Transplantation from living or deceased donors has been limited by donor availability that is opposed to the increasing demand and by the risk of allograft loss rejection and immunosuppressive therapy toxicity. In recent years, xenotransplantation of metanephroi has offered a novel solution for the unlimited supply of human donor organs. However, even if in a most favourable and idyllic situation in which the organ availability and its demand could be balanced using transplantation of animal embryonic organs, the future of this treatment would still be compromised without proper long-term storage procedure. Thus, based on the ongoing long-term storage necessities, this study was designed to investigate the effect of two specific time “window” of the metanephroi development (15 days-old and 16 days-old) on the in vivo developmental capacity and the developed morphologically normal glomeruli of vitrified metanephroi in non-immunosuppressive rabbits. Metanephroi originating from 15 and 16 days old rabbit embryos were vitrified using M22 solution and Cryotop® as a device. After three months of storage in liquid nitrogen, metanephroi were transplanted into non-immunosuppressed adult hosts by laparoscopy surgery. Twenty-one days after allotransplantation, 6 (32%) and 7 (35%) “new kidneys” were recovered. All the “new kidneys” recovered exhibited significant growth and mature glomeruli. However, histomorphometry analysis revealed that “new kidneys” developed from 16 days-old metanephroi exhibit a greater degree of maturity compared with 15 days-old metanephroi. Results obtained in the present study point out that, in rabbit model, vitrified 16 days-old metanephroi could be stored in liquid nitrogen, achieving good in vivo developmental capacity and developing morphologically normal glomeruli after laparoscopy transplantation into non-immunosuppressed hosts.
metanephros, cryopreservtion, xenotransplant, kidney disease, cryobanks
Abbreviations
BM: Base Medium; BS: Bowman’s space; DMSO: Dimethyl Sulphoxide; EEC: Environment European Commission; EG: Ethylene Glycol; FBS: Foetal Bovine Serum; G: Glomerulus; MP: 3-methoxy-1,2-propanediol; NMF: N-methylformamide; PVP: Polyvinylpyrrolidone; RC: Renal Corpuscle
In end-stage kidney disease, the kidneys no longer adequately clarify the blood of wastes and remove the excess fluids [1]. Then, organ transplantation provided the ideal method to restoring full physiological organ function [2]. However, transplantation from living or deceased donors has been limited by donor availability that is opposed to the increasing demand [2,3]. For this reason, patients with end-stage kidney disease are usually forced to receive dialysis treatment to support the lost kidney function. However, none of these treatments including peritoneal dialysis cure end-stage renal disease. It does not replace the kidneys’ role in metabolism regulation, endocrine function or homeostasis, includes blood pressure, electrolyte balance, acid-base balance, etc. [4-9]. Furthermore, dialysis is an expensive treatment, supposing a cost of 1518 million € in countries like Spain or 1.2 billion in United Kingdom [10-12]. In other countries like Canada the estimated annual per-patient costs were $56,000 for peritoneal dialysis, $71,000 to $90,000 for home haemodialysis and $95,000 to $107,000 for in-centre or satellite unit haemodialysis [13]. Furthermore, the dialysis centre capacity is limited and resources make impossible to provide more frequent or longer treatment sessions to patients [13-16]. In addition, the diet changes that patients must make, and the need to attend to receive dialysis treatment, affect negatively the life quality of patients and deplete its health [17]. For this, many patients define dialysis as more life to his years, but no more years to his life [18].
