Abstract
Diabetes mellitus remains as a pandemic disease, associated to progressive and irreversible complications including lower extremity ulcerations, derived from a predisposition to ischemia, neuropathy and an intrinsic wound healing failure. The molecular operators supporting wound chronicity remain elusive, but a local deficit of growth factors is invoked as a common cause for proliferative arrest, apoptosis, and cells senescence. The prodegradative environment of these lesions contributes to reduce growth factors availability and receptors’ physiology. The introduction of growth factors in the clinical arena was precocious since critical pieces of chronicity pathophysiology had not been identified. The topical administration of these agents failed by the effect of local proteolysis, narrow bioavailability window, inadequate diffusion, and a harsh polymicrobial biofilm. To circumvent these pharmacodynamic limitations, we envisioned an intra-ulcer infiltrative delivery route for epidermal growth factor (EGF), based on the rationale derived from experimental evidences. The clinical development program with this procedure has comprised from a proof-of-concept to post-marketing studies in poor-prognosis, ischemic, neuropathic, and neuroischemic wounds, involving more than 300 000 patients along 20 years. Pharmacovigilance studies demonstrated that infiltrated EGF is therapeutically effective and safe to circumvent the limitations of the classic topical administration. This pharmacological intervention has remained as an adjuvant therapy to conventional treatments and wound care protocols. Generation of EGF nanovesicles is envisioned as a promising future direction to trigger the re-epithelialization of stagnant, non-resurfaced diabetic wounds upon topical administration.
Keywords
chronic wounds, diabetic complications, diabetic foot ulcer, growth factors, epidermal growth factor (EGF)
Introduction
Diabetic foot ulcer (DFU) is an example of chronic complex wound, demographically approaching to pandemic proportions, and still remaining as an unmet medical need [1]. Despite decades of research efforts and resources investments, diabetic population contributes to 80% of all non-traumatic lower extremity amputation around the world, leading to disability, social exclusion, and early mortality [2,3]. Diabetic patients exhibit a failure in their healing mechanism as skin cells are severely affected by the toxic effects of an abnormal glucose burden and adjacent biochemical derivatives [4-6]. Thus, diabetic wounds are transversed by numerous limiting factors that impair the ordered progression of overlapping cellular and molecular healing events. In this milieu converge and synergize a prolonged inflammatory reactivity, an excessive production of proteolytic enzymes, an uncontrolled spillover of free radicals, a deficiency of fibroangiogenic growth factors (GFs), a reduction in stem cells recruitment, and an increased number of senescent cells [7-10]. Ultimately, these factors pave the way for the onset of the chronicity phenotype, clinically expressed as a torpid granulation response, abnormal angiogenesis, reduced contraction rate, and stagnant re-epithelialization [8,11,12].
The sequential discovery of GFs at the beginning of the 60’s and their biological capability to enhance cells proliferation, migration, and to circumvent cells cycle arrest [13,14], founded the rationale for their introduction to treat a variety of acute and chronic wounds. During the 80’s and the 90’s, a myriad of enlightening articles appeared describing the beneficial effects of topically administered GFs to different experimental biomodels of skin wounds [15-25]. Nonetheless, the clinical and molecular complexity of a human chronic wound is yet far to be recapitulated in an animal model [26-28]. At the times of GFs hyperenthusiasm, there was an incomplete understanding of the molecular basis supporting tissue repair, and particularly of its failure. Elemental concepts for chronic wound management had not yet come to light. For example, TIME (tissue, infection/inflammation, moisture balance, and edge of wound) was first implemented at the early 2000’s, as an attempt to offer a framework for a structured and systematic approach to chronic wound bed preparation [29-31]. Of note, however, TIME principles are only a part of the systematic and complete evaluation of each patient at every wound assessment, which was later acknowledged [29-31]. More advanced studies suggested that the use of GFs was somewhat precipitated, as subsequent findings showed that chronic wound milieu may impair the response to GFs, by preventing the binding and subsequent activation of the receptors for transducing the signal. It was the case of the down-regulation of epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor beta (TGF-β) receptors autophosphorylation, and downstream signaling in diabetic and venous ulcers [32-34].
Since Regranex (PDGF-BB isoform) approval by the USA Food and Drug Administration (FDA) in 1997, no other GF has been approved for DFU treatment. Regranex progress was additionally shadowed, when in 2008 the FDA included a “black box warning” of increased mortality rates secondary to malignancy in patients after treatment with three or more vials (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116909.htm).
Although during the past 20 years there was an increasing amount of novel, basic science-based approaches and developments that include wound dressings, living cells equivalents, and smart GF formulations for an efficient local delivery; some of these approaches still need clinical validation and others vanished along the way due to limited therapeutic impact [35-38].
The interventional procedure based on the infiltrative delivery of EGF directly into the bed and contours of diabetic complex, high-grade, neuropathic and ischemic lower extremity wounds, including both ulcers, and stagnant amputation residual bases; emerged and consolidated as a successful alternative to circumvent the limitations of the topical application route [39]. This innovation pursued the efficacious intra-tissue delivery of a stabilized and integral molecule with proven tissue repair-enhancing effects. This EGF injectable formulation is not the “standalone” within the medical resources for complex diabetic wounds. Its polyvalent effects on cellular populations are largely integrated and supported by the conventional pharmacological interventions, and medical management protocols for this pathology.
Twenty years of medical practice has shown that despite the complexity of the repair response, the appropriate delivery of the target GF may restore the “acute healing phenotype” of a hard-to-heal wound.
This narrative review describes the line of thoughts and fundamentals that encouraged us to foster the hypothesis that by simply injecting EGF down into the lesion bed, would translate in unprecedented clinical outcomes. Our views are largely substantiated by the retrospective analysis of a group of classic articles from the 80’s and 90’s, which were fundamental to understand the conundrum of GFs molecular pharmacology into an ulcer bed.
Overview of diabetic foot ulcers (DFU)
Diabetes mellitus is characterized by the onset and progression of multi-organs complications resulting from biochemical derangements and epigenetic factors, that ultimately translate into irreversible morphofunctional changes as a response to glucooxidative stress [5,40]. Of all these complications, DFUs are amongst the most common, frightened and debilitating [2], ultimately leading to amputation-disability, and early mortality [2,41].
According to experts’ opinion, 19-34% of diabetic patients develops DFU, whereas the five years post-amputation mortality rate is greater than 70%, only preceded by lung cancer [42,43]. Accordingly, the International Working Group on Diabetic Foot has reported that a diabetic foot amputation is done every 20 seconds for over a million of patients every year [44].
The onset of DFU is driven by two major intrinsic predisposing factors: peripheral neuropathy (distal sensorimotor polyneuropathy) and peripheral arterial diseases [45-49]. Besides, diabetic individuals are also affected by cutaneous and mucosal infections given a dysregulation in primary surveillance, recognition, activation, and neutralization mechanisms, all processes of the innate immune response [50-52].
The glucotoxic environment and its distal effectors impair and disrupt the flow of overlapping healing phases, leading to a stagnant wound, chronically arrested in an unproductive inflammatory phase [11,53-55]. Diabetes predisposes to inflammation, which is more a condition than a transient reaction, while impairment in its resolution also perpetuates the inflammatory stage [56-58]. This model of chronic complex wound is therefore overloaded by a network of inflammatory cytokines, an uncontrolled production of local proteases, cytotoxic reactive oxygen and nitrogen species, and a polymicrobial biofilm [59]. Mechanistically speaking, this phenotype seems to be driven by three major factors: precocious cellular senescence, proliferative arrest, and unscheduled apoptosis of granulation tissue-productive cells [5,57,60-64].
