The present investigation aimed to study the protective effect of tomato and carrot against acrylamide on histopathological sections of some organs in mice. In materials and groups, 7 each. First group was considered as negative control. The remaining mice were subjected for daily oral administration of acrylamide (40 μg/kg body weight) for 8 weeks. Second group was considered as positive control. Both negative and positive groups were fed on basal diet. The other groups were given basal diets with 20% of dried tomato and carrot (Groups 3 and 4, respectively). At the end of experiment, the relative organs' weights were calculated. Brain, prostate and small intestines were histopathologically examined. Results showed insignificant differences among feed intakes, initial, final & gain% of body weight, feed efficiency ratios and organs' weights. Groups fed on diets with carrot and tomato showed significant improvement in almost all the parameters studied compared to the positive control. No alteration and normal histopathological structure in different organs (small intestine, prostate and brain) of the normal group (negative control) was observed. Very severe alterations were noticed in small intestine, prostate and brain of the positive control treated with acrylamide. The other groups detected slight alterations such as moderate and mild (in small intestines and prostate gland) and showed a normal histopathological structure (in brain) organs.
carrot, tomato, histopathological alterations, acrylamide
Diets rich in vegetables reduced cancer risk, but definitive controlled trials on the prevention of specific cancers by antioxidants and phytochemicals were not completed [1]. The Mediterranean diet, which is rich in vegetables and fruits, including tomatoes, has been suggested to be responsible for the lower cancer rates in that region. Dietary intake of tomatoes and tomato products has been found to be associated with a lower risk of a variety of cancers in several epidemiological studies [2].
Epidemiologic evidence has been suggesting an association between the consumption of vegetables and reduced incidence of cancer [3,4]. It has been estimated that 30–40 percent of all cancers could be prevented by lifestyle and dietary measures alone. When a diet was compiled according to the guidelines, it might be at least a 60–70% decrease in breast, colorectal, and prostate cancers, and 40–50 % decrease in lung cancer, and cancers at other sites. Diet not only would be conductive to prevent cancer but also might favor recovery from cancer as well [5].
A variety of components in plant foods included micronutrients, polyunsaturated fatty acids, and secondary metabolites such as glucosinolates and flavonoids, which could inhibit cell proliferation and induce apoptosis, and might well act synergistically when combined in the human diet [6].
Co
Compelling evidence have been continued to strengthen the link between diet and cancer [7-12]. In addition, the review by an international panel of experts provided a limited evidence of World Cancer Research Fund Association /American Institute for Cancer Research, 2007 [13].
Dietary factors accounted for about 35% of cancer deaths in the United States. Recent meta-analyses suggested an association of colorectal, prostate, and brain cancers with certain dietary patterns. Not only food components might be associated with cancer risk, but also cooking methods, the direct impact of food on the human gastrointestinal mucosa, and individual susceptibility to dietary carcinogens could significantly increase cancer risk [14].
The estimates from the American Cancer Society and the International Union Against Cancer indicated that 12 million cases of cancer were diagnosed, with 7 million deaths worldwide that would be expected to double by 2030 (27 million cases with 17 million deaths). Extensive research, however, revealed that cancer could be a preventable disease requiring major changes in life style. One third of all cancers assigned to Tobacco, another third to diet, and remaining third to the environment [15].
The most important lesson for nutrition research should be recognizing the need for discovering the role of nutrition and diet in disease prevention. Lycopene is among the major caretenoids and one of the protective factors in vegetable-rich diets [16,17].
Much research attention has been focused on discovering the role of nutrition in disease prevention. The present investigation aimed to study the effect of carrot and tomato on preventing the histopathological alterations in brain, prostate and small intestine induced by acrylamide in mice. Such study could be an indicator for using such fruits and vegetables as protective superset against acrylamide as cancer promoting substance. This study would provide a promising guide to people's diet for the purpose of reducing the harmful effect of acrylamide.
Materials and chemicals
Vegetables: Carrot (Caucus carota), Tomato (Lycopersicum esculentum) were purchased from local market Giza, Egypt.