Paradoxically, for these reasons that evidence the superior benefits of kidney transplantation over dialysis and the growing incidence of end-stage renal disease have led to an exponential increase in the need for kidney transplantation worldwide [19]. In addition, it is known that five-year survival was considerably better after living-donor kidney transplantation (94%) or after cadaveric-kidney transplantation (76%) than on chronic dialysis (60%) [20]. These facts that causes that the lack of these organs becomes in a very serious problem of global health and causes patients to be on long waiting lists to obtain a transplant. Only in USA, the length of waiting list has doubled over the past decade, reaching around 100.000 patients and a median waiting time of 4.5 years [21]. In 2014, only 17.814 kidneys were transplanted, being 12.279 from deceased donors and 5535 from living donors [22]. Thus, as long as the patient does not get an organ it will be dependent on dialysis, which have a higher risk for morbidity and mortality [19], dying nearly 5000 patients every year in this country [21]. In fact, the long‐term mortality risk was 68% lower in the case of the patient receives a transplant when compared with patients remaining on the waiting list [23]. However, it is not only a problem of USA. In European Union in late 2011, more than 42.000 patients were on waiting list and only 18.712 transplants were performed, being a 20.6% from living donors [24]. Actually, in 2015 the situation isn’t better. Only 19,426 kidney transplants were performed, being a 68% from deceased donors, a 31% from living donors and 1% from an unknown donor type [22]. So taking into account all this information, seeking alternative solutions to this grave problem is indispensable.
In this point, regenerative medicine has garnered great attention in the last years because it has the potential to generate new organs for transplantation. Particularly, human kidney exhibits a remarkable complexity, coupled with the presence of at least 30 different specialized cells that have to be able to function together, for which their proper spatial distribution is indispensable [25]. Thus, the need for recapitulation the three-dimensionally integrated kidney structure is the reason because cell therapies with individual cells fail in restoring kidney function [26]. However, there is an open line of research that retakes the field of xeno-transplants as a possible solution to organ shortage. To date, rejection and zoonosis have limited the application of this kind of treatments [27,28]. However, transplantation of embryonic kidneys into non-immunosuppressed adult hosts can mature and growth as if they had not been extracted from the embryo. These embryonic kidneys or metanephroi are able to attract the formation of a vascular system from host (angiogenesis), undergoing maturation and exhibiting functional renal properties [26,29]. Dekel et al. [30] transplanted embryonic kidneys from both human and pig origins into mice, demonstrating that metanephroi were differentiated into functional nephrons evidenced by the dilute urine that they produce. However, it’s known that new renal tissue developed from metanephroi not only provide an excretion function but also an endocrine function, synthesising renal hormones such as renin and erythropoietin [31,32]. Furthermore, if metanephroi are transplanted beside bladders developed from cloacas, and if cloacal-developed bladder was connected to the host ureters, new metanephro-developed kidney produces and excretes urine through the recipient ureter, avoiding hydronephrosis and allowing the nascent kidney to continue their growth [33]. These findings, together with the scientific capability to produce specific pathogen-free animals [34] suggest than metanephroi transplantation could be a possible solution for kidney need [26,35].
Our group has been working on the development of a novel technique that allows the metanephroi transplantation procedure thought a minimal invasive procedure, presenting recently an effective way by laparoscopy [24,36-42]. To our best knowledge, all the previous studies were performed through open surgery, a fact that has limited the experiments on larger animals and should be avoided aimed at clinical application. However, even if in a most favourable and idyllic situation, in which the organ availability and its demand could be balanced using embryonic xeno-transplants, the future of this treatment would still be compromised without proper cryopreservation procedures [36,37]. Effective cryopreservation can permit long-term storage of the transplantable organ, dissociating the recuperation and transplantation time, allowing an adequate inventory control and quality assurance thought the pertinent laboratory analysis whose requires time to organ examination. To date, only small structures such as small ovaries, heart valves or corneas are the only macroscopic structures that retain the capacity to recover, at least in part, after vitrification [38]. A historical case was reported by Fahy et al., [38], who describe how one whole rabbit kidney survive and supported indefinitely the life of a recipient animal after vitrification procedure using M22 vitrification medium. Based on this knowledge, we recently described a method to cryopreserve metanephroi effectively using VM3 vitrification solution and Cryotop® as device [36,37]. Previously to our works, only Bottomley et al., [39] examined the cryopreservation of metanephroi, but only under in vitro conditions. In an effort to advance in organ cryopreservation, this study investigate the effect of two specific time “window” of the metanephroi development (15 days-old and 16 days-old) on the in vivo developmental capacity and the developed morphologically normal glomeruli of vitrified metanephroi in non-immunosuppressive rabbits.
