in the gastrointestinal tract and significant levels in systemic circulation. The chemical analysis of Storg-I and Storg-Zn is shown in Tables 4 and 5.
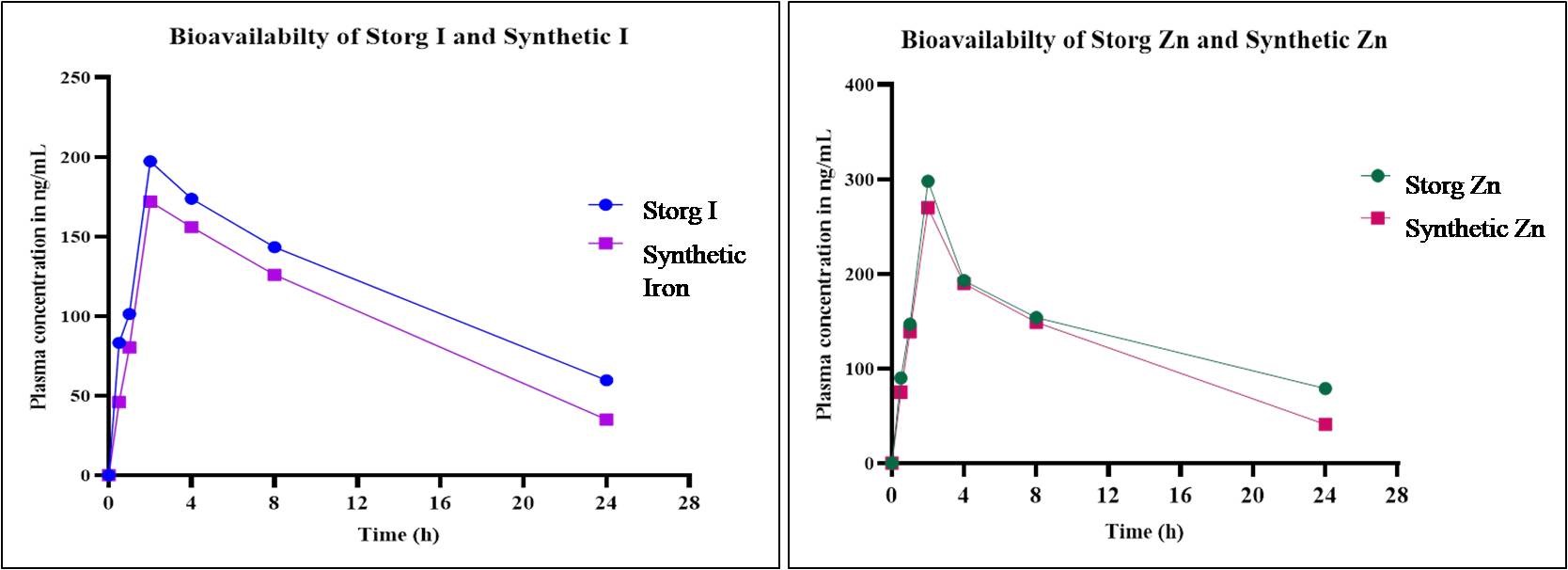
Figure 2. Mean plasma concentration time profile of storg I, synthetic Iron, storg Zn and synthetic Zn. Mean ± SD (n=6)
Discussion
The growing interest in natural products as alternatives to synthetic drugs is driven by the increasing awareness of the potential side effects and toxicity of synthetic compounds. Natural sources, particularly plant extracts, offer a rich repository of bioactive molecules that can provide therapeutic benefits with fewer adverse effects [24]. Recent studies have highlighted the biofortification, antioxidant, immunomodulatory, and bioavailability-enhancing properties of various plant-derived compounds, with Murraya koenigii (curry leaves) and Psidium guajava (guava leaves) being prime examples. Plant extracts like Murraya koenigii, rich in essential nutrients, are increasingly explored for their potential to combat inflammation, microbial infections, and free radical-induced diseases [25]. Similarly, guava leaves (Psidium guajava) are packed with antioxidants, fiber, vitamins, and minerals, providing medicinal benefits such as improved digestion, heart health, immunity, blood sugar control, and weight management [26]. According to these results, Storg-I and Storg-Zn, which are natural sources of iron and zinc, may be superior to synthetic supplements, which frequently require unique formulations to enhance absorption. Other phytochemicals in plant extracts may work in concert to increase the bioavailability of certain minerals, which is advantageous when treating immune-related disorders and nutritional deficiencies.