Several evidences demonstrate that the local deficit of GFs is, among other factors, responsible for these three proximal inducers of wound chronification in diabetes, and other types of chronic wounds [5,65-68]. Classic results support this notion: (1) decreased expression of GFs by chronic wound cells [36,67,69,70]; (2) decreased expression and reduced functionality of GFs receptors in diabetic ulcers fibroblasts [32]; (3) decreased responsiveness to GFs-induced proliferation by cultured fibroblasts in an ulcer-age dependent fashion [71-73]; (4) proliferative arrest, and onset of biochemical and morphological traits of senescence by chronic wounds fibroblasts [5,74-76]; and (5) failure to stimulate DNA synthesis and proliferative commitment of fibroblasts, keratinocytes, and vascular endothelial cells when exposed to chronic wounds fluid [77,78].
GFs intervention for problem wounds
The primary attempts to administer GFs in chronic wounds were likely driven by the concept of “replacement therapy”, aiming to restore local wound cells biological competence, and ultimately resume the physiological healing trajectory [39]. Since a senescent phenotype depends on the downregulation of proliferation regulatory-positive genes (i.e., MYC, FOS, CDK2, and CCNs), ordinarily controlled by paracrine secretion of GFs [79,80], the early idea that locally administered GFs could reverse this arrest in wound cells, appeared thoughtfully justified [79-83].
GFs are defined as biologically active polypeptides that interact with specific cell surface receptors inducing DNA synthesis, cell division, migration, differentiation, survival, and phenotypic transition [84]. Most of these events are essential for the dynamic progression of the healing phases [66], and contribute to granulation tissue formation and contraction, organization of a novel angiogenic network, sustain re-epithelialization, and ultimately remodeling of the scar [14,85]. Thus, each GF is endowed with more than one biological role along the healing process, which depends on the target cell and the time phase of the wound [86,87]. GFs are also ubiquitous ingredients in most epithelial and mesenchymal mammals tissues; not only controlling tissue healing events, but ensuring epithelial cells populations’ homeostatic turnover and biological resilience [88]. In general terms, GFs have a major role in coordinating and integrating tissue physiology [89-92]. Several GFs are known to be involved in the wound healing process. These include PDGF, EGF, fibroblast growth factor, insulin-like growth factors 1 and 2, vascular endothelial growth factor, TGF-β, and keratinocyte growth factor [70,93,94].
The history of GFs pharmacology, as an alternative to combat wound chronicity, has not progressed along a smooth road upon time. So far, PDGF in its pharmaceutical jelly presentation (Regranex, https://www.smith-nephew.com/professional/products/all-products/regranex/), remains as the only GF approved by the FDA to be topically administered to low grade neuropathic DFU (https://www.fda.gov/media/76010/download). To the best of our knowledge the first clinical intervention with a recombinant human GF dates back to 1989, when Brown and co-workers topically administered EGF to accelerate the epidermal regeneration of skin graft donor sites in burn patients [95]. Experimental studies at that time, however, began to emphasize that certain pharmacokinetic requisites had to be met, for an effective outcome with EGF topical administration [96]. The initial expectations about GFs topical application as “magic bullets” for accelerating resurfacing of at least partial thickness injuries [97], soon disappeared following two clinical studies. In a chronological sequence, Falanga failed to show a significant re-epithelialization of chronic venous ulcers upon topically administering EGF for a maximum of 10 weeks [98]. Subsequently, Cohen showed in a rigorously controlled study with healthy volunteers, that topical administration of EGF failed to enhance re-epithelialization of partial-thickness, dermatome-induced acute wounds [99]. These unexpected results warned about the need for additional research in GFs physiology and pharmacology, as in the understanding of the wound milieu biochemistry [98,100].
Thereafter, numerous investigations emphasized about the necessity of modifying wound local factors through wound bed preparation, in order to ensure an appropriate GFs pharmacodynamic response (for review see: [31]). Others claimed the need for GFs combinations as the optimal tool to restore the healing trajectory in chronic wounds [100,101]. The debut of GFs in the clinical arena seemed premature in relation to the basic science supporting its molecular pharmacology [102]. This statement is likely based on the observation by Jeff Davidson's group in 1985, which demonstrated that EGF wound healing enhancement was solely promoted under a prolonged, sustained, in situ slow release system [15]. The biological significance of a prolonged bioavailability of EGF and the ensued interaction with its receptor, were further validated when the GF was incorporated into multilamellar liposomes, which enhanced incisional wounds tensile strength over 200% as compared to controls [18]. Conclusively, according to Davidson’s group data, EGF could stimulate the mitogenic response in wound cells if the receptors were exposed/stimulated for at least 8 to 12 hours [15].
These basic science results prompted the need to introduce novel local delivery systems for EGF, a polypeptide with three disulfide bonds, largely sensitive to proteolysis, and that required a prolonged half-life within the wound milieu to express its biological bounties. Thus, the topical application method employed so far appeared proved not to be an ideal delivery for chemically-sensitive polypeptide GFs. This represented a challenge, as it was known that even the acute wounds environment is harsh for locally applied proteins, and particularly because diabetic wounds milieu contains all the detrimental ingredients against GFs chemical stability, bioavailability, and ultimately therapeutic capability [103]. Although experimental studies at the early 90’s clearly showed the importance of formulating EGF with protease inhibitors, these combinations never progressed to a clinical trial [82,104-106].
EGF biological effects to counteract DFU chronicity
EGF is perhaps the most widely studied GF in mammals biology, and we have upraised the hypothesis that this GF is biologically competent to largely counteract the molecular drivers of the chronicity phenotype [107,108]. Since the 60’s interpretation that its exogenous administration to mice, reprogrammed chronologically-specified skin cells mitogenic and differentiation events; EGF was applied to repair multiple forms of wounds in both peripheral and internal tissues [107,109,110]. Aside from the classic mitogenic, motogenic and cytoprotective actions during healing events [107], EGF biological spectrum involves a potential anti-senescent effect. Experimental evidences postulate that EGF aborts cellular senescence programs [111,112]. In line with this, a groundbreaking study revealed that cell cultures depleted of EGF progress toward a senescent phenotype with elevated SA-β-gal activity, decreased proliferation, reduced Rb phosphorylation, and elevated p21 expression. These results advise that cultured cells may depend on EGF as a mechanism to escape from senescence and ensure proliferation, thus placing EGF as a central mediator in preserving mitogenic competence, and avoiding senescence [113,114]. Likewise, EGF showed to activate the expression of human telomerase reverse transcriptase in cultured cells, via Ets-2, a cancer-specific transcription factor that appears to depend on EGF receptor (EGFR)-mediated Erk and Akt activation [115]. Experimental studies suggest that EGF enhances cell survival and tissue replenishment in otherwise lethal scenarios, by controlling oxidative stress and by mitigating cellular senescence [116-119].