All chemicals, unless otherwise stated, were reagent- grade and purchased from Sigma-Aldrich Química S.A. (Alcobendas, Madrid, Spain). All the experimental procedures used in this study were performed in accordance with Directive 2010/63/EU EEC for animal experiments and reviewed and approved by the Ethical Committee for Experimentation with Animals of the Polytechnic University of Valencia, Spain (research code: 2015/VSC/PEA/00170).
Animals
New Zealand white females, 5 months old, were used as embryo donors and metanephroi recipients. The animals used came from the experimental farm of the Universidad Politécnica de Valencia. The rabbits were kept in conventional housing (with light alternating cycle of 16 light hours and eight dark hours, and under controlled environmental conditions: average daily minimum and maximum temperature of 17.5 and 25.5°C, respectively). All rabbits had free access to fresh food and water.
Metanephroi recovery and vitrification
Metanephroi were surgically dissected from a 15 and 16-day-old rabbit embryo under a dissecting microscope using previously described techniques [24,36-37,40-42] and vitrified within 1 h following the minimum essential volume method using Cryotop [40] as device and M22 [38] as vitrification solution.
Metanephroi were first exposed for 3 min to 2.5 ml equilibration solution containing 1.68% w/v ethylene glycol (EG), 1.28% w/v formamide, 2.23% w/v dimethyl sulphoxide (DMSO), 0.3% w/v N-methylformamide (NMF), 0.4% w/v 3-methoxy-1,2-propanediol (MP), 0.28% w/v PVP K12 (polyvinylpyrrolidone of Mr 5000Da) and 0.1% and 0.2% w/v final concentrations respectively of commercially available SuperCool X-1000 and SuperCool Z-1000 (ice blockers) in base medium (BM: DPBS + 20% foetal bovine serum, FBS) for 3 min. Then, the metanephros were submerged into 2.5 ml of solution containing 4,82% w/v EG, 3.68% w/v formamide, 6.38% w/v DMSO, 0.86% w/v NMF, 1.14% w/v MP, 0.8% w/v PVP K12 and 0.29% w/v with 0.57% w/v final concentrations respectively of SuperCool X-1000 and SuperCool Z-1000 ice blockers in BM for 1 min.
Finally, metanephroi were submerged into 2.5 ml of vitrification solution consisting of 16.84% w/v EG, 12.86% w/v formamide, 22.31% w/v DMSO, 3% w/v NMF, 4% w/v MP, 2.8% w/v PVP K12 and 1% w/v with 2% w/v final concentrations respectively of SuperCool X-1000 and SuperCool Z-1000 ice blockers in BM before being loaded into Cryotop devices and directly plunged into liquid nitrogen within 1 min. All manipulations were performed at room temperature (25 ± 1°C) and all the media were used at room temperature, except for the first warming solution, which was used at 37.5°C.
Metanephros transplantation surgery
After 3 months of storage in liquid nitrogen, metanephroi were warmed and transplanted into recipients. For warming, metanephroi were submerged into 2.5 ml of a solution containing 1.25 M sucrose in BM for 1 min and later transferred stepwise into decreasing sucrose solutions (0.6, 0.3 and 0.15 M sucrose in BM) for 30 s before and then washed twice in BM for 5 min. Then, metanephroi were transplanted within 45 min after warming or collected (fresh).
Host females were sedated by intramuscular injection of xylazine (5 mg/kg) (Rompun, Bayer AG, Leverkusen, Germany) and morphine chloride (3 mg/kg) (Morfina, B. Braun, Barcelona, Spain). As surgical preparation, anaesthesia was performed by intravenous injection of ketamine hydrochloride (35 mg/kg) (Imalgene®, Merial, S.A., Lyon, France) into the marginal ear vein. Firstly, animals were placed on an operating table in a vertical position (head down at 45-degree angle). Only one endoscope trocar was inserted into the abdominal cavity.
