Titanium dioxide absorbs light in the ultraviolet region. Shifting of the absorption from UV to visible light is very important to attain high efficiency. In this paper this was achieved by incorporation of cobalt into the titanium dioxide lattice of cobalt/titanium dioxide hollow spheres. Titanium dioxide hollow sphere and cobalt/titanium dioxide hollow sphere were prepared by a hydrothermal method in the presence of cyclohexylamine as a surfactant. For comparison purposes, titanium dioxide nanoparticles and cobalt/titanium dioxide nanoparticles were prepared by the same method in absence of the surfactant. The prepared materials were characterized by many techniques. The photocatalytic activity of cobalt/titanium dioxide hollow spheres is better than titanium dioxide hollow spheres, cobalt/titanium dioxide nanoparticles and titanium dioxide nanoparticles for hydrogen production by 1.25, 3.3 and 33.3 times, respectively. In addition, cobalt/titanium dioxide hollow spheres exhibit photocatalytic stability for hydrogen production which enable many time reuse of the photocatalyst.
hollow sphere, nanoparticles, TiO2, Cobalt, visible light, hydrogen production
Photocatalysis has attracted interest in many fields of scientific research and industrial applications. In photocatalysis, a catalyst is used in reactions under illumination of ultraviolet (uv) or visible radiation. The use of visible radiation allows conducting reactions in environmentally friendly fashion with no need to energy from other sources. Scientists are interested in designing catalysts with band energy that fall within the visible frequency. Other required properties in catalysts include nontoxicity, stability, possible reuse, ease of separation, favorable magnetic and electric properties along with other characteristics depending on the application required.
TiO2 is widely used photocatalyst as it possesses properties that include chemical stability, nontoxicity, possible reuse, possible incorporation in other materials, and favorable physical properties. However its wide band gab (Eg=3.2 eV) makes it active when used in reactions that involve uv light which constitute about 3%–5% of the solar spectrum; for this, researchers always try to manipulate its structure to narrow the band gab which in turn makes the derived material active in the natural or artificial sunlight [1].
TiO2 hollow spheres have a structure that attracted attention. Scientists developed Many approaches to synthesize TiO2 hollow spheres and this area of research is still active [2] Yan et al. fabricated TiO2 hollow spheres through a template-free solvothermal route and applied it to rhodamine degradation [3]. Eiden and Maret reported the synthesis of TiO2 hollow spheres consisting exclusively of crystalline rutile structure [4]. Wang et al. prepared anatase titania hollow micro spheres using styrene-acrylic acid copolymer latex particles as template and showed characterization of these spheres [5]. Lei et al. prepared TiO2 hollow spheres via a facile hydrothermal method without the use of any template agent and demonstrated improved performance in dye-sensitized solar cells [6]. Zhang et al. reported Controlled fabrication of nanosized TiO2 hollow sphere particles via acid catalytic hydrolysis/hydrothermal treatment and tested the catalyst on phenol removal [7]. Fing et al. reported catalyst-free hydrothermal method for the preparation of titania hollow spheres [8]. Lin et al. reported Synthesis of hollow spherical TiO2 for dye-sensitized solar cells and demonstrated the enhanced light harvesting efficiency of the structure [9]. Wang et al. reported the synthesis of TiO2 hollow microspheres with mesoporous surface via a facile template-assisted solvothermal reaction and demonstrated a superior adsorption performance for dye removal [10]. Ye et al. reported hydrothermal synthesis of TiO2 hollow microspheres for the photocatalytic degradation of 4-chloronitrobenzene [11].