Diabetes is associated to a particular deficit of circulating [120] and salivary levels of EGF [121], which synergizes with the glucotoxic environment to drive multicellular systems demise [122-126]. The early 90’s experiments, in which we examined the response to locally injected EGF to injured sciatic nerves in rats, became a turning point and paved the way to what was therapeutically achievable by an efficient delivery method. At the same time, this animal model itself somehow recapitulated the diabetic-like combination of neuropathy and tissues hypoxia. At the end, EGF local infiltration restored the neurological response, enhanced hind limbs soft tissues survival, and reduced the onset of plantar ulcers and toes necrosis [127]. We subsequently showed in a variety of pathological models that single or repeated EGF systemic injections or local infiltrations, stimulated “clear-cut” cytoprotective and proliferative responses, supporting the intrinsic ability of EGF at supraphysiological concentrations to promote tissue repair [107,128,129].
Evidences supporting EGF intralesional infiltration rationale
Injecting EGF down into the base and contours of the wounds, including the dermo-epidermal junction, appears to: (1) ensure the direct delivery of the GF to the responsive cells, (2) reduce its local degradation, (3) jump over the diffusion limiting barriers from the wound surface to the deeper stratum, and (4) ensure EGF bioavailability for a prolonged interaction with the receptor in a deep layer cells. The immunohistochemical demonstration on the existence of a cellular distribution of EGFR and other cell proliferation regulators, along the longitudinal axis of neuropathic DFU granulation tissue bed, turned a crucial hint for the rationale of the local infiltration treatment. In parallel, the finding contributed to explain why topical administration of semisolid formulations had failed. As shown in Figure 1, EGFR is not detected on the wound surface cells layer, in contrast to deeper cells strata where it is intensely expressed.
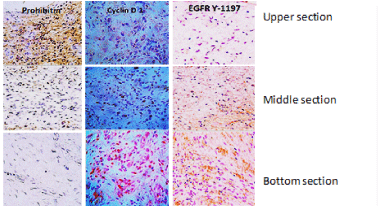
Figure 1. Cell proliferation cycle controllers in granulation tissue of neuropathic ulcers
Three vertical sections (≈2-3mm length) are immunohistochemically distinguished in biopsies materials by immunolabeling with antibodies against prohibitin as a cell cycle negative regulator, cyclin D1 as a master switch controlling G1–S transition in response to growth factor stimulation. The active EGF-receptor phosphorylated in tyrosine 1197 as a critical substrate for multiple physiological functions of the receptor is also shown. Prohibitin is far more abundant on the upper section corresponding to the most superficial stratum of the wound. Conversely, Cyclin D1 and EGFR Y-1197 appear marginally labeled. It is noticeable that proliferative Cyclin D1 and the phosphorylated form of the EGFR are by large abundantly detected in bottom section – the deepest wound layer where no prohibitin is detected.
Similarly, cyclin D1, the G1-S phase cell cycle promoter [131] is not expressed by wound superficial cells but in lower cells layers. On the contrary, the wound surface cells exhibit abundant expression of prohibitin, a well-known tumor suppressor and cell cycle arrest protein [132]. Other early studies of our group showed that 125I-EGF formulated in a semisolid vehicle, appeared to be rapidly cleared from the application site, probably by protease-driven cleavage and receptor-mediated endocytosis. Mean residence time values suggested that over 60% of the amount administered, may have disappeared as early as two hours after its administration [133]. This local pharmacokinetic modeling based on EGF topical administration, indicated that the receptors dynamics does not fulfill the requisite of prolonged receptor/ligand interaction for wound cells proliferation [15]. Our rationale was also supported by the evidences offered by Cross and Roberts in 1999, which showed that EGF only penetrated slightly into the upper granulating layers of the wound; with a subsequent exponential decline in solute concentration with tissue depth. This limited absorption kinetic also contributed to explain why topically administered GFs failed in the clinical scenario [134]. We enlarged the list of researchers [29,135,136] who showed the limited in-wound bioavailability of EGF due to the effect of locally secreted proteases. Of note, our finding derived from experimentally induced acute, controlled, full-thickness wounds in pigs under laboratory conditions. This suggested that proteases contained in the physiological exudate of clean, non-chronic wounds may also threaten EGF molecular integrity and stability [137].
It is likely that the most substantial support to our approach of intralesional EGF injection derived from a time-point immunoelectron microscopy kinetic study addressed to characterize the intracellular trafficking of the EGFR in ulcers-collected fibroblasts. This study showed that locally infiltrated EGF into Wagner’s 3 and 4 neuropathic ulcers resulted in (a) dramatic increase of the EGFR expression 15 minutes after the EGF infiltration as compared prior to the intervention point, which indicated the induction of the receptor by the high-affinity ligand; (b) immediate endocytosis of the EGFR/ligand complex; (c) translocation and biodistribution to different cytoplasmic organelles from 15 minutes to 24 hours after the infiltration; (d) nuclear translocation of the receptor and its binding to DNA, which appeared to last up to 24 hours after the treatment; (e) a concomitant activation of the proliferating cell nuclear antigen (PCNA) (a cell cycle promoting protein) gene transcription, since a high expression of this protein was detected following EGF intervention, even 24 hours after the injection; (f) a significant and intriguing accumulation of the receptor in mitochondria which lasted for 24 hours after the infiltration; (g) significant accumulation of the receptor bound to collagen fibers within the extracellular matrix [138]. All these data endorse the EGF delivery procedure as an effective mean to stimulate the receptor for hours, indirectly promoting PCNA gene expression and consequently cell proliferation [138].
Clinical validation of EGF infiltration
The animal experiments carried out during the 90’s [107,128,129], supported the hypothesis to initiate in 2001 a pilot study with 29 diabetic patients with high-grade (Wagner scale III and IV), poor-prognostic, and comprising ischemic lower limb wounds (ulcers and non-healing amputation residual bases). In this study, recombinant human EGF was intralesionally infiltrated into the bottom and contours of the wounds three times a week, along with sharp debridement, and other conventional medical interventions [139]. The fact that from this initial intervention 17 subjects were healed and prevented from amputation; paved the way for subsequent clinical trials [140-142], the development of an injectable lyophilized formulation, and the onset of a nationwide program that included two pharmacovigilance studies with more than 2000 patients [143,144]. Clear glass data confirmed the clinical usefulness of this delivery route to trigger and sustain the healing process. In terms of figures, it was shown that infiltrated EGF elicited 75% full granulation response, 61% of wound closure, and 71% reduction of amputation-relative risk, as well as positive benefit-risk balance. Of major clinical and social relevance is that recurrences were reported as an exceptional event (approximately 5%) upon a 12-month follow-up period [143,144] which had been anticipated since the proof-of-concept trial. This is a meaningful advantage of the EGF intralesional infiltration. Recent investigations disclose that roughly 40% of patients have a recurrence within 1 year after ulcer healing, almost 60% within 3 years, and 65% within 5 years [42]. Other international groups, who have introduced EGF intralesional infiltration in the daily practice, converge to report that EGF infiltration triggers an exceptional healing response with low amputation rates [145-147].
As just mentioned, the outcomes observed in the initial study in 2001 urged the need for the development of a high-standard injectable pharmaceutical formulation, which required to be a lyophilized form, given that EGF is labile in aqueous systems [148]. At the end of a series of pre-formulation studies, the freeze-dried formulation proved to be an appropriate technical solution for stabilizing and preserving EGF for the intralesional delivery route [149].