Then, an epidural 17G needle (for 15-days-old metanephroi) or Cistofix® needle (for 16-days-old metanephroi) was inserted into the inguinal region. After identify a renal vessel in the retroperitoneal fat, a hole was performed adjacent to the vessel. Then, kidney precursor was aspirated in appropriate catheter using previously described techniques [24,36,37,41,42], and the catheter was introduced through the corresponding needle and insert into the performed hole. Between 3 to 4 kidney precursors were transplanted in each host without immunosuppression (one metanephros per hole). After surgery, analgesia was administered for 3 days (0.03 mg/kg of buprenorphine hydrochloride, Buprex®, Esteve, Barcelona, Spain, each 12 hours, and 0.2 mg/kg of meloxicam, Metacam®, 5 mg/mL; Norvet; Barcelona, Spain, every 24 hours). Also, all the recipients were treated with antibiotics (4 mg/kg of gentamicine (10% Ganadexil, Invesa, Barcelona, Spain) every 24-h for 3 days). No immunosuppression was given to recipients. Metanephroi transplantation was assessed in three sessions.
Growth of metanephroi and histomorphometry of the renal corpuscle
The animals receiving implants were euthanatized at 3 weeks after transplantation. New kidneys were then removed, fixed in 4% paraformaldehyde solution and embedded in paraffin wax. New kidneys were cut into 5-μm histological sections and stained with haematoxylin and eosin. Kidneys from a 5-weeks (approximately 36 days) old rabbit were used as controls. The stained sections were examined with light microscopy for histological and histomorphometric analysis according with previous works [36,37]. In the histomorphometric measurements, 20 renal corpuscle and glomeruli on each sample were measured (area and perimeter) in each of the groups – control and experimental. Photomicrographs were taken at total magnification of x1000. In addition, the glomerular tuft cellularity was estimated by counting the total number of nuclei of each glomerulus. Photomicrographs were measured using ImageJ analysis software (free software http://rsb.info.nih.gov/ij/). Kidneys originating from a 5-week-old rabbit (coeval with the metanephroi age) were used as controls.
Statistical analysis
The renal corpuscle and glomeruli measured (area and perimeter), and the glomerular tuft cellularity were compared by analysis of variance ANOVA with sample type (fresh and vitrified) as a fixed factor and replicate as random factor. The replicate was non-significant and was removed from the model. Differences of p<0.05 were considered significant. Data are shown as means ± standard error means (S.E.M.). All analyses, except mixed model (SAS Institute Inc., Cary, NC, USA) were performed with SPSS 21.0 software package (SPSS Inc., Chicago, Illinois, USA, 2002) (Table 1).
Table 1. Histomorphometric quantification of renal corpuscle of kidneys developed after allotransplantation of vitrified and fresh metanephroi.
Metanephroi age |
Group |
n |
Renal corpuscle |
Glomerulus |
Area
(μm2) |
Perimeter
(μm) |
Area
(μm2) |
Perimeter
(μm) |
Cells number |
|
Control |
4 |
2778 ± 199.8c |
188.5 ± 5.53c |
2221 ± 152.1c |
169.1 ± 4.96c |
54 ± 2a |
15 days |
Fresh |
7 |
3222 ± 200.8c |
200.5 ± 5.53c |
2267 ± 154.3c |
167.9 ± 4.96c |
42 ± 2c |
Vitrified |
6 |
3834 ± 214.2b |
223.1 ± 5.93b |
2726 ± 162.9b |
189.3 ± 5.32b |
50 ± 2a,b |
16 days |
Fresh |
12 |
6164 ± 233.8a |
283.4 ± 6.34a |
4498 ± 176.7a |
242.4 ± 5.69a |
47 ± 2b |
Vitrified |
7 |
5716 ± 229.2a |
271.3 ± 6.30a |
4045 ± 174.3a |
231.5 ± 5.66a |
52 ± 2a |
n: Number of new kidneys or control kidneys. Data are expressed as mean ± SD. a,b,c: Data in the same column with uncommon letters are different (p < 0.05).