Preparations that include variety of crystal structures and various dopants on the TiO2 hollow spheres are also of interest. Zhang et al. prepared hollow core/shell CeO2@TiO2 photocatalysts via precipitation-co-hydrothermal method and applied it in the removal of rhodamine B as a model dye pollutants [12]. Fang et al. prepared hollow carbon-doped titania composite spheres through a template-assisted method and illustrated its potential application prospect in the development of electrophoretic ink [13]. Qu et al. prepared TiO2 and TiO2: Gd3+ hollow spheres with Gd doping with the assistance of the carbon sphere templates and demonstrated enhanced photocatalytic activity [14]. Liu et al. solvothermally synthesized hybrid TiO2 hollow spheres using tetrabutyl titanate and hydrated metal sulfates as soft templates and reported excellent efficiency and durability in photo-decomposition of methyl orange (MO) under visible-light irradiation [15]. Geng et al. reported a facile route for the controllable design of fluorine-doped carbon-treated TiO2 hollow spheres with mesoporous shells for improved lithium storage [16]. Zhang et al. reported preparation carbon coated TiO2 hollow composite spheres with enhanced visible photocatalytic performance in the degradation of rhodamine B dye [17]. Cho et al. reported preparation and photocatalytic activity of nitrogen-doped TiO2 hollow nanospheres [18]. Wang et al. reported one-step template-free fabrication of mesoporous ZnO/TiO2 hollow microspheres and demonstrated the enhanced photocatalytic activity on degradation of methyl orange [19]. Tang et al. reported α-Fe2O3/TiO2 composite hollow spheres synthesis by a template-assisted precipitation reaction and the activity of the catalyst was tested via the photocatalytic decolorization of RhB aqueous solution [20]. Li et al. synthesized WO3/TiO2 composite, hollow-sphere photocatalyst using a template method and demonstrated improved photocatalytic activity [21]. Chattopadhyay et al. studied hydrogen production by the application of tin doped titania hollow spheres [22]. Zhang et al. developed an in-situ synthesis of c-doped titania hollow spheres with high photoctalytic activity [23].
In an effort to produce clean and environmentally friendly energy, scientists showed great interest in the production of hydrogen from water splitting using various materials [24,25]. TiO2 is important material in this area because of its aforementioned properties. Researchers try to manipulate this material’s properties to enhance its ability in the water splitting reaction. Scientists synthesized materials of various crystal structures and different morphologies for hydrogen production [26- 30]. Doping with various metals and non-metals is an important way to narrow the band gap and attain good properties [31-36]. Method of synthesis of a material could also impact the properties of the product [37].
In this paper we report the synthesis of a new Co doped TiO2 hollow spheres through the hydrothermal method in which cyclohexamine was used for the first time as a template and we apply this material in H2 production reaction.
Preparation of photocatalysts
- °C for 24 h after which the autoclave was left to cool to room temperature. The product was filtered and washed with distilled water then ethanol for several times, dried in vacuum at 80 °C for 10 h. TiO2 hollow spheres (THS) were synthesized in the same fashion except in the absence of cobalt nitrate hexahydrate.
For comparison purposes Cobalt/TiO2nanoparticles (Co/TN) and TiO2 nanoparticles (TN) were prepared by hydrothermal method. Co/TN was synthesized by dissolving 8.0 g of tetrabutyl titanate and 1.5 g cobalt nitrate hexahydrate in a mixed solvent composed of 40 mL absolute ethanol, 3 mL of distilled water and 0.3 mL 1M HNO3 under magnetic stirring for 30 min. The mixture was transferred into Teflon-lined stainless steel autoclave, which was sealed and maintained at 80 °C for 24 h. The autoclave was left to cool down to room temperature and the product was filtered and washed with distilled water then ethanol for several times, dried in vacuum at 80 °C for 10 h. TiO2 nanoparticles were synthesized following the same procedure in the absence of cobalt nitrate hexahydrate.
Characterization
In order to obtain morphological structure, the material of interest was suspended in ethanol and ultrasonicated for 30 m. portion of the suspension was dried on a carbon coated copper grid and loaded into a JEOL-JEM-1230 transmission electron microscope (TEM). Surface area was obtained from N2-adsorption measurements using a Nova 2000 series Chromatech apparatus at 77K. the crystalline phase of the four composites was determined using Bruker axis D8 with Cu Kα radiation (λ = 1.540 Å) at room temperature. A Thermo Scientific K-ALPHA spectrometer was utilized to obtain X-ray photoelectron spectroscopy (XPS) measurements. Uv-visible diffuse reflectance spectra (UV-Vis-DRS) was exploited to obtain band gap information utilizing a UV-Vis-NIR spectrophotometer (V-570, Jasco, Japan) at room temperature. Absorption was measured over 200-800 nm range. A Shimadzu RF-5301 fluorescence spectrophotometer was used to record photoluminescence emission spectra (PL).