Finally, we have also demonstrated in two independent and extemporaneous studies that the intralesional administration of EGF has a systemic translation with beneficial effects (Figure 2). Following 3-4 weeks of treatment, there was a systemic reduction of oxidative stress and a concomitant recovery of antioxidant reserve markers. The decrease in circulating levels of matrix metalloproteases and their tissue inhibitors, as the systemic attenuation of several acute phase pro-inflammatory and innate immunity reactants were also evidenced. Interestingly, local EGF infiltration was also shown to significantly reduce circulating levels of pro-apoptotic inducers and the levels of advanced glycation end products [150,151].
Chronic and complex acute wounds may act as a pro-inflammatory and a pro-oxidative superimposed organ that establish a dynamic bidirectional reciprocity circuit with the host. Nine local infiltrations of EGF into the wounds, accounted for a reduction in systemic pro-inflammatory biomarkers as erythrosedimentation rate, C-reactive protein, interleukin-6, soluble FAS, and macrophage inflammatory protein 1-alpha. EGF infiltrative therapy also decreased redox and nitrosilative stress biomarkers, while increased the circulating levels of the soluble receptor for the advanced glycation end-products. Behind the healing success achieved upon EGF infiltration, there is an extensive profile of pharmacodynamic effects, given by the systemic restoration of multiple internal metabolites and functions and interrogatively facilitate the resumption of a physiologic healing trajectory.
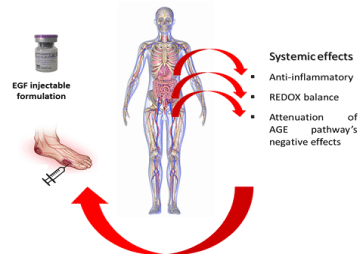
Figure 2. Systemic translation of locally infiltrated EGF
Future directions
Wound care and management is an age-old practice. The two most traditional approaches for wound healing entail the topical (superficial) administration of active healing ingredients, and the occlusion of the wound by dressings [152]. Contemporary wound healing science has embraced nanomedicine in the form of nanoparticles, scaffolds, and composites that, to a significant extent, have been able to assist the repair process [153]. Biomaterials with controlled-release of signaling molecules as growth factors have turned a promising method for diabetic wound healing [154]. Dr. Nora Ventosa’s laboratory at the Institute of Materials Science of Barcelona (ICMAB-CSIC, Spain) has developed non-liposomal nanovesicles, named quatsomes, composed of ionic surfactants and cholesterol derivatives [155-158]. Quatsomes formulations are stable upon dilution, and have high vesicle-to-vesicle homogeneity in terms of size, morphology and chemical composition. These innovative nanovesicles can be used for the nanoformulation of therapeutically active small synthetic molecules or biomolecules [159,160]. Interestingly, quatsomes are prepared by a one-step eco-efficient process, named DELOS-SUSP [161,162], and have built-in anti-microbial and anti-biofilm properties which can help to protect skin from infections [163]. Overall, this is a promising platform for nanomedicine applications.
The collaborative work among our groups has yielded an innovative nanotherapy for diabetic wound healing based on EGF-loaded quatsomes (EGF@Quatsomes). These nanoconjugates exhibited (1) extraordinary colloidal stability, (2) increased EGF mitogenic activity as compared to free EGF, (3) high resistance to proteolytic degradation of EGF, (4) prolonged cutaneous retention of EGF, and (5) antimicrobial activity against gram-positive bacteria, yeast, and fungi; all of which may translate in a satisfactory availability of bioactive EGF within the wound milieu and ultimately a broader pharmacodynamics [164]. Our small pilot clinical assessment of the EGF@Quatsomes topical administration (3 times/week) yielded an encouraging outcome: stagnantly granulated, non-reepithelialized diabetic wounds of both ischemic and neuropathic nature with an evolution time from 5–60 months; were triggered a steady and progressive epithelial response with complete resurfacing in approximately 90 days. Of note, immunohistochemical studies based on small granulation tissue biopsies, indicated the activation of the EGF receptor signaling axis and the downstream transducing pathways involved in cellular proliferation and survival [164]. Additional basic science and pharmaceutical development studies are in progress in pursuant for a definitive clinical positioning.
Concluding remarks
Skin fibroblasts, vascular cells and keratinocytes become biochemically and epigenetically imprinted with the particular “diabetes seal”. Not less significant is the damage induced by hyperglycemia and its associate by-products on the mesenchymal stem cell niches. Thus, skin cells of diabetic individuals exhibit an abnormal behavior and a short replicative life span, displaying a senescent phenotype even under ideal in vitro culture conditions. This replicative arrest that remains in the cellular memory is one of the major drivers of wound chronicity. Diabetic wound is conceptually an additional source of circulating toxic, pro-inflammatory and catabolic mediators that establish a self-perpetuating loop. More recently, new pathological elements have arisen with the recent identification and characterization of dozens of miRNA released from the diabetic wound realm. The microenvironment of these wounds has proved to be hostile for GFs and their receptors in terms of physicochemical integrity and physiology. Despite the initial promise for optimal wound management and despite long years of research efforts, GFs have not fully integrated the pharmacological armamentarium. For the particular case of EGF, it was the first in line used by topical administration in acute and chronic wounds. Unfortunately, the results were either controversial or neutral in both basic and clinical studies. Despite these outcomes, there is no question about its intrinsic biological potency in mitogenic commitment for most epithelial and mesenchymal-derived cells. The experiments based on the local infiltration of EGF in rats injured hind limbs, were crucial in showing the big gap existing between the topical and the infiltrative delivery routes in terms of a clear-cut tissue healing response. This example of translational science has contributed to safely heal more than 300 000 patients, most of them with low recurrence rates, reductions of amputations, and prolonged survival. An incoming generation of EGF nanovesicles for topical use is already designed for reluctant-to-reepithelialize granulated wounds.
Author declaration
- There is no conflict-of-interest or any type of competing interest.
- All the authors enlisted are aware of the content of the manuscript and abide to it.
- This review manuscript has not been partially or totally submitted before and that it is not under the consideration of any editorial.
- No funds were solicited or used to write this review manuscript.
References
- Muhammad Ibrahim A (2018) Diabetic Foot Ulcer: Synopsis of the Epidemiology and Pathophysiology. Int J Diabetes Endocrinol 3: 23.
- Costa RHR, Cardoso NA, Procopio RJ, Navarro TP, Dardik A, et al. (2017) Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Syndr 11: 583-587. [Crossref]
- Shin JY, Roh SG, Sharaf B, Lee NH (2017) Risk of major limb amputation in diabetic foot ulcer and accompanying disease: A meta-analysis. J Plast Reconstr Aesthet Surg 70: 1681-1688. [Crossref]
- Wilkinson HN, Clowes C, Banyard KL, Matteuci P, Mace KA, et al. (2019) Elevated Local Senescence in Diabetic Wound Healing Is Linked to Pathological Repair via CXCR2. J Invest Dermatol 139: 1171-1181. [Crossref]
- Berlanga-Acosta JA, Nieto GEG, Rodriguuez NR, Mari YM, Vega MLB, et al. (2020) Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity. Front Endocrinol (Lausanne) 11: 573032. [Crossref]
- Xiao S (2020) Diabetes-induced glucolipotoxicity impairs wound healing ability of adipose-derived stem cells-through the miR-1248/CITED2/HIF-1α pathway. Aging (Albany NY) 12: 6947-6965. [Crossref]
- Veith AP (2019) Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev 146: 97-125.