Six rabbit females were used as embryo donors. Three of the females were euthanatized 15 days since artificial insemination (to obtained 15-days-old metanephroi) and the rest were euthanatized after 16 days since artificial insemination (obtaining 16-days-old metanephroi). In total, 35 metanephroi from 15-days-old embryos and 42 from 16-days-old embryos were carefully micro-dissected. After this, 19 vitrified and 16 fresh metanephroi from 15-days-old embryos and 22 vitrified and 20 fresh metanephroi from 16-days-old were transplanted into 20 host. Twenty-one days after transplantation procedure, the “new kidneys” were recovered and analysed (Figure 1). In total, 6 (32%) vitrified and 7 (44%) fresh metanephroi from 15-days-old embryos and 7 (35%) vitrified and 12 (55%) fresh metanephroi from 16-days-old were successfully grown. All the new kidneys, developed from both ages (15 and 16 days) independently of the experimental group (fresh or vitrified), underwent differentiation and developed histological mature glomeruli (Figure 1). Histomorphometry analysis showed similar values between vitrified and fresh metanephroi from 16-days-old and between fresh metanephroi from 15-days-old embryos and control samples. All the parameters of “new kidneys” from vitrified and fresh metanephroi from 16-days-old were statistically higher than vitrified and fresh metanephroi from 15-days-old (p < 0.05). Similar glomerular tuft cellularity was observed between “new kidneys” from vitrified metanephroi for both ages. In addition, in all the new kidneys parameters were significantly higher than in control group.
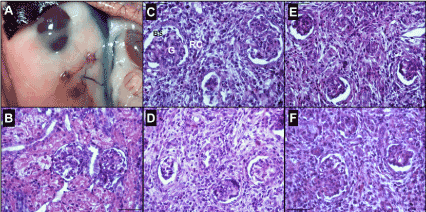
Figure 1. Metanephroi Development. (A) New-kidney developed from metanephroi. Representative photomicrograph of the renal corpuscles (400x): G; Glomerulus. RC; Renal Corpuscle. BS; Bowman’s space. (B) Renal corpuscle of the control kidney originating from 5-week-old rabbit (coeval with metanephros age). (C) Renal corpuscle of a fresh 15 days kidney precursor 3 weeks after transplantation. (D) Renal corpuscle of vitrified 15 days kidney precursor 3 weeks after transplantation. (E) Renal corpuscle of a fresh 16 days kidney precursor 3 weeks after transplantation. (F) Renal corpuscle of vitrified 16 days kidney precursor 3 weeks after transplantation.
To our best acknowledge, this is the first study that evaluated the effect of the metanephroi age on the in vivo developmental capacity and the developed morphologically normal glomeruli after long-term storage period. After transplant, both metanephroi ages (15 and 16 days old) underwent differentiation and growth, became vascularised by host blood vessels, and developed histological and morphologically mature glomeruli. Previously to our recent studies [24,36,37,41] only one study had evaluated the metanephroi cryoconservation [39], but the difference in methodology (in vitro vs. in vivo) to test the metanephroi viability after its vitrification difficult the comparison between the studies. However, vitrification was presented as the more promising technique. The results of the present work are consistent with our previous experiments [24,36-37,41].