Chemicals: Acrylamide was obtained from Sigma-Aldrich Laborchemikalien GmbH (Milwaukee, WI). Minerals, casein, cellulose, starch, vitamins, ascorbic acid, and acrylamide were purchased from El-Naser Pharm. & Chem. Ind. Comp. Cairo, Egypt.
Experimental animals: A total of 28 adult male Swiss albino mice strain with average body weight of 25 ± 2 g were obtained from the animal house of Research Institute of Ophthalmology, Ministry of Scientific Research, and Giza, Egypt. Rats were caged individually in wire bottomed stainless steel cages and kept under normal healthy laboratory conditions at constant temperature (22°C - 24°C). Water was consumed ad libitum.
Methods
Preparation of the raw materials: Carrot and tomato were separately washed with tap water, chopped into small pieces and blanched with water vapor then (freeze drying) according to A.O.A.C. (2005). The dried materials were separately milled into powder and sieved through 100-mesh sieve, then packed in polyethylene bags at 4°C till used.
Biological evaluation: For biological evaluation 28 male mice were fed on a basal diet for three weeks. Basal diet was composed according A.O.A.C. (2005) [18]. After adaptation period animals were randomly divided into 4groups, (each comprised of 7 mice). The first group (7 rats) was considered as negative control (group 1). The other three groups (21 mice) were subjected for daily oral administration of acrylamide (40 μg/1kg body weight) for 8 weeks. The second group was considered as positive control. Both negative and positive groups were fed on basal diet throughout the experimental period (8 weeks). The other two groups were given basal diets with 20% of dried tomato and 20% of dried carrot (Groups 3, and 4, respectively). After elapse of experimental period, rats were fasted 12 hours. Then the mice were anaesthetized and sacrificed. At the end of experiment, brain, prostate and small intestines were histopathologically examined. The compositions of experimental diets are shown in Table 1.
Table 1. Composition of experimental diets
Ingredients (g/kg) |
Experimental diets |
Basal diet |
Dried Tomato |
Dried Carrot |
Casein |
140 |
111 |
129.14 |
Soy bean oil |
40 |
39.02 |
37.74 |
Sucrose |
100 |
100 |
100 |
Salt mix. |
35 |
14.98 |
24.68 |
Vitamin mix. |
10 |
10 |
10 |
Cellulose |
50 |
- |
- |
Corn starch |
620.7 |
520.7 |
494.14 |
Dried Tomato |
- |
200 |
- |
Dried Carrots |
- |
- |
200 |
Histopathological changes of examined organs: Brain, small intestines and prostate samples were histopathologically examined at the Histology Laboratory, Faculty of Veterinary Medicine, Cairo University according to the method [19].
Statistical analysis: The data were expressed as means ± S.D. The statistical analysis was performed using SPSS for Windows (SPSS, Inc.). P values less than 0.05 was considered to be significant [20].
Results showed insignificant differences among feed intakes, initial, final & gain% of body weight, feed efficiency ratios and organs' weights. Groups fed on diets with carrot and tomato showed significant improvement in almost all the parameters studied compared to the positive control.
Group 1 of mice kept as negative control
In brain, there was no histopathological alteration observed and the normal histological structure of the meninges, cerebral cortex, cerebral striatum, hippocampus, and cerebellum as seen in Figure 1A. In small intestine, there was no histopathological alteration observed and the normal histological structure of the mucosal layer, submucosa and muscularis were recorded in Figure 1B. Prostate gland, there was no histopathological changes (Figure 1C).
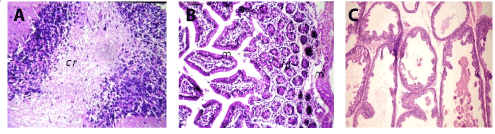
Figure 1. A. Brain of mice in group 1 showing normal histopathological structure of the cerebellum (cr) (H and E X64. B. Small intestine of mice in group 1 showing normal histopathological structure of the mucosal (m), lamina propria (lp) and muscularis (ml) (H and E X40). C. Prostate gland of mice in group 1 showing normal histopathological structure (H and E X200).