- Wang Z, Shi C (2020) Cellular senescence is a promising target for chronic wounds: a comprehensive review. Burns & Trauma 8.
- Yang R (2020) Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int 2020: 9148310.
- Dvorak HF (2021) Reconciling VEGF With VPF: The Importance of Increased Vascular Permeability for Stroma Formation in Tumors, Healing Wounds, and Chronic Inflammation. Front Cell Dev Biol 9: 660609. [Crossref]
- Dasari N, Jiang A, Skochdopole A, Chung J, Reece EM, et al. (2021) Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin Plast Surg 35: 153-158. [Crossref]
- Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, et al. (2021) Immunology of Acute and Chronic Wound Healing. Biomolecules 11: 700. [Crossref]
- Maddaluno L, Urwyler C, Werner S (2017) Fibroblast growth factors: key players in regeneration and tissue repair. Development 144: 4047-4060. [Crossref]
- Qing C (2017) The molecular biology in wound healing & non-healing wound. Chin J Traumatol 20: 189-193. [Crossref]
- Buckley A, Davidson JM, Kamerath CD, Wolt TB, Woodward SC (1985) Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci U S A 82: 7340-7344. [Crossref]
- Brown GL, Curtsinger 3rd L, Brightwell JR, Ackerman DM, Tobin GR, et al. (1986) Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exper Med 163: 1319-1324. [Crossref]
- Laato M, Niinikoski J, Lebel L, Gerdin B (1986) Stimulation of wound healing by epidermal growth factor A dose-dependent effect. Ann Surg 203: 379-381. [Crossref]
- Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, et al. (1988) Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann surg 208: 788-794. [Crossref]
- Tsutsumi OA, Oka T (1988) Epidermal growth factor-like, corneal wound healing substance in mouse tears. J Clin Invest 81: 1067-1071. [Crossref]
- Lynch SE, Colvin RB, Antoniades HN (1989) Growth factors in wound healing Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest 84: 640-646. [Crossref]
- Greenhalgh DG, Sprugel KH, Murray MJ, Ross R (1990) PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 136: 1235-1246. [Crossref]
- Mustoe TA, Pierce GF, Morishima C, Deuel TF (1991) Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest 87: 694-703. [Crossref]
- Schultz G, Clark W, Rotatori DS (1991) EGF and TGF-α in wound healing and repair. J Cell Biochem 45: 346-352. [Crossref]
- Danilenko DM, Ring BD, Tarpley JE, Morris B, Van GY, et al. (1995) Growth factors in porcine full and partial thickness burn repair Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. Am J Pathol 147: 1261-1277. [Crossref]
- Matsuda H, Koyama H, Sato H, Sawada J, Itak A, et al. (1998) Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med 187: 297-306. [Crossref]
- Pastar I, Wong LL, Egger AN, Canic MT (2018) Descriptive vs mechanistic scientific approach to study wound healing and its inhibition: Is there a value of translational research involving human subjects? Exp Dermatol 27: 551-562. [Crossref]
- Dunyach-Remy C (2021) Pressure ulcers microbiota dynamics and wound evolution. Sci Rep 11: 18506.
- Pichlsberger M, Jerman UD, Obradovic H, Tratnjek L, Macedo AS, et al. (2021) Systematic Review of the Application of Perinatal Derivatives in Animal Models on Cutaneous Wound Healing. Front Bioeng Biotechnol 9: 742858. [Crossref]
- Schultz GS, Barillo DJ, Mozingo DW, Chin GA (2004) Wound bed preparation and a brief history of TIME. Int Wound J 1: 19-32. [Crossref]
- Boersema GC, Smart H, Cilliers MGCG, Mulder M, Weir GR, et al. (2021) Management of Nonhealable and Maintenance Wounds: A Systematic Integrative Review and Referral Pathway. Adv Skin Wound Care 34: 11-22. [Crossref]
- Sibbald RG, Elliot JA, Jaimangal RP, Goodman L, Armstrong DG, et al. (2021) Wound Bed Preparation 2021. Adv Skin Wound Care 34: 183-195. [Crossref]
- Portero-Otín M, Pamplona R, Bellmunt MJ, Ruiz MC, Prat J, et al. (2002) Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes 51: 1535-1542. [Crossref]
- Pastar I (2010) Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med 16: 92-101. [Crossref]
- Liu Y, Liu Y, Deng J, Li W, Nie X (2021) Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front Endocrinol (Lausanne) 12: 744868. [Crossref]
- Ren X, Zhao M, Lash B, Martino MM, Julier Z (2019) Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front Bioeng Biotechnol 7: 469. [Crossref]
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound Healing: A Cellular Perspective. Physiol Rev 99: 665-706. [Crossref]
- Mochizuki M (2020) Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat Biomed Eng 4: 463-475. [Crossref]
- Tan JL (2021) Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun Biol 4: 422.
- Berlanga-Acosta J, Rodríguez HC, Marí YM, Cama VF, Ojalvo AG, et al. (2020) Epidermal Growth Factor in Healing Diabetic Foot Ulcers: From Gene Expression to Tissue Healing and Systemic Biomarker Circulation. MEDICC Rev 22: 24-31. [Crossref]
- Forbes JM, Fotheringham AK (2017) Vascular complications in diabetes: old messages, new thoughts. Diabetologia 60: 2129-2138. [Crossref]
- Everett E, Mathioudakis N (2018) Update on management of diabetic foot ulcers. Ann N Y Acad Sci 1411: 153-165. [Crossref]
- Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic Foot Ulcers and Their Recurrence. N Engl J Med 376: 2367-2375. [Crossref]
- Armstrong DG (2020) Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res 13: 16. [Crossref]
- Schaper NC, van Netten JJ (2020) Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 1: e3266. [Crossref]
- Edmonds ME, Foster AVM (2006) Diabetic foot ulcers. BMJ 332: 407-410.
- Feldman EL (2019) Diabetic neuropathy. Nat Rev Dis Primers 5: 42.