Temperature, humidity, and photosynthetic activity influence seasonal fluctuations in carbon isotope deposition (δ¹³C) in guava plants and curry leaves. Studies imply that δ¹³C readings can represent variations in water-use efficiency and stomatal conductance throughout seasons. During limited water supply or high temperatures, δ¹³C levels increase, indicating less stomatal opening and enhanced water-use efficiency. During colder and wetter seasons, δ¹³C might drop due to open stomata and less selective carbon absorption. Cooler and wetter seasons are ideal for collecting leaves due to decreased carbon deposition in plant extracts, resulting in lower δ¹³C values. This is owing to higher stomatal conductance and improved selectivity against carbon-13 during photosynthesis, making these leaves suited for applications requiring lower isotope Concentrations [27].
Storg-Zn (Guava) and Storg-I are potent natural antioxidants known for their ability to scavenge free radicals, thanks to their rich content of minerals and antioxidants that play a critical role in maintaining overall health and regulating oxidative stress. In this study, guava and curry leaf extracts were obtained using an aqueous solvent to isolate the mineral compounds, and their antioxidant activity was measured using the DPPH assay, a method that evaluates the ability of compounds to reduce the absorbance of DPPH, a stable free radical, at 517 nm [28]. Both Storg-I and Storg-Zn demonstrated significant antioxidant activity. For example, Storg-Zn IC50 of 113.96 µg/mL indicated that it was effective at scavenging free radicals, exhibiting significant scavenging activity when compared with the synthetic compound, which had an IC50 of 113.97 µg/mL. Additionally, Storg-I, with an IC50 of 127.95 µg/mL, indicated substantial antioxidant activity when compared with synthetic iron, which had an IC50 of 111.05 µg/mL. Despite the higher IC50, the guava extract exhibited comparable antioxidant activity to BHT, suggesting that zinc extracts from Psidium guajava are promising natural alternatives for managing diseases linked to oxidative stress and free radical damage. In some studies, natural antioxidants have shown greater antioxidant efficiency than synthetic antioxidants.
The humoral immunity and immunostimulant effects of guava leaf extract were assessed using hemagglutination and hematological analysis. Cyclophosphamide, an alkylating agent, suppresses immune function by causing DNA alkylation, which results in decreased WBC and differential leukocyte counts. However, the administration of guava leaf extract demonstrated a significant immunomodulatory effect, preventing mortality associated with myelosuppression [21]. The results showed that guava leaf zinc extract enhanced adaptive immunity by stimulating B lymphocytes, which are crucial for both innate and adaptive immune responses. Zinc plays a vital role in B cell proliferation, leading to the production of immunoglobulins that activate humoral immunity. At a dose of 100 mg/kg, the treatment group exhibited a markedly improved immune response compared to levamisole, a standard immunomodulator, indicating that guava leaf extract holds promise as an effective immunomodulator. Similarly, the immune system, a complex network defending the body against infections and disease-causing agents, can be modulated through stimulation or suppression to maintain health and prevent diseases. Additionally, the study demonstrated that Murraya koenigii leaf extract significantly increased the antibody titer in rats, thereby enhancing the humoral immune response to SRBCs. This suggests that the extract boosts B lymphocyte function, which is critical for antibody production. Furthermore, previous research has shown that Murraya koenigii leaf extract regulates oxidative stress metabolism, particularly in diabetic mice, further enhancing its immunomodulatory effects [29]. The immunostimulant effects of the aqueous extract of Murraya koenigii leaves demonstrate its ability to enhance both specific and non-specific immune responses. Central to humoral immunity are antibodies produced by plasma cells and B lymphocytes, which are crucial in pathogen defense. SRBCs, a T-dependent antigen, were used in this study to assess the humoral immune response [5]. The study also compared the immune-stimulating effects of Storg-I and synthetic iron in immune-suppressed rats. The humoral antibody titer in rats treated with Storg-I (100 mg/mL) was comparable to that of levamisole, a standard immune stimulant. Synthetic iron also showed significant immune-boosting effects, indicating the potential of Storg-I as an immune stimulant, especially in immune-compromised individuals.