Group 2 of mice kept as positive control
In brain, Focal as well as diffuse gliosis were detected in the cerebrum (Figures 2A and 2B), while the medullas oblongata showed perivascular cuffing (Figure 2C).
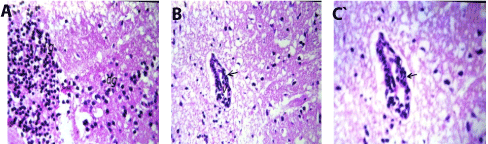
Figure 2. A. Brain of mice in group 2 showing focal (fg) as well as diffuse (dg) gliosis in the cerebrum (H and E X80). B. Brain of mice in group 2 showing perivascular cuffing surrounding the blood vessels (arrow) and medullas oblongata (H and E X80). C. Brain of mice in group 2 showing magnification of (Fig. 30) to identify the perivascular cuffing surrounding the blood vessels (arrow) in medullas oblongata (H and E X40).
In small intestine, the lamina propria of the mucosal layer showed focal inflammatory cells infiltration with edema and hypertrophy of the muscularis (Figures 3A and 3B). There was lymphoid hyperplasia in the submucosal layer (Figure 3C).
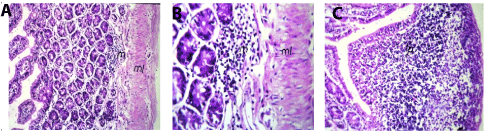
Figure 3. A Small intestine of mice in group 2showing normal focal inflammatory cells infiltration (m) in lamina propria with odema and hypertrophy in the muscularis (ml) (Hand EX40). B. Small intestine of mice in group 2 showing magnification of (Picture7) to identify the focal inflammatory cells infiltration (m) in lamina propria with edema and hypertrophy in the muscularis (ml) (H and E X80). C. Small intestine of mice in group 2 showing lymphoid hyperplasia (lp) (H and E X40).
Meanwhile, prostate gland of mice from positive control revealed atrophy of epithelial lining (Figure 4A), necrosis and fibrosis of epithelium (Figure 4B), interstitial edema and desquamated epithelium in the lumen (Figure 4C).
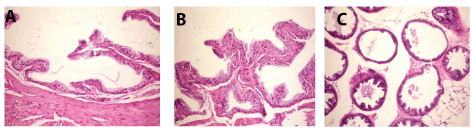
Figure 4. A. Prostate gland of mice in group 2 showing atrophy of epithelial lining (H and E X200). B. Prostate gland of mice in group 2 showing necrosis and fibrosis of epithelium (H and E X200). C. Prostate gland of mice in group 2 showing interstitial edema and desquamated epithelium in the lumen (H an E X200).
Group 3 of mice fed on diet with tomato
In brain, there was no histopathological alteration observed in the meninges, cerebral cortex and striatum as recorded in Figure 5A. In small intestine, inflammatory cells infiltration with edema were noticed in the lamina propria of the villi associated with hypertrophy and edema in the muscularis (Figures 5B and 5C) and follicular lymphoid hyperplasia in the submucosal layer (Figure 5D). Meanwhile, prostate gland revealed atrophy of epithelial lining, thickening of muscular layer (Figure 5E) and slight interacineredema (Figure 5F).