Photo-catalytic tests
An important application in which the synthesized catalyst can be used is hydrogen production from water splitting. In the experimental setup a known weight of the photocatalyst was added into 450 mL aqueous solution containing 10 vol% methanol as a scavenger. The reaction system was sealed and the experiments were conducted at room temperature and atmospheric pressure. The heat from the lamp was prevented from affecting the reaction by placing a jacketed cooler made of quartz between the reactor and lamp. A dispersion using an ultrasonic cleaner at 100 W for 15 min was carried out. The slurry was aerated by N2 for 30 min and irradiated by visible light generated from 500 W Xenon lamp under continuous stirring. The evolved hydrogen gas generated from the reaction was analyzed by Agilent GC 7890 A gas chromatography system using N2 as carrier gas. Blank reactions with illumination in the absence of the photocatalyst and with photocatalyst in the dark were carried out. Both cases yielded no evolution of hydrogen gas.
Characterizations of materials
Figure 1 shows XRD patterns of the four synthesized composites, TN, Co/TN, THS, and Co/THS. The patterns reveal pure TiO2 anatase phase in all four composites with no peaks of pure cobalt or cobalt oxides which could be explained by low percentage of the cobalt present. The patterns also show s shift to right in peaks of Co/TN, and Co/THS which means incorporation of the cobalt ions within the titanium dioxide lattice. The crystallite sizes of TN, Co/TN, THS, and Co/THS are 22, 18, 14, 10 nm respectively as calculated by the Scherrer formula. It is clear that cobalt doping results in a decrease of crystallite and hollow spheres sizes.
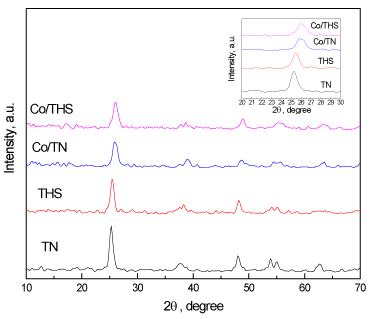
Figure 1. XRD patterns of TN, Co/TN, THS, and Co/THS samples.
Figure 2 shows TEM images of TN, Co/TN, THS, and Co/THS samples. Co/TN and TN are spherical in shape with sizes of 130 and 160 nm, respectively. However, Co/THS, and THS are hollow spherical in shape with shell thicknesses of 15-35 and 20-45 nm, respectively and core diameter of 190 and 240 nm, respectively. This reveals that the addition of cobalt ion decreases size of Co/THS and Co/TN samples.
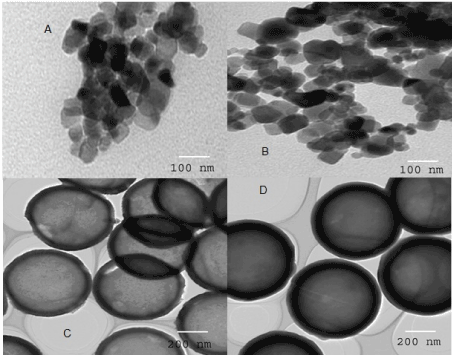
Figure 2. TEM images of (A)TN, (B) Co/TN, (C)THS, and (D) Co/THS samples.
Figure 3 shows XPS spectra of Co2p species for the Co/THS sample. It shows two peaks for Co 2p1/2 and Co2p3/2 at 794.9 and 779.7 eV, respectively confirming the cobalt ion (Co2+ ion) and that it is incorporated into TiO2 lattice.
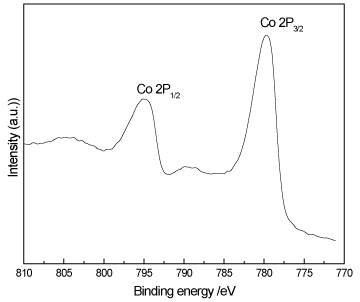
Figure 3. XPS spectra of Co 2p for Co/THS sample.
Adsorption-desorption isotherms of TN (A), Co/TN (B), THS (C), and Co/THS (D) samples are shown in Figure 4. TN and Co/TN have an isotherm of type II. While THS and Co/THS show an isotherm of type IV. This is interpreted as that samples THS and Co/THS have mesoporous materials.