Comparative studies of vitamins (Zn and Iron) suggest that while synthetic versions offer benefits, they cannot fully replicate the advantages of natural sources [30-32]. Bioavailability refers to the extent to which an intake enters the bloodstream and is available for physiological use. The solubility of zinc in the intestinal lumen is critical for absorption and is influenced by its chemical form and the presence of absorption enhancers or inhibitors. In this study, Storg Zn exhibited higher bioavailability compared to synthetic zinc, with an AUC of 2522 ng•h/mL at a 200 mg/kg dose and a peak concentration of 298 ng/mL at 2 hours, indicating efficient absorption and high bioavailability into the bloodstream, supporting its therapeutic potential. Storg-I (Cmax 197.3 ng/mL) showed better absorption than synthetic iron (Cmax 172 ng/mL), suggesting that the iron in Storg-I is more bioavailable. Iron from Aspergillus oryzae-enriched sources was absorbed similarly to ferrous sulfate, with slower release and fewer side effects [33]. Nonetheless, the encouraging findings of this investigation suggest that Storg-I and Storg-Zn have a great deal of promise for becoming innovative, plant-based medicines.
Conclusion
The findings of this study highlight the significant therapeutic potential of natural products derived from Psidium guajava and Murraya koenigii. The mineral extract of zinc from guava (Storg-Zn) demonstrated immunostimulatory effects, evidenced by an increased antibody titer and enhanced lymphocyte activity. This indicates that Storg-Zn boosts immunity and acts as an antioxidant with improved intestinal absorption and immunomodulatory properties, making it a promising therapeutic option. Additionally, Storg-I, an aqueous extract of Murraya koenigii leaves, exhibited notable antioxidant capacity, potential immunomodulatory activity, and showed higher bioavailability than synthetic iron, with reduced side effects. In conclusion, Storg-Zn and Storg-I represent innovative natural products with significant potential for enhancing immunity, acting as antioxidants, and supplementing individuals with iron deficiency.
Author contributions
FHM have made the conceptualization of the study. SCT & AT have done the data curation and formal analysis. SCT supervised the study, SCT, AT, SS & S have done the methodology and validation. AT, SS, S, ZFH wrote the manuscript, reviewed and edited. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare there are no conflicts of interest.
Funding
The authors declare no specific funding for this work.
Acknowledgements
We are very thankful to Bharathi College of pharmacy Mandya, Karnataka, India, helped us with in-vivo pharmacological studies.
References
- Harnafi H, Amrani S (2008) Spectrophotometric methods for determination of plant polyphenols content and their antioxidant activity assessment: An overview. Pharmacognosy review 2: 20.
- Hooda P, Malik R, Bhatia S, Al-Harrasi A, Najmi A, et al. (2024) Phytoimmunomodulators: A review of natural modulators for complex immune system. Heliyon 10: e23790. [Crossref]
- Pathak S, Fialho J, Nandi D (2022) Plant-based Immunomodulators and their potential therapeutic actions. J Explor Res Pharmacol 7: 243-256.
- Divya C (2019) Comparative study of synthetic vs. natural antioxidants in inflammatory diseases. EJCM 9: 65-68.
- Shah AS, Wakade AS, Juvekar AR (2008) Immunomodulatory activity of methanolic extract of Murraya koenigii (L) Spreng. leaves. Indian J Exp Biol 46: 505-509.
- Laily N, Kusumaningtyas RW, Sukarti I, Rini MR (2015) The potency of guava Psidium guajava (L.) leaves as a functional immunostimulatory ingredient. Procedia Chem 14: 301-307.
- Jain M, Gilhotra R, Singh RP, Mittal J (2017) Curry leaf (Murraya Koenigii): A spice with medicinal property. MOJ Biol Med 2: 236-256.
- Franyoto YD, Nurrochmad A, Fakhrudin N (2024) Murraya koenigii L. Spreng: An updated review of chemical composition, pharmacological effect, and toxicity studies. J Appl Pharm Sci 14: 011-027.
- Kumar M, Tomar M, Amarowicz R, Saurabh V, Nair MS, et al. (2021) Guava (Psidium guajava L.) Leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Foods 10: 752. [Crossref]
- Njoku OU, Joshua PE, Ossai EC, Agu CV, Ugwuanyi JO (2011) Antioxidant properties of Murraya koenigii. Asian J Res Chem 4: 1549-1552.