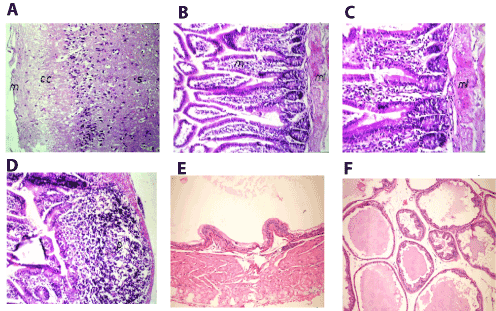
Figure 5. A. Brain of mice in group 3 showing intact normal histopathological structure in the meninges(m), cerebral cortex (cc) and cerebral striatum (s) (H and E X40) B. Small intestine of mice in group 3 showing inflammatory cells infiltration in lamina propria of the villi (m) with hypertrophy and edema and muscularis (ml) (H and E X40). C. Small intestine of mice in group 3 showing magnification of (Picture 17) to identify the inflammatory cells infiltration in lamina propria of the villi (m) with hypertrophy and edema in the muscularis (ml) (H and E X64). D. Small intestine of mice in group 3 showing lymphoid hyperplasia in the submucosal layer (p) (H and E X40). E. Prostate gland of mice in group 3 showing atrophy of epithelial lining and thickening of muscular layer (H and E X200). F. Prostate gland of mice in group 3 showing slight interacineredema (H and E X200).
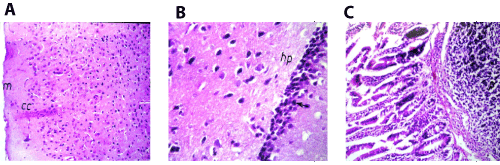
Figure 6. A. Brain of mice in group 4 showing intact normal histopathological structure in the meninges (m), cerebral cortex (cc) and cerebral striatum (s) (H and E X40). B. Brain of mice in group 4 showing intact normal histopathological structure in the hippocampus (hp) (H and E X80). C. Small intestine of mice in group 4 showing lymphoid hyperplasia in the submucosal layer (p) (H and E X40).
Group 4 of mice fed on diet with carrot
In brain, there was no histopathological alteration observed in the meninges cerebral cortex, striatum and hippocampus as recorded in (Figures 6A and 6B). Small intestine, Follicular lymphoid hyperplasia was detected in the submucosal layer (Figure 6C).
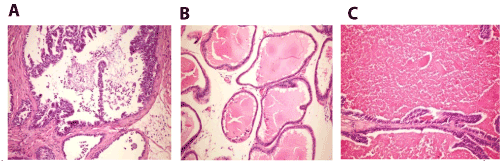
Figure 7. A. Prostate gland of mice in group 4 showing desquamation of epithelial cells with formation of corpora amylacea in the lumen (H and E X200). B. Prostate gland of mice in group 4 showing interaciner edema (H and E X200). C. Prostate gland of mice in group 4 showing marked distension of the acini with colloid protein (H and E X200).
Prostate gland sections showed desquamation of epithelial cells with formation of corpora amylacea in the lumen (Figure 7A), interaciner edema (Figure 7B) and marked distension of the acini with colloid protein (Figure 7C).
The severity of the reaction according to histopathological alterations in different organs of different groups
Table 2 illustrates the severity of histopathological alterations in organs of different groups.
Table 2. The severity of the reaction in different organs according to histopathologica lalterations
Groups |
Organs |
|
|
4: Carrot group |
3: Tomato group |
2:Positive control |
1: Negative control |
|
- |
- |
+++ |
- |
Brain |
|
+ |
++ |
+++ |
- |
Small Intestine |
|
++ |
+ |
+++ |
- |
Prostate gland |
+ + + Severe, + + Moderate, + Mild, - Nil
The results of histopathology examinations (Table 2) showed no alteration and normal histopathological structure in different organs (small intestine, prostate and brain) of the normal group (negative control) was observed. Very severe alterations were noticed in small intestine, prostate and brain of the positive control treated with acrylamide. The other groups fed on diets with dried vegetables detected slight alterations such as moderate and mild (in small intestines and prostate gland) and showed a normal histopathological structure (in brain) organs.
No alterations in brain of groups 3 and 4 fed on diets with vegetables (tomatoes and carrots) were also found. Group 3 and 4 fed on diet with tomato and carrot showed histopathological alterations in small intestine either mild (groups 4) or moderate (group 3). In prostate, the changes varied from mild (in group 3) to modrate in groups 4. Thus, it could be noted that adding dried vegetables to the diets ameliorated the effect of acrylamide and delayed its determenal damege on different tissues in mice. Such might be related to the components found in dried fruits and vegetsbles (lycopene and phenols).