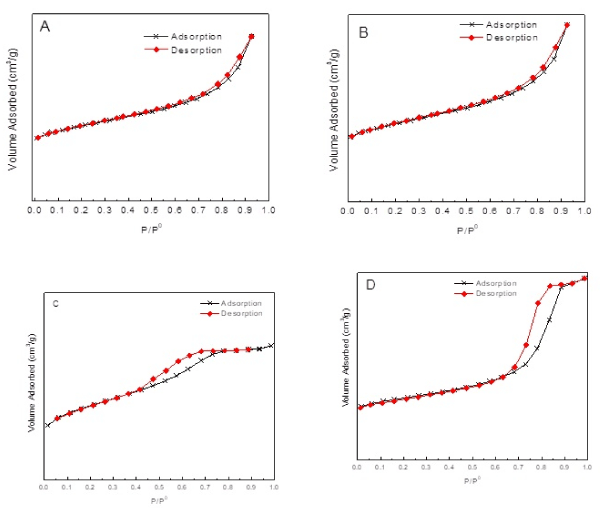
Figure 4. Adsorption-desorption isotherms of (A)TN, (B) Co/TN, (C)THS, and (D) Co/THS samples.
Pore size distribution of Co/THS is shown in Figure 5. It shows very narrow distribution around 1.9 nm indicating that hollow sphere samples may have a high surface area. The specific surface area of TN, Co/TN, THS, and Co/THS samples was measured by Nova 2000 resulting in values of 70, 80, 140 and 155 m2/g respectively. Therefore, the hollow sphere structure increases BET surface area of titanium dioxide. These results show that there are two factors affecting the photocatalytic activity of titanium dioxide, namely the higher surface area due to hollow spherical structure and the presence of doped cobalt ion.
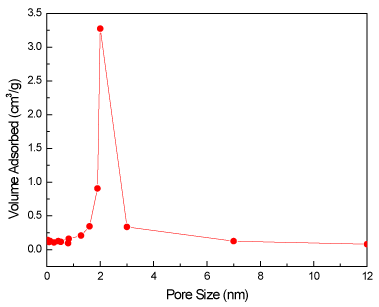
Figure 5. Pore size distribution of Co/THS sample.
UV-Vis spectra of TN, Co/TN, THS, and Co/THS samples (Figure 6) reveal a red shift of absorption edges of titanium dioxide toward higher wavelengths going from nanoparticles to hollow spherical structure and also by presence of cobalt ion in the titanium dioxide lattice. The values of band gap energy of TN, Co/TN, THS, and Co/THS samples calculated from their respective UV-Vis spectra were 3.2, 3.01, 2.89 and 2.72 eV respectively, Showing narrowing in the band gap, hence more efficiency toward visible light photocatalysis.
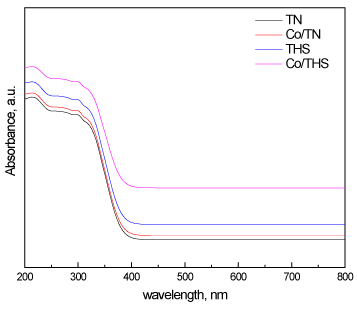
Figure 6. UV-Vis spectra of TN, Co/TN, THS, and Co/THS samples.
Pl spectra of TN, Co/TN, THS, and Co/THS samples (Figure7) show peak intensity decrease in the following order TN ˃ Co/TN ˃ THS ˃ Co/THS which again shows that the red shift because the change of phase of titanium dioxide from nanoparticles to hollow spheres also because of the incorporation of the cobalt ion into titanium dioxide lattice. The values of band gap energy of TN, Co/TN, THS, and Co/THS samples calculated from their Pl emission spectra were 3.2, 3.02, 2.88 and 2.71 eV respectively confirming data observed from the UV-Vis spectra.
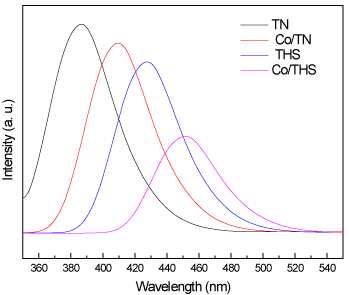
Figure 7. Pl spectra of TN, Co/TN, THS, and Co/THS samples.