Cryobiology is the science that studies the effects of low temperatures on living organism and its aim is to achieve cells become immortals at low temperatures though stopping its metabolism. One of the major problems to obtain this goal is the need to avoid the ice crystal formation [43,44] that causes cell death. Presumably, the cause for our improvement was due to the vitrification technique, in which the liquids in a living system are turned into the glassy state without ice crystal formation [41]. In this study, we evaluate the vitrification solution named M22, which had already demonstrated its effectiveness in the preservation of renal tissue [38]. In addition, we combined this solution with Cryotop® device [40], which minimise the volume to be vitrified, facilitating the rapid transfer of heat to liquid nitrogen [36,37] that allows the vitrification procedure. Marco-Jimenez, et al. [36] and Garcia-Dominguez et al. [37] had already observed that the small size of metanephroi could allow an adequate diffusion and equilibration of cryoprotectants within the organ cells and the vitrification medium, a fact that could explain also the effectiveness in the vitrification procedure. This is in concordance with the fact that to date, only small biological structures had been vitrified and recovered later. Another point in favour of the metanephroi survival after it be thawed is that this embryonic organ is avascular and attract blood vasculature directly from the host not requiring immediate anastomosis as in the case of mature vascularized adult organs [41,42].
We previously demonstrated that the time window in which rabbit metanephroi can implant and develop into new kidneys are 15 and 16 days [24,36,37]. In our previous metanephroi vitrification studies, based on VM3 vitrification solution, metanephroi from 15 days-old embryos exhibit better growth rate than metanephroi from 16 days-old embryos. The result could be explained due to larger size. However, in the present study, no differences were observed between both metanephroi ages. A plausible explanation could be that M22 vitrification medium includes components that might help in the diffusion of cryoprotective agents into the whole metanephroi structure. However, this study demonstrated a clear effect of metanephroi age on the renal histomorphometry. Specifically, developed new kidneys from 16 days-old vitrified metanephroi exhibited similar renal glomerular histomorphometry than fresh metanephroi. Furthermore, glomerulus cell density was maintained in from 16 days-old vitrified metanephroi compared with control. In contrat, vitrification of 15 days-old metanephroi disrupts histomorphometric parameters compared with fresh metanephroi. Nevertheless, our results indicates that vitrification procedure neither affect negatively the development capacity of 15 days-old metanephroi. In general, all the histomorphometric parameters relating to new kidneys are higher than control samples. In part, these results could be explained by the fact that these new renal structures were not connected to the host’s urinary system. Under this condition, unconnected metanephroi become hydronephrotic, accumulating the liquid inside the new kidney dilating the glomerular structures [36,37,41,42]. In this paper we demonstrated than metanephroi vitrification using M22 medium as vitrification solution support the developmental capability of both 15 days-old and 16 days-old metanephroi. However, although we have focused the study solely from the point of the cryobiological effect, it is important to say that some authors have already shown that transplants of fresh kidney precursors are able to filter blood producing urine [33,35] and synthetizing renal hormones [31,32] such as renin and erythropoietin. In the future, it would be interesting demonstrate the same capacities in developed kidneys from vitrified metanephroi. This study, together with our previous results [24,36,37,41,42], reinforce the idea that it is possible to create a biobank of renal precursors, a fact that would facilitate inventory control and give the necessary time to guarantee the safety of the transplant.
In conclusion, the present study shows that storing vitrified 16 days-old metanephroi in liquid nitrogen is an effective long-term storage option that maintains metanephroi developmental viability and could achieve good developmental capacity and developing morphologically normal glomeruli after laparoscopy transplantation into non-immunosuppressed hosts. Further studies are needed in order to support our results and understand the potential future implication of this technique in the clinical practice.
This work was supported by funds from the Generalitat Valenciana Research Programme (PrometeoII 2014/036), ALCER-TURIA foundation and PRECIPITA CROWDFUNDING. English text version was revised by N. Macowan English Language Service.
- Leigh-Ann Topfer (2017) Wearable artificial kidneys for end-stage kidney disease. Ottawa: CADTH. (CADTH issues in emerging health technologies; issue 150).