- Hui-Yin C, Gow C Y (2007) Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidiumguajava L.) leaves. Food Chemistry 101: 686-694.
- Balakrishnan R, Vijayraja D, Jo SH, Ganesan P, Su-Kim I, et al. (2020) Medicinal Profile, phytochemistry, and pharmacological activities of Murraya koenigii and its primary bioactive compounds. Antioxidants (Basel) 9: 101. [Crossref]
- Anderson GH, Zlotkin SH (2000) Developing and implementing food-based dietary guidance for fat in the diets of children. Am J Clin Nutr 72: 1404S-1409S. [Crossref]
- Janet R Hunt (2003) Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr 72: 633S-639S. [Crossref]
- Haase H, Rink L (2009) The immune system and the impact of zinc during aging. Immun Ageing 6: 9 [Crossref]
- Hambidge KM, Krebs NF (2007) Zinc deficiency: A special challenge. J Nutr 137: 1101-1105. [Crossref]
- Aydinoglu S (2022) Iron and zinc determination in dietary supplements by flame atomic absorption spectrophotometry. Braz J Pharm Sci 58: e21094.
- Piskin E, Cianciosi D, Gulec S, Tomas M, Capanoglu E (2022) Iron Absorption: Factors, limitations, and improvement methods. ACS Omega 7: 20441-20456. [Crossref]
- Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181: 1199-1200.
- Abebe D, Karim A, Bitew H, Periasamy G (2022) In-vivo evaluation of immunomodulatory activity of crude extract and solvent fractions of Cyphostemma adenocaule (Steud. exA. Rich). Heliyon 8: e12377. [Crossref]
- Shabbir A, Butt HI, Shahzad M, Arshad H, Waheed I (2016) Immunostimulatory effect of methanolic leaves extract of Psidium guajava (Guava) on humoral and cell-mediated immunity in mice. J Anim Plant Sci 26: 1492-1500.
- Rajani J, Ashok BK, Patgiri BJ, Prajapati PK, Ravishankar B (2012) Immunomodulatory activity of Āmalaki Rasāyana: An experimental evaluation. Anc Sci Life 32: 93-98. [Crossref]
- Stasys T, Laura S, Rolandas K (2004) Determination of iron in natural and mineral waters by flame atomic absorption spectrometry. J Serb Chem Soc 69: 393-402.
- Chaachouay N, Zidane L (2024) Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 3: 184-207.
- Harish KH, Prashanth KJ, Shruthi SD (2010) Pharmacognostic and phytochemical studies on the leaves of Murraya koenigii L Spreng. Pharmacophore 13: 231-238.
- Kareem AT, Kadhim EJ (2024) Psidium guajava: A review on its pharmacological and phytochemical constituents. Biomed Pharmacol J 17:1079-1090.
- Leavitt SW, Danzer S R (1992) Radiocarbon: Proceedings of the 14th international radiocarbon conference 34: 783-791.
- Szabo M, Idiţoiu C, Chambre D, Lupea AJ (2007) Improved DPPH determination for antioxidant activity spectrophotometric assay. Chemical Papers 61: 214-216.
- Paul S, Bandyopadhyay TK, Bhattacharyya A (2011) Immunomodulatory of leaf extract of Murraya koenigii in diabetic mice. Immunopharmacol Immunotoxicol 33: 691-699. [Crossref]
- Nelson EW, Streiff RR, Cerda JJ (1975) Comparative bioavailability of folate and vitamin C from a synthetic and a natural source. Am J Clin Nutr 28: 1014-1019. [Crossref]
- Pereira R, Costa M, Velasco C, Cunha LM, Lima RC, et al. (2022) Comparative analysis between synthetic vitamin E and natural antioxidant sources from tomato, carrot and coriander in diets for market-sized dicentrarchus labrax. Antioxidants 11: 63. [Crossref]
- Hatcher HC, Singh RN, Torti FM, Torti SV (2009) Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med Chem 1: 1643-1670. [Crossref]
- Reddy MB, Armah SM, Stewart JW, O’Brien KO (2018) Iron absorption from iron-enriched Aspergillus oryzae is similar to ferrous sulfate in healthy female subjects. Curr Dev Nutr 2: nzy004. [Crossref]