Evolution of photocatalytic performance
Type of the photocatalyst, dose of the Co/THS photocatalyst, Recycling and reuse of Co/THS were studied to measure the photocatalytic performance for hydrogen production under visible light conditions.
Effect of type of photocatalyst on amount of hydrogen evolution was studied under the following conditions: light source is 500 W Xe lamp; reaction time is 4 h; dose of photocatalyst is 0.8 g/L; volume of aqueous solution is 450 mL. Figure 8 shows the effect of type of photocatalyst on amount of hydrogen evolution. TN sample almost has no photocatalytic activity, as TN absorbs in the UV region and reaction was carried out under visible light. Performance of Co/TN sample for hydrogen evolution was increased from 6 to 60 µmol, respectively, due to decrease band gap of TN from 3.2 to 3.01 eV by cobalt doping. We observed that photocatalytic performance of THS sample for hydrogen evolution was increased from 6 to 160 µmol, respectively, due changing phase of titanium dioxide from nanoparticles to hollow spherical structure. Also, the pphotocatalytic performance of Co/THS sample for hydrogen evolution was increased from 160 to 200 µmol by cobalt doping. Therefore, morphology of titanium dioxide affects the photocatalytic performance along with doping of cobalt ions.
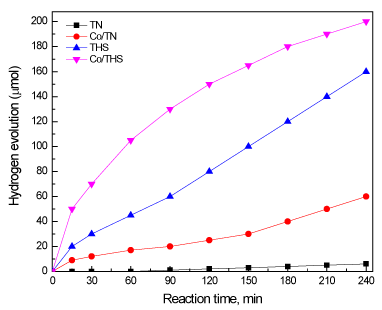
Figure 8. Effect of type of photocatalyst on amount of hydrogen evaluation .
Effect of dose of Co/THS photocatalyst on amount of hydrogen evolution was studied under the following conditions: light source is 500 W Xe lamp; reaction time is 4 h; dose of photocatalyst is changed from 0.4 to 2.0 g/L; volume of aqueous solution is 450 mL. Figure 9 shows effect of dose of Co/THS photocatalyst on amount of hydrogen evolution. Hydrogen evolution increased from 150 to 250 µmol by increased dose of Co/THS photocatalyst from 0.4 to 1.6 g/L, respectively. This could be explained by the increase in number of available sites for photocatalytic reaction as the dose increases resulting in more photocatalytic activity. Upon increasing the dose of photocatalyst above 1.6 g/L the amount of hydrogen evaluation drops to 190 µmol. This may be resulting from hindrance of light penetration due to high concentration of photocatalysts particles in the reaction solution.
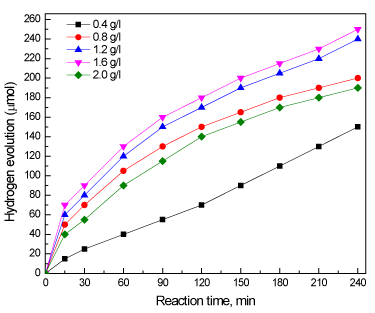
Figure 9. Effect of dose of Co/THS photocatalyst on amount of hydrogen evaluation.
Recycling and reuse of Co/THS photocatalyst on amount of hydrogen evaluation was studied under the following conditions: light source is 500 W Xe lamp; reaction time is 4 h; dose of photocatalyst is 1.6 g/L; volume of aqueous solution is 450 mL. Figure 10 shows Recycling and reuse of Co/THS photocatalyst on amount of hydrogen evaluation. It is clear that Co/THS photocatalyst has photocatalytic stability and can be used and recycled many times.
Figure 10. Recycling and reuse of Co/THS photocatalyst on amount of hydrogen evaluation.
Titanium dioxide hollow spheres and cobalt/titanium dioxide hollow spheres were prepared by a hydrothermal method in the presence of cyclohexylamine as a surfactant. For comparison purposes, titanium dioxide nanoparticles and cobalt/titanium dioxide nanoparticles were prepared by the same method in absence of surfactant. The hydrothermal method in the presence of a surfactant results in the formation of titanium dioxide hollow spheres and the incorporation of cobalt into titanium dioxide lattice. A red shift is also observed because of the hollow spherical structure and the doping of Co ions. The photocatalytic activity of cobalt/titanium dioxide hollow spheres is better than titanium dioxide hollow spheres, cobalt/titanium dioxide nanoparticles and titanium dioxide nanoparticles for hydrogen production by 1.25, 3.3 and 33.3 times, respectively. Cobalt/titanium dioxide hollow spheres show photocatalytic stability for hydrogen production for repetitive use.