Processed tomato products appeared to be good dietary sources of lycopene [21,22]. The Mediterranean diet, which is rich in vegetables, including tomatoes, has been suggested to be responsible for the lower cancer rates in that region. Dietary intake of tomatoes and tomato products has been found to be associated with a lower risk of a variety of cancers in several epidemiological studies [2].
In this respect, Kuriyama, et al. [23] noted that the purified fraction with the major glycolipids from some dried vegetables (included carrots) was an inhibitor of DNA polymerase α (pol α) in vitro and also the proliferation of human cancer cells. They noticed a significant correlation between sulfoquinovosyl diacyloglycerol (SQDG) content and inhibition of DNA polymerase. The inhibition of pol α activity by SQDG might lead to cell growth suppression.
Flavone, a flavonoid proved to be an effective apoptosis inducer in colon cancer cells in culture, affected the development of aberrant crypt foci (ACFs) in C57BL/6J mice in vivo when preneoplastic lesions were induced by the carcinogen 1, 2 dimethylhydrazine (DMH). Flavone applied daily at either a low dose (15 mg) or a high dose (400 mg) per kg body wt significantly reduced the numbers of ACFs, regardless it was supplied simultaneously with the carcinogen (blocking group) or subsequent to the tumor induction phase (suppressing group). Flavone reduced the number of ACFs in DMH-treated mice at doses that could be achieved for flavonoids by a diet rich in vegetables. Mitochondrial substrate oxidation was increased by flavone in colonic cells in vivo as already observed in HT-29 cells in vitro as the prime mechanism underlying tumor cell apoptosis induction by flavone [24].
The anti-neoplastic effect might be exerted by induction of apoptosis and autophagy by Mori, et al. [25] who noticed anti-neoplastic effect of MK615 , an extract from the Japanese apricot (Prunus mume), against colon cancer cells. Wang, et al. [26] assured that Fig fruit latex (FFL)exhibited potent cytotoxicity in some human cancer cells with little effect in normal cells at certain concentration. The mechanism for such effects might be associated with the inhibition of DNA synthesis, induction of apoptosis, and cell cycle arrest of cancer cells.
The anti-neoplastic effect might be exerted by induction of apoptosis and autophagy by Wang, et al. [26] assured that FFL exhibited potent cytotoxicity in some human cancer cells with little effect in normal cells at certain concentration. The mechanism for such effects might be associated with the inhibition of DNA synthesis, induction of apoptosis, and cell cycle arrest of cancer cells.
In this respect, Tharappel et al. [16] examining the effects of several antioxidant phytochemicals on the tumor promoting activity of tetrachlorobiphenyl (PCB-77) in female Sprague Dawley rats proved that rats, which received PCB-77 alone showed an increase in the number and size of positive foci in the liver. Lycopene significantly decreased the number of foci, while curcumin and CoQ10 decreased the size of the foci. In contrast, ellagic acid increased the number but decreased the size of the foci.
Cancer found to be a complex disease to treat and the treatments have not significantly progressed in the last few years. Proof of concept of chemoprevention has been shown with the non-steroidal anti-inflammatory drugs (NSAIDs). However there has been significantly more interest in plant and naturally available compounds for chemoprevention [27]. Lycopene was also associated with a reduced risk of prostate cancer and decreased cell apoptosis rates [17].
2021 Copyright OAT. All rights reserv
Severe effects of acrylamide adminestration in the treated tissues of the positive control was noticed. However, in the other groups fed on diets with dried fruits and vegetables demonstrated that the severity of alterations was different (normal, mild and moderate).
- Byers T (1999) What can randomized controlled trials tell us about nutrition and cancer prevention? CA Cancer J Clin 49: 353- 361. [Crossref]
- Giovannucci E (1999) Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst 91:317-31.