- Lauri C, Leone A, Cavallini M, Signore A, Giurato L, et al. (2020) Diabetic Foot Infections: The Diagnostic Challenges. J Clin Med 9: 1779. [Crossref]
- Miranda C, Da Ros R, Marfella R (2021) Update on prevention of diabetic foot ulcer. Arch Med Sci Atheroscler Dis 6: 123-131. [Crossref]
- Ouyang W, Jia Y, Jin L (2021) Risk factors of diabetic foot ulcer in patients with type 2 diabetes: a retrospective cohort study. Am J Transl Res 13: 9554-9561. [Crossref]
- Daryabor G (2020) The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol 11: 1582. [Crossref]
- Rodriguez-Rodriguez N, Jiménez IM, Ojalvo AG, Mari YM, Nieto GG, et al. (2021) Wound Chronicity, Impaired Immunity and Infection in Diabetic Patients. MEDICC Rev 24: 44-58. [Crossref]
- Chávez-Reyes J, Escárcega-González CE, Chavira-Suárez E, Buitimea AL, León PV, et al. (2021) Susceptibility for Some Infectious Diseases in Patients with Diabetes: The Key Role of Glycemia. Front public health 9: 559595-559595. [Crossref]
- Theocharidis G (2020) Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes 69: 2157-2169. [Crossref]
- Ramirez HA (2018) Staphylococcus aureus Triggers Induction of miR-15B-5P to Diminish DNA Repair and Deregulate Inflammatory Response in Diabetic Foot Ulcers. J Invest Dermatol 138: 1187-1196. [Crossref]
- Deng L, Du C, Song P, Chen T, Rui S, et al. (2021) The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev 2021: 8852759. [Crossref]
- Ruiz-Bedoya CA, Gordon O, Mota F, Abhisekh S, Tucker EW, et al. (2019) Molecular Imaging of Diabetic Foot Infections: New Tools for Old Questions. Int J Mol Sci 20. [Crossref]
- Rubitschung K, Sherwood A, Crisologo AP, Bhavan K, Haley RW, et al. (2021) Pathophysiology and Molecular Imaging of Diabetic Foot Infections. Int J Mol Sci 22. [Crossref]
- Versey Z (2021) Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front Immunol 12: 648554. [Crossref]
- Theocharidis G, Baltzis D, Roustit M (2020) Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes 69: 2157-2169. [Crossref[OT1] ]
- Berlanga-Acosta J (2013) Glucose Toxic Effects on Granulation Tissue Productive Cells: The Diabetics’ Impaired Healing. BioMed Res Int 2013: 256043.
- Zhang P, Song X, Dong Q, Zhou L, Wang L (2020) miR-27-3p inhibition restore fibroblasts viability in diabetic wound by targeting NOVA1. Aging 12. [Crossref]
- Jeong SR, Lee KW (2021) Methylglyoxal-Derived Advanced Glycation End Product (AGE4)-Induced Apoptosis Leads to Mitochondrial Dysfunction and Endoplasmic Reticulum Stress through the RAGE/JNK Pathway in Kidney Cells. Int J Mol Sci 22.
- Mponponsuo K, Sibbald RG, Somayaji R (2021) A Comprehensive Review of the Pathogenesis, Diagnosis, and Management of Diabetic Foot Infections. Adv Skin Wound Care 34: 574-581. [Crossref]
- Peng Y, Xiong RP, Zhang ZH, Ning YN, Zhao Y, et al. (2021) Ski promotes proliferation and inhibits apoptosis in fibroblasts under high-glucose conditions via the FoxO1 pathway. Cell Prolif 54: e12971. [Crossref]
- Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 25: 304-314. [Crossref]
- Yamakawa S, Hayashida K (2019) Advances in surgical applications of growth factors for wound healing. Burns & Trauma 7: 10. [Crossref]
- Zhang C (2019) Platelet-Rich Plasma with Endothelial Progenitor Cells Accelerates Diabetic Wound Healing in Rats by Upregulating the Notch1 Signaling Pathway. J Diabetes Res 2019: 5920676. [Crossref]
- Farooq M, Khan AW, Kim MS, Choi S (2021) The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 10. [Crossref]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair and Regeneration 16: 585-601. [Crossref]
- Park JW, Hwang SR, Yoon IS (2017) Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 22. [Crossref]
- Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E (1999) Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J investigative dermatol 112: 463-469. [Crossref]
- Rognoni E (2018) Fibroblast state switching orchestrates dermal maturation and wound healing. Mol Syst Biol 14: e8174. [Crossref]
- Ruiz-Canada C, Garcia AB, Liarte S, Valiente MR, Nicolas FJ (2021) Chronic Wound Healing by Amniotic Membrane: TGF-beta and EGF Signaling Modulation in Re-epithelialization. Front Bioeng Biotechnol 9: 689328. [Crossref]
- Vande Berg JS, Rudolph R, Hollan C, Reid PLH (1998) Fibroblast senescence in pressure ulcers Wound Repair and Regeneration. 6: 38-49. [Crossref]
- Broszczak DA, Sydes ER, Wallace D, Parker TJ (2017) Molecular Aspects of Wound Healing and the Rise of Venous Leg Ulceration: Omics Approaches to Enhance Knowledge and Aid Diagnostic Discovery. Clin Biochem Rev 38: 35-55. [Crossref]
- Wang Z, Shi C (2020) Cellular senescence is a promising target for chronic wounds: a comprehensive review. Burns & trauma 8: 021.
- Bucalo B, Eaglstein WH, Falanga V (1993) Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen 1: 181-186. [Crossref]
- Bodnár E, Bakondi E, Kovacs K, Hegedus C, Lakatos P, et al. (2018) Redox Profiling Reveals Clear Differences between Molecular Patterns of Wound Fluids from Acute and Chronic Wounds. Oxi Med Cell Longev 2018: 1-12. [Crossref]
- Brooks RF (2021) Cell Cycle Commitment and the Origins of Cell Cycle Variability. Front Cell Dev Biol 9: 698066.
- Kumari R, Jat P (2021) Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol 9: 645593. [Crossref]
- Shao M, Hussain Z, Thu HE, Khan S, De Matas M, et al. (2017) Emerging Trends in Therapeutic Algorithm of Chronic Wound Healers: Recent Advances in Drug Delivery Systems, Concepts-to-Clinical Application and Future Prospects. Crit Rev Ther Drug Carrier Syst 34: 387-452. [Crossref]
- Boeringer T, Gould LJ, Koria (2020) Protease-Resistant Growth Factor Formulations for the Healing of Chronic Wounds. Adv Wound Care (New Rochelle) 9: 612-622. [Crossref]
- Ko KI, Sculean A, DT Graves (2021) Diabetic wound healing in soft and hard oral tissues. Transl Res 236: 72-86. [Crossref]
- Miricescu D (2021) Growth Factors, Reactive Oxygen Species, and Metformin-Promoters of the Wound Healing Process in Burns? Int J Mol Sci 22: 9512. [Crossref]
- Singh S, Young A, McNaught CE (2017) The physiology of wound healing. Surg (Oxford) 35: 473-477. [Crossref]
- Chicharro-Alcántara D, Zaragoza MR, Gimenez ED, Poveda JMC, Serrato BC, et al. (2018) Platelet Rich Plasma: New Insights for Cutaneous Wound Healing. Management J Funct Biomat 9: 10. [Crossref]
- Shi GJ, Shi GR, Zhou JY, Gao CY, Jiang YP, et al. (2018) Involvement of growth factors in diabetes mellitus and its complications: A general review. Biomed Pharmacother 101: 510-527. [Crossref]
- Tarnawski AS, Ahluwalia A (2021) The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells 10: 1964. [Crossref]
- Di Domenico M, Giordano A (2017) Signal transduction growth factors: the effective governance of transcription and cellular adhesion in cancer invasion. Oncotarget 8: 36869-36884. [Crossref]
- Gross SM, Rotwein P (2017) Quantification of growth factor signaling and pathway cross talk by live-cell imaging. Am J Physiol Cell Physiol 312: C328-C340. [Crossref]
- Zhang F, Zheng L, Cheng S, Peng Y, Fu L, et al. (2019) Comparison of the Interactions of Different Growth Factors and Glycosaminoglycans. Molecules 24. [Crossref]
- Onesto MM (2021) Growth Factors as Axon Guidance Molecules: Lessons From in vitro. Studies Frontiers in Neuroscience 15. [Crossref]
- Castano O, Amodio SP, Requena CN, Timoneda MAM, Engel E (2018) Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv Drug Deliv Rev 129: 95-117. [Crossref]
- Sharma P, Kumar A, Dey AD, Behl T, Chadha S (2021) Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci 268: 118932. [Crossref]
- Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, et al. (1989) Enhancement of Wound Healing by Topical Treatment with Epidermal Growth Factor. New Eng J Med 321: 76-79. [Crossref]
- Brown GL (1988) Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg 208: 788-794.