This Project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. P-11-130-436. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
- Chen J, Qiu F, Xu W, Cao S, Zhu H (2015) Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Applied Catalysis A: General 495: 131-140.
- Zhang P, Li A, Gong J (2015) Hollow spherical titanium dioxide nanoparticles for energy and environmental applications. Particuology 22: 13-23.
- Yadav HM, Kolekar TV, Pawar SH, Kim JS (2016) Enhanced photocatalytic inactivation of bacteria on Fe-containing TiO2 nanoparticles under fluorescent light. J Mater Sci: Mater Electron 27: 4068-4073.
- Eiden S, Maret G (2002) Preparation and Characterization of Hollow Spheres of Rutile. Journal of Colloid and Interface Science 250: 281-284.
- Wang D, Song C, Lin Y, Hu Z (2006) Preparation and characterization of TiO2 hollow spheres. Materials Letters 60: 77-80.
- Lei BX, Zhang P, Qiao HK, Zheng XF, Zhang XX (2014) A facile template-free route for synthesis of anatase TiO2 hollow spheres for dye-sensitized solar cells. Electrochimica Acta 143: 129-134.
- Zhang Q, Li W, Liu S (2011) Controlled fabrication of nanosized TiO2 hollow sphere particles via acid catalytic hydrolysis/hydrothermal treatment. Powder Technology 212: 145-150.
- Feng X, Yang L, Liu Y (2010) Preparation of titania hollow spheres by catalyst-free hydrothermal method and their high thermal stabilities. Applied Surface Science 257: 756-761.
- Lin XP, Song DM, Gu XQ, Zhao YL, Qiang YH (2012) Synthesis of hollow spherical TiO2 for dye-sensitized solar cells with enhanced performance. Applied Surface Science 263: 816-820.
- Wang R, Cai X, Shen F (2014) TiO2 hollow microspheres with mesoporous surface: Superior adsorption performance for dye removal. Applied Surface Science 305: 352-358.
- Ye M, Chen Z, Wang W, Shen J, Ma J (2010) Hydrothermal synthesis of TiO2 hollow microspheres for the photocatalytic degradation of 4-chloronitrobenzene. Journal of Hazardous Materials 184: 612-619.
- Zhang L, Zhang J, Jiu H, Zhang X, Xu M (2015) Preparation of hollow core/shell CeO2@TiO2 with enhanced photocatalytic performance. J Mater Sci 50: 5228-5237.
- Fang Y, Wang J, Li J, Li L, Lin J, et al. (2016) Preparation and application of black hollow carbon-doped titania composite spheres for electrophoretic displays. J Mater Sci: Mater Electron 27: 6115-6121.
- Qu X, Yan X, Hou Y, Wang P, Song H (2015) Preparation of Gd-doped TiO2 hollow spheres with enhanced photocatalytic performance. J Sol-Gel Sci Technol (2015) 76: 699-707.
- Ruiping L, Feng R, Jinlin Y, Weiming S, Zhiming S, et al. (2016) One-step synthesis of hierarchically porous hybrid TiO2 hollow spheres with high photocatalytic activity, Front Mater Sci 10: 15-22.
- Geng H, Ming H, Ge D, Zheng J, Gu H (2015) Designed fabrication of fluorine-doped carbon coated mesoporous TiO2 hollow spheres for improved lithium storage. Electrochimica Acta 157: 1-7.
- Zhang Z, Zhou Y, Zhang Y, Sheng X, Xiang S (2013) A spontaneous dissolution approach to carbon coated TiO2 hollow composite spheres with enhanced visible photocatalytic performance. Applied Surface Science 286: 344-350.
- Cho HJ, Hwang PG, Jung D (2011) Preparation and photocatalytic activity of nitrogen-doped TiO2 hollow nanospheres. Journal of Physics and Chemistry of Solids 72: 1462-1466.