- Salvatori M, Peloso A, Katari R, Orlando G (2014) Regeneration and bioengineering of the kidney: current status and future challenges. Curr Urol Rep 15: 379. [Crossref]
- D'Agati VD (2012) Growing new kidneys from embryonic cell suspensions: fantasy or reality? J Am Soc Nephrol 23: 1763-1766. [Crossref]
- Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH (2014) The bioartificial kidney: current status and future promise. Pediatr Nephrol 29: 343-351. [Crossref]
- Kim S, Fissell WH, Humes DH, Roy S (2015) Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed) 7: 215-228. [Crossref]
- Kooman JP, Joles JA, Gerritsen KG (2015) Creating a wearable artificial kidney: where are we now? Expert Rev Med Devices 12: 373-376. [Crossref]
- Mitsides N, Keane DF (2014) Technology innovation for patients with kidney disease. J Med Eng Technol 39: 424-433. [Crossref]
- Jansen J, Fedecostante M, Wilmer MJ, van den Heuvel LP, Hoenderop JG, et al. (2014) Biotechnological challenges of bioartificial kidney engineering. Biotechnol Adv 32: 1317-1327. [Crossref]
- Davenport A (2012) Portable or wearable peritoneal devices--the next step forward for peritoneal dialysis? Adv Perit Dial 28: 97-101. [Crossref]
- Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, Cuervo J, Rebollo P, Otero A, Arrieta J. (2011) Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 26: 3709-3714.
- Dilworth MR, Clancy MJ, Marshall D, Bravery CA, Brenchley PE, et al. (2008) Development and functional capacity of transplanted rat metanephroi. Nephrol Dial Transplant 23: 871-879. [Crossref]
- Clancy MJ, Marshall D, Dilworth M, Bottomley M, Ashton N, Brenchley P (2009) Immunosuppression Is Essential for Successful Allogeneic Transplantation of the Metanephroi. Transplantation. 88: 151-159.
- Klarenbach SW, Tonelli M, Chui B, Manns BJ (2014) Economic evaluation of dialysis therapies. Nat Rev Nephrol 10: 644-652. [Crossref]
- Davenport A, Gura V, Ronco C, Beizai M, Ezon C, et al. (2007) A wearable haemodialysis device for patients with end-stage renal failure: a pilot study. Lancet 370: 2005-2010. [Crossref]
- ECRI Institute (2015) U.S. clinical trial data announced on wearable artificial kidney prototype. Health Technol Trends. 27: 1-3.
- Pauly RP, Komenda P, Chan CT, Copland M, Gangji A, et al. (2014) Programmatic variation in home hemodialysis in Canada: results from a nationwide survey of practice patterns. Can J Kidney Health Dis 1: 11. [Crossref]
- Davenport A (2015) Portable and wearable dialysis devices for the treatment of patients with end-stage kidney failure: Wishful thinking or just over the horizon? Pediatr Nephrol 30: 2053-2060. [Crossref]
- Iñaki Saralegui, Arantza Arrausi, Oscar García Uriarte, Elena Montoya, Yolanda Martínez, et al. (2014) La suspensio´n de la Dia´lisis en pacientes con Insuficiencia Renal Cro´nica Avanzada: ¿Que´ opinan los enfermos?. Enferm Nefrol 17: 110-119.
- Wu DA, Watson CJ, Bradley JA, Johnson RJ, Forsythe JL, et al. (2017) Global trends and challenges in deceased donor kidney allocation. Kidney Int. [Crossref]
- Medin C, Elinder CG, Hylander B, Blom B, Wilczek H (2000) Survival of patients who have been on a waiting list for renal transplantation. Nephrol Dial Transplant 15: 701-704. [Crossref]
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, et al. (2015) OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 15 Suppl 2: 1-34. [Crossref]
- Wang JH, Skeans MA, Israni AK (2016) Current Status of Kidney Transplant Outcomes: Dying to Survive. Adv Chronic Kidney Dis 23: 281-286. [Crossref]
- Briggs JD (2001) Causes of death after renal transplantation. Nephrol Dial Transplant 16: 1545-1549. [Crossref]
- Vera-Donoso CD, García-Dominguez X, Jiménez-Trigos E, García-Valero L, Vicente JS, Marco-Jiménez F (2015) Laparoscopic transplantation of metanephroi: A first step to kidney xenotransplantation. Actas Urol Esp. 39: 527-534.