- Schultz GS, White M, Mitchell R, Brown G, Lynch J, et al. (1987) Epithelial Wound Healing Enhanced by Transforming Growth Factor-; and Vaccinia Growth Factor. Science 235: 350-352. [Crossref]
- Falanga V (1992) Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol 19: 667-672. [Crossref]
- Cohen IK, Crossland MC, Garrett A, Diegelmann RF (1995) Topical application of epidermal growth factor onto partial-thickness wounds in human volunteers does not enhance reepithelialization. Plast Reconstr Surg 96: 251-254. [Crossref]
- Bennett NT, Schultz GS (1993) Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg 165: 728-737. [Crossref]
- Jimi S, Jaguparov A, Nurkesh A, Sultankulov B, Saparov A (2020) Sequential Delivery of Cryogel Released Growth Factors and Cytokines Accelerates Wound Healing and Improves Tissue Regeneration. Front Bioengineer Biotechnol 8: 345. [Crossref]
- Falanga V (2005) Wound healing and its impairment in the diabetic foot. Lancet 366: 1736-1743. [Crossref]
- Berry-Kilgour C, Cabral J, Wise L (2020) Advancements in the Delivery of Growth Factors and Cytokines for the Treatment of Cutaneous Wound Indications. Adv Wound Care 10: 596-622. [Crossref]
- Okumura K (1990) Improvement in Wound Healing by Epidermal Growth Factor (EGF) Ointment I Effect of Nafamostat, Gabexate, or Gelatin on Stabilization and Efficacy of EGF. Pharma Res 7: 1289-1293. [Crossref]
- Kiyohara Y, Okumora K, Komada F, Iwakawa S, Hirai M, et al. (1991) Improvement in wound healing by epidermal growth factor (EGF) ointment II Effect of protease inhibitor, nafamostat, on stabilization and efficacy of EGF in burn. J Pharmacobiodyn 14: 47-52. [Crossref]
- Ishihara J, Ishihara A, Fukunaga K, Sasaki K, White MJV, et al. (2018) Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat Commun 9: 2163. [Crossref]
- Berlanga-Acosta J, Cowley JG, Saura PL, Lopez TG, Santana MDC, et al. (2009) Epidermal growth factor in clinical practice - a review of its biological actions, clinical indications and safety implications. Int Wound J 6: 331-346. [Crossref]
- Jeong S, Kim BW, Park M, Ban E, Lee SH, et al. (2020) Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics 12. [Crossref]
- Hardwicke J (2008) Epidermal growth factor therapy and wound healing -past, present and future perspectives. The Surg 6: 172-177.
- Gushiken LFS, Besarra FP, Bastos JK, Jackson CJ, Pellizzon CH (2021) Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life (Basel) 11: 665. [Crossref]
- Wang M, Morsbach F, Sander D, Gheorghiu L, Nanda A, et al. (2011) EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Res 71: 6261-6269. [Crossref]
- Singh B, Carpenter G, Coffey RJ (2016) EGF receptor ligands: recent advances. F1000Res 5: 2270. [Crossref]
- Alexander PB, Yuan L, Yang P, Sun T, Chen R, et al. (2015) EGF promotes mammalian cell growth by suppressing cellular senescence. Cell Res 25: 135-138. [Crossref]
- Ai G, Shao X, Meng M, Song L, Qui J, et al. (2017) Epidermal growth factor promotes proliferation and maintains multipotency of continuous cultured adipose stem cells via activating STAT signal pathway in vitro. Medicine (Baltimore) 96: e7607. [Crossref]
- Hsu CP, Lee LW, Tang SC, Hsin IL, Lin YW, et al. (2015) Epidermal growth factor activates telomerase activity by direct binding of Ets-2 to hTERT promoter in lung cancer cells. Tumour Biol 36: 5389-5398. [Crossref]
- Feng Y (2017) Interdependency of EGF and GLP-2 Signaling in Attenuating Mucosal Atrophy in a Mouse Model of Parenteral Nutrition. Cell Mol Gastroenterol Hepatol 3: 447-468. [Crossref]
- Ali R, Brown W, Purdy SC, Davisson VJ, Wendt MK, et al. (2018) Biased signaling downstream of epidermal growth factor receptor regulates proliferative versus apoptotic response to ligand. Cell Death Dis 9: 976. [Crossref]
- Tang X, Liu B, Wang X, Yu Q, Fang R (2018) Epidermal Growth Factor, through Alleviating Oxidative Stress, Protect IPEC-J2 Cells from Lipopolysaccharides-Induced Apoptosis. Int J Mol Sci 19: 848. [Crossref]
- Fang T, Zhang Y, Chang VY, Roos M, Termini CM, et al. (2020) Epidermal growth factor receptor-dependent DNA repair promotes murine and human hematopoietic regeneration. Blood 136: 441-454. [Crossref]
- Astaneie F, Afsari M, Mojtahedi A, Mostafalou S, Zamani MJ, et al. (2005) Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch Med Res 36: 376-381. [Crossref]
- Oxford GE, L Tayari, Barfoot MD, Peck AB, Tanaka Y, et al. (2000) Salivary EGF levels reduced in diabetic patients. J Diabetes Complications 14: 140-145. [Crossref]
- Stefano GB, Challenger S, Kream RM (2016) Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur J Nutr 55: 2339-2345. [Crossref]
- Alharbi MA, Zhang C, Lu C, Milovanova TN, Yi L, et al. (2018) FOXO1 Deletion Reverses the Effect of Diabetic-Induced Impaired Fracture Healing. Diabetes 67: 2682-2694. [Crossref]
- Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R (2020) Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev 16: 442-449. [Crossref]
- Giese IM, Schilloks MC, Degroote RL, Weigand M, Renner S, et al. (2020) Chronic Hyperglycemia Drives Functional Impairment of Lymphocytes in Diabetic INS (C94Y) Transgenic Pigs. Front Immunol 11: 607473. [Crossref]
- Yin M, Zhang Y, Yu H, Li X (2021) Role of Hyperglycemia in the Senescence of Mesenchymal Stem Cells. Front Cell Dev Biol 9: 665412. [Crossref]
- Prats P (1998) El factor de crecimiento epidérmico en lesiones del sistema nervioso periférico. Rev Mex Cienc Farm 29: 17-23.
- Berlanga-Acosta J, Mella-Lizama C (1998) Some physiological considerations of epidermal growth factor in relation to its pharmacological applications. Biotecnología Aplicada 15: 141-148.