- RiboliE, Norat T (2003) Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk, Am J Clin Nutr 78 (suppl.): 69S-559S. [Crossref]
- Temple NJ, Gladwin KK (2003) Fruit, vegetables, and the prevention of cancer: research challenges. Nutrition 19: 467–470. [Crossref]
- Donaldson MS (2004) Nutrition and cancer: A review of the evidence for an anti-cancer Diet. Nutrition J 19: 1475-2891. [Crossref]
- Johnson IT (2004) New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis: 551: 9-28. [Crossref]
- Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, et al. (2000) Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. J. Cancer Epidemiol Biomarker Prev: 795–804. [Crossref]
- Greenwald P (2001) Clinical trials of breast and prostate cancer prevention. J Nutr 131: 176S–178S. [Crossref]
- WHO (2004) Fruit and vegetables for health: Report of a Joint FAO/WHO Workshop. Kobe, Japan.
- Adams K, Campbell J, Zaripheh S, Jeffery E, Erdaman J (2005) The tomato as a functional food. J Nutr 135: 1226-1230. [Crossref]
- Vainio H, Weiderpass E (2006) Fruit and Vegetables in Cancer Prevention. Nutr Cancer: 111–142. [Crossref]
- Gonzalez CA, Riboli E (2006) Diet and cancer prevention: where we are, where we are going. Nutr Cancer56: 225–231. [Crossref]
- World Cancer Research Fund/American Institute for Cancer Research (2007) Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC, USA.
- Kravchenko JS (2008) Diet and Cancer. Int Encyclopedia Public Health:169-181.
- Aggarwal BB, Danda D, Gupta S, Gehlot P (2009) Models for prevention and treatment of cancer: Problems vs promises. Biochem Pharmacol 78: 1083-1094. [Crossref]
- Tharappel JC, Lehmler H, Srinivasan C, Robertson LW, Spear BT, et al. (2008) Effect of antioxidant phytochemicals on the hepatic tumor promoting activity of 3, 3′, 4, 4′-tetrachlorobiphenyl (PCB-77). Food Chem Toxicol 46: 3467–3474. [Crossref]
- Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW (2011) Lycopene and Apo-12'-Lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. J Nutr Cancer 63 : 256 – 263. [Crossref]
- A.O.A.C. (2005) Association of Official Analytical Chemist, Official Methods Of Analytical Chemists' 18th Edn., A. O. A. C., Washington, USA.
- Banchroft JD, Stevens A, Turner DR (1996) Theory and Practice of Histological Techniques. 4th Edn. Churchill Livingstone, New York, USA.
- Steel RG, Torrie JH (1980) Principles and Procedures of Statistic. 2nd Edn McGraw-Hill, Inc, USA.
- Stahl W, Sies H (1992) Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr 122: 2161-2166. [Crossref]
- Gärtner C, Stahl W, Sies H (1997) Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr 66: 116- 122. [Crossref]
- Kuriyama I, Musumi K, Yonezawa Y, Takemura M, Maeda N, et al. (2005) Inhibitory effects of glycolipids fraction from spinach on mammalian DNA polymerase activity and human cancer cell proliferation. J Nutr Biochem 16:594-601. [Crossref]
- Winkelmann I, Diehl D, Oesterle D, Daniel H, Wenzel U (2007) The suppression of aberrant crypt multiplicity in colonic tissue of 1,2-dimethylhydrazine-treated C57BL/6J mice by dietary flavone is associated with an increased expression of Krebs cycle enzymes. J Carcinogenesis 28: 1446–1454.
- Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K (2007) New anti-proliferative agent, MK615, from Japanese apricot “Prunusmume” induces striking autophagy in colon cancer cells in vitro. World J Gastroenterol 13: 6512-6517. [Crossref]
- Wang J, Wang X., Jiang S, Lin P, Zhang J, et al. (2008). Cytotoxicity of fig fruit latex against human cancer cells. Food and Chemical Toxicology, 46: 1025-1033. [Crossref]
- Saunders FR, Wallace HM (2010) On the natural chemoprevention of cancer. Plant Physiology and Biochemistry 48: 621-626. [Crossref]