- Montserrat N, Garreta E, Izpisua Belmonte JC (2016) Regenerative strategies for kidney engineering, FEBS J. IN PRESS. doi:10.1111 /febs.13704.?
- Hammerman MR (2007) Transplantation of renal primordia: renal organogenesis. Pediatr Nephrol 22: 1991-1998. [Crossref]
- Cooper DK (2012) A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 25: 49-57. [Crossref]
- Costa MR, Fischer N, Gulich B, Tönjes RR (2014) Comparison of porcine endogenous retroviruses infectious potential in supernatants of pro- ducer cells and in cocultures. Xenotransplantation. 21: 162– 73.
- Takeda S, Rogers SA, Hammerman MR (2006) Differential origin for endothelial and mesangial cells after transplantation of pig fetal renal primordia into rats. Transpl Immunol. 15: 211–5. ?
- Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, et al. (2003) Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9: 53-60. [Crossref]
- Yokote S, Yokoo T, Matsumoto K, Utsunomiya Y, Kawamura T, Hosoya T (2012) The effect of metanephroi transplantation on blood pressure in anephric rats with induced acute hypotension. Nephrol Dial Transplant. 27: 3449-3455.
- Matsumoto K, Yokoo T, Yokote S, Utsunomiya Y, Ohashi T, et al. (2012) Functional development of a transplanted embryonic kidney: effect of transplantation site. J Nephrol 25: 50-55. [Crossref]
- Yokote S, Matsunari H, Iwai S, Yamanaka S, Uchikura A, et al. (2015) Urine excretion strategy for stem cell-generated embryonic kidneys. Proc Natl Acad Sci U S A 112: 12980-12985. [Crossref]
- Yasutomi Y (2010) Establishment of specific pathogen-free macaque colonies in Tsukuba Primate Research Center of Japan for AIDS re- search. Vaccine. 28: 75-77.
- Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, et al. (2003) Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9: 53-60. [Crossref]
- Marco-Jiménez F, Garcia-Dominguez X, Jimenez-Trigos E, Vera- Donoso CD, Vicente JS (2015) Vitrification of kidney precursors as a new source for organ transplantation. Cryobiology. 70: 278-82.
- Garcia-Dominguez X, Vicente JS, Vera-Donoso C, Jimenez-Trigos E, Marco-Jiménez F (2016) First steps towards organ banks: vitrification of renal primordia. Cryo Letters. 37: 47-52.
- Fahy GM, Wowk B, Pagotan R, Chang A, Phan J, et al. (2009) Physical and biological aspects of renal vitrification. Organogenesis 5: 167-175. [Crossref]
- Bottomley MJ, Baicu S, Boggs JM, Marshall DP, Clancy M, et al. (2005) Preservation of embryonic kidneys for transplantation. Transplant Proc 37: 280-284. [Crossref]
- Kuwayama M, Vajta G, Ieda S, Kato O (2005) Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online 11: 608-614.
- García-Domínguez X, Vera-Donoso CD, García-Valero L, Vicente JS, Marco-Jimenez F (2016) Embryonic Organ Transplantation: The New Era of Xenotransplantation. Capitulo 2. In: Abdeldayem H, El-Kased AF, El-Shaarawy A (Eds) "Frontiers in Transplantology" 25-46.
- García-Domínguez X, Vicente JS, Vera-Donoso CD, Marco-Jimenez F (2017) Current Bioengineering and Regenerative Strategies for the Generation of Kidney Grafts on Demand. Curr Urol Rep 18: 2. [Crossref]
- Mazur P (1963) Kinetics of Water Loss from Cells at Subzero Temperatures and the Likelihood of Intracellular Freezing. J Gen Physiol 47: 347-369. [Crossref]
- Edgar DH, Gook DA (2012) A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update 18: 536-554. [Crossref]