- Berlanga J (1999) The role of the epidermal growth factor in cell and tissue protection. Med Clin (Barc) 113: 222-229. [Crossref]
- Berlanga-Acosta J, Montequin JF, Perez CV, Gutierrez WS, Mari YM, et al. (2017) Diabetic Foot Ulcers and Epidermal Growth Factor: Revisiting the Local Delivery Route for a Successful Outcome. BioMed Res Inter 2017: 2923759. [Crossref]
- Ao J, Ma Z, Li R, Zhang S, Gao X, et al. (2021) Phospho-Tudor-SN coordinates with STAT5 to regulate prolactin-stimulated milk protein synthesis and proliferation of bovine mammary epithelial cells. Anim Biotechnol 2021: 1-11. [Crossref]
- Koushyar S, Economides G, Zaat S, Jiang W, Bevan CL, et al. (2017) The prohibitin-repressive interaction with E2F1 is rapidly inhibited by androgen signalling in prostate cancer cells. Oncogenesis 6: 333-333. [Crossref]
- Prats PA, Duconge J, Valenzuela C, Berlanga J, Edrosa CR, et al. (2002) Disposition and receptor-site binding of (125) I-EGF after topical administration to skin wounds. Biopharm Drug Dispos 23: 67-76. [Crossref]
- Cross SE, Roberts MS (1999) Defining a model to predict the distribution of topically applied growth factors and other solutes in excisional full-thickness wounds. J Invest Dermatol 112: 36-41. [Crossref]
- Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, et al. (1997) Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 5: 23-32. [Crossref]
- McCarty SM, Percival SL (2013) Proteases and Delayed Wound Healing. Adv Wound Care 2: 438-447. [Crossref]
- Berlanga-Acosta J (1998) Epidermal growth factor stimulated re-epithelialization in pigs: The possible role of acute wound proteases. Biotecnología Aplicada 15: 83-87.
- Cama V, Mayola MF, Mari YM (2016) Epidermal Growth Factor based Therapy Promotes Intracellular Trafficking and Accumulation of its Receptor in the Nucleus of Fibroblasts from Diabetic Foot Ulcers. J Diabet Comp Med 01.
- Acosta JB, Savinge W, Valdez C, Franco N, Alba JS, et al. (2006) Epidermal growth factor intralesional infiltrations can prevent amputation in patients with advanced diabetic foot wounds. Int wound J 3: 232-239. [Crossref]
- Fernandez-Montequin JI, Infante-Cristiá E, Valenzuela-Silva C, Franco-Pérez N, Gutierrez WS, et al. (2007) Intralesional injections of Citoprot-P (recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 4: 333-343. [Crossref]
- Valenzuela-Silva CM (2013) Granulation response and partial wound closure predict healing in clinical trials on advanced diabetes foot ulcers treated with recombinant human epidermal growth factor. Diabet Care 36: 210-215. [Crossref]
- Fernandez-Montequin JI, Silva CMV, Diaz OG, Savinge W, Soutelo NS, et al. (2009) Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo-controlled, double-blind study. Int Wound J 6: 432-443. [Crossref]
- López-Saura PA (2013) Medical practice confirms clinical trial results of the use of intralesional human recombinant epidermal growth factor in advanced diabetic foot ulcers. Adv Pharmacoepidem Drug Safet 2: 128.
- Yera-Alos IB, Carbonell LA, Silva CMV, Iglesias ADT, Martinez MM, et al. (2013) Active post-marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacol Toxicol 14: 44. [Crossref]
- Gomez-Villa R, Rebolledo FA, Platonoff AL, Soto JMT, Victoriano MRF, et al. (2014) Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial. Wound Repair Regen 22: 497-503. [Crossref]
- Ertugrul BM, Buke C, Ersoy OS, Ay B, Demirez DS, et al. (2015) Intralesional epidermal growth factor for diabetic foot wounds: the first cases in Turkey. Diabet Foot Ankle 6: 28419. [Crossref]
- Ertugrul BM (2017) An Assessment of Intralesional Epidermal Growth Factor for Treating Diabetic Foot WoundsThe First Experiences in Turkey. J Am Podiatr Med Assoc 107: 17-29. [Crossref]
- Santana H (2013) Screening for stability and compatibility conditions of recombinant human epidermal growth factor for parenteral formulation: effect of pH, buffers, and excipients. Int J Pharm 452: 52-62. [Crossref]
- Santana H (2014) Stabilization of a recombinant human epidermal growth factor parenteral formulation through freeze-drying. Biologicals 42: 322-333. [Crossref]
- Ojalvo AG, Acosta JB, Mari YM, Mayola MF, Perez CV, et al. (2017) Healing enhancement of diabetic wounds by locally infiltrated epidermal growth factor is associated with systemic oxidative stress reduction. Int Wound J 14: 214-225. [Crossref]
- Garcia-Ojalvo A, Acosta JB, Martinez AF, Romero MB, Mari YM, et al. (2019) Systemic translation of locally infiltrated epidermal growth factor in diabetic lower extremity wounds. Int Wound J 16: 1294-1303. [Crossref]
- Kosaric N, Kiwanuka H, Gurtner GC (2019) Stem cell therapies for wound healing. Expert Opin Biol Ther 19: 575-585. [Crossref]
- Banerjee K, Madhyastha R, Nakajima Y, Maruyama M, Madhyastha H (2021) Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects. Int J Mol Sci 22: 4748. [Crossref]
- Bai Q, Han K, Dong K, Zheng C, Zhang Y, et al. (2020) Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int J Nanomedicine 15: 9717-9743. [Crossref]
- Ferrer-Tasies L, Calvo EM, Sarabia MC, Arzo MA, Lesieur S, et al. (2013) Quatsomes: vesicles formed by self-assembly of sterols and quaternary ammonium surfactants. Langmuir 29: 6519-6528. [Crossref]
- Grimaldi N (2016) Lipid-based nanovesicles for nanomedicine. Chem Soc Rev 45: 6520-6545.
- Vargas-Nadal G (2020) MKC-Quatsomes: a stable nanovesicle platform for bio-imaging and drug-delivery applications. Nanomedicine 24: 102136.
- Ballell-Hosa L, Mira EG, Santana H, Folch JM, Masip MM, et al. (2022) DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics 14: 199. [Crossref]
- Rossetti M (2021) Engineering DNA-Grafted Quatsomes as Stable Nucleic Acid-Responsive Fluorescent Nanovesicles. Adv Functional Mater 31: 2103511.
- Boloix A, Gracia NF, Kober M, Repetto J, Pascarella R, et al. (2021) Engineering pH-Sensitive Stable Nanovesicles for Delivery of MicroRNA Therapeutics Small 18: 2101959. [Crossref]
- Merlo-Mas J, Melero JT, Corchero JS, Mira EG, Font A, et al. (2021) Application of Quality by Design to the robust preparation of a liposomal GLA formulation by DELOS-susp method. J Supercrit Fluids 173: 105204. [Crossref]
- Cabrera I, Elizondo E, Esteban O, Corchero JL, Malgarejo M, et al. (2013) Multifunctional nanovesicle-bioactive conjugates prepared by a one-step scalable method using CO2-expanded solvents. Nano Lett 13: 3766-3774. [Crossref]
- Dong D, Thomas N, Ramenarpour M, Psaltis A, Huang S, et al. (2020) Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations. Exp Biol Med (Maywood) 245: 34-41. [Crossref]
- Ferrer-Tasies L, Santana H, Puig CI, Mira EG, Hosa LB, et al. (2021) Recombinant Human Epidermal Growth Factor/Quatsome Nanoconjugates: A Robust Topical Delivery System for Complex Wound Healing. Adv Therapeutics 4.