- Wang Y, Zhu S, Chen X, Tang Y, Wang H (2014) One-step template-free fabrication of mesoporous ZnO/TiO
2 hollow microspheres with enhanced photocatalytic activity. Applied Surface Science 307: 263-271.
- Tang H, Zhang D, Tang G, Ji X, Yang X (2013) Hydrothermal synthesis and visible-light photocatalytic activity of a-Fe2O3/TiO2 composite hollow microspheres. Ceramics International 39: 8633-8640.
- Lv K, Li J, Qing X, Li W, Chen Q (2011) Synthesis and photo-degradation application of WO3/TiO2 hollow spheres. Journal of Hazardous Materials 189: 329-335.
- Chattopadhyay J, Kim HR, Moon SB, Pak D (2008) Performance of tin doped titania hollow spheres as electrocatalysts for hydrogen and oxygen production in water electrolysis. Int J Hydrogen Energy 33: 3270-3280.
- Zhang Y, Zhao Z, Chen J, Cheng L, Cao S (2015) C-doped hollow TiO2spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Applied catalysis B: Environmental 165: 715-722.
- Ozbay B, Genc N, Ozbay I, Baghaki B, Zor S (2016) Photocatalytic activities of polyaniline-modified TiO2 and ZnO under visible light: an experimental and modeling study. Clean Techn Environ Policy 18: 2591-2601.
- Ameen S, Akhtar MS, Kim YS, Yang OB, Shin HS (2011) An effective nanocomposite of polyaniline and ZnO:preparation, characterizations, and its photocatalytic activity. Colloid Polym Sci 289: 415-421.
- Sharma S, Singh S, Khare N (2016) Synthesis of polyaniline/CdS (nanoflowers and nanorods)nanocomposites: a comparative study towards enhanced photocatalytic activity for degradation of organic dye. Colloid Polym Sci 294: 917-926.
- Min S, Wang F, Han Y (2007) An investigation on synthesis and photocatalytic activity of polyaniline sensitized nanocrystalline TiO2 composites. J Mater Sci 42: 9966-9972.
- Olad A, Nosrati R (2013) Preparation, characterization, and photocatalytic activity of polyaniline/ZnO nanocomposite. Res Chem Intermed 39: 3969-3979.
- Cheng Y, An L, Zhao Z, Wang G (2014) Preparation of polyaniline/TiO2 composite nanotubes for photodegradation of AZO dyes. Journal of Wuhan University of Technology-Mater Sci Ed 29: 468-472.
- Wei J, Zhang Q, Liu Y, Xiong R, Pan C, et al. (2011) Synthesis and photocatalytic activity of polyaniline–TiO2 composites with bionic nanopapilla structure. J Nanopart Res 13: 3157-3165.
- Olad A, Behboudi S, Entezami AA (2012) Preparation, characterization and photocatalytic activity of TiO2/polyaniline core-shell nanocomposite. Bull Mater Sci 35: 801-809.
- Agarwal S, Tyagi I, Gupta VK, Golbaz F, Golikand AN (2016) Synthesis and characteristics of polyaniline/zirconium oxide conductive nanocomposite for dye adsorption application. Journal of Molecular Liquids 218: 494-498.
- Patil UV, Ramgir NS, Karmakar N, Bhogale A, Debnath AK, et al. (2015) Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Applied Surface Science 339: 69-74.
- Salvatierra RV, Zitzer G, Savu SA, Alves AP, Zarbin AJG, et al. (2015) Carbon nanotube/polyaniline nanocomposites: Electronic structure, doping level and morphology investigations. Synthetic Metals 203: 16-21.
- Onn TM, Ramirez LA, Monai M, Soh T, Talati M, et al. (2016) Modification of Pd/CeO2 catalyst by Atomic Layer Deposition of ZrO2. Applied Catalysis B: Environmental 197: 280-285.
- Tang C, Sun B, Sun J, Hong X, Deng Y, et al. (2016) Solid state preparation of NiO-CeO2 catalyst for NO reduction. Catal Today 281: 575-582.
- Kim TH, Seon H, Park DW (2016) Synthesis of CeO2 nanocrystalline powders using DC non-transferred thermal plasma at atmospheric pressure. J Sol-Gel Sci Technol 27: 2012-2018.










