Keywords
Intestinal transplantation; surgical complications; rats; microsurgery
Abstract
Small Bowel transplantation in rats is a highly complex microsurgical procedure because several technical complications may lead to recipient mortality and transplant failure. Our aim was to report the most common complications associated with orthotopic and heterotopic intestinal transplantation in rats in order to identify the “pitfalls” of the procedure and prevent them. A retrospective multicenter study was performed. All participant centers have established rodent transplant procedures and trained surgeons. Two hundred ninety-three complications from 264 unsuccessful intestinal transplants were reported, representing an overall failure rate of 15% of the procedures performed. Recipient complications were most frequent than donor (257 vs. 36 p<0.0001). Excessive surgical time (11/36); severe hemorrhage (12/36) and inappropriate infusion of the preservation solution in the intestinal graft (11/36) were the most common donor complications. Arterial anastomosis bleeding (50/257), venous anastomosis bleeding (35/257) and portal vein stenosis (26/257) were the most common intraoperative complications in the recipient. To maximize success rate, surgeons should optimize time and avoid bleeding during graft dissection in the donor surgery. After performing a bloodless vascular anastomosis an adequate post-operative management of the animal is mandatory to guarantee survival.
Introduction
Since Monchik and Rusell first described the technique to perform small bowel transplantation (SBTx) in rats, it has become one of the most commonly used in experimental models [1]. In general terms, it is used to study intestinal ischemia-reperfusion injury, graft rejection and assess immunosuppressive therapies [2-4]. SBTx in rats is a feasible, replicable and inexpensive procedure when compared to large animals models [5].
In spite of these advantages, SBTx in rats is a highly complex microsurgical procedure. Several technical complications and non-optimized animal care may lead to recipient mortality and transplant failure [6]. Therefore, surgeons should be trained not only in microsurgery but also in both pre and post-transplant management of the rodents to achieve prolonged recipient survival.
The difficulties to succeed in this challenging procedure urged the design of different surgical techniques; considering how the engraftment is performed in relation to the native organs, intestinal transplantation (ITx) may be performed orthotopically or heterotopically when the graft is placed in the anatomical location of the native organ removed during the surgery or if it is placed in a non-native location, respectively [7]. Orthotopic intestinal transplantation (OITx) is the approach of choice for physiological SBTx studies, and it may be performed in one step (as reported by Kort et al.) or, as described by Deltz and Thiede, in two surgical stages [8-10]. In the two-stage OITx, the intestinal graft is first placed in a heterotopic position, and then anastomosed to the recipient´s naïve intestine three weeks after the first procedure. Heterotopic intestinal transplantation (HITx) is the best option to obtain successive samples of the graft, and have permanent access to the transplanted intestine lumen [11].
Other differences in experimental SBTx models are based on the vascular anastomosis. Although the hand-sewn vascular anastomosis is the most frequently reported, other techniques were developed in order to shorten warm ischemia time. In general terms, these techniques replace the sutures for cuffs [12,13]. In addition, improvements in terms of anesthesia, analgesia, pre and post-surgical care, proper handling of animal environment, recipient end-point application, among others have contributed to better outcomes [14].
Despite the different reported ITx techniques and the improvement in rat handling, SBTx remains a challenging procedure and many complications may lead to failure. The manuscript published along with the development of this technique reports clearly the measures taken for a successful procedure, as well as some of the complications during the learning curve. However, to the best of our knowledge, very few manuscripts have focused on the complications that lead to procedure failure once learning curve has been completed. In order to identify the most common complications and provide a useful guide for surgeons or researchers wanting to perform SBTx in rats, we have retrieved information from four centers where expert microsurgeons have performed the procedure on a regular basis.
Materials and methods
This is a retrospective study performed by 4 active microsurgical research laboratories (Multiorgan Transplant Institute, Argentina; Organ and Tissue Transplant Laboratory, Argentina; La Paz University Hospital, Spain and Biomedical Research Center from Aragón, Spain) with extensive experience in experimental microsurgery and microsurgical SBTx techniques [10,15,16-20].
All animal experiments were performded according to guidelines set by USPHS and/or European Union policy (National Research Council, National Academy Press, Washington DC, 2010, and/or European Union Directive for Animal Experiments 2010/63/EU).
In all cases, OITx and HITx procedures were performed identically by all participant surgeons in the four participating centers. The graft was obtained following the same surgical steps in OITX and HITx procedures. Briefly, a median laparotomy was performed and the small bowel from the Treitz to 3 cm from the ileocecal valve was dissected. Ileocecocolic, colic and duodenal vessels, pyloric vein, splenic vein and celiac trunk were transected to prepare a graft vascular pedicle consisting of superior mesenteric artery and portal vein. Finally, infrarrenal aorta was cannulated and the graft was perfused with 5-7 ml of cold Ringer Lactate.
In the recipient surgery, engraftment by end-to-side arterial and vein anastomosis was performed. In HITx, the graft was externalized by two ostomies on the recipient right abdominal wall. On the other hand, after a recipient enterectomy, reperfused graft was incorporated to the recipient gastrointestinal tract by two end-to-end intestinal anastomosis.
A common database was designed. The information requested included: donor surgery time, use of bipolar coagulator or ties for intestinal donor dissection, anesthetic protocol (donor and recipient), type of preservation solution, description of donor surgery complications, graft use (if transplanted or not), cold and warm ischemia times, suture material, type of SBTx (OITX or HITx), recipient complications and outcome from the intrasurgical stage to 5th post-transplant day.
Data were reported from procedures that fulfilled the following conditions:
1- Unsuccessful SBTx procedures, defined as non-accepted graft for transplant, intraoperative recipient death and recipient sacrifice or death within the first 5 post-transplant days.
2- Unsuccessful SBTx procedures performed in an experimental context were considered. No practice procedures developed during surgeon’s learning curve were reported.
3- Isolated ITx (graft should include Jejunum-Ileum).
4- Transplanted intestines were preserved in Ringer Lactate at 4ºC. Cold ischemia time should not be longer than 90 minutes.
5- Engraftments were performed in one stage, HITx and OITx´s performed by hand-sewn end-to-side arterial (donor superior mesenteric artery with recipient infrarenal aorta) and vein (donor portal vein (PV) with recipient infrarenal vena cava) anastomosis with 8-0 or 9-0 monofilament Nylon. All the procedures were performed under microscopic magnification (6, 10 and 26X). Also, ostomies in HITx and intestinal anastomosis in OITx procedures were performed with 7-0 monofilament nylon.
6- Inhalation anesthesia (2% Isoflurane or 4% Sevoflurane for intrasurgical maintenance) was used for donors and recipient procedures. Moreover, post-transplant antibiotic and analgesics were administered to the recipient.
SBTx procedure data not meeting these criteria were not included in the database.
The eight microsurgeons who participated in the study were all high proficient in performing experimental transplantation. They all received basic training in microsurgery and specific-intensive training in rats SBTx, achieving successful results regarding graft and recipient survival. We only included data provided by surgeons who currently achieve more than 70% recipient survival. Also, these surgeons participate actively and regularly in experimental protocols that involve OITx and HITx in rats.
Statistics: Continuos variables were analyzed using T-Test (GraphPad software version 5.00, San Diego, Calif, United States). Discrete variables were analyzed using z-Test (website http://epitools.ausvet.com.au/).
Results
Considering all the participating institutions, the current global average of recipient prolonged survival (more than 5 days after surgery) is 85%. From 2007 to 2015, 293 complications from 264 unsuccessful ITx procedures were reported (Figure 1) with an average of 1.1 complications / surgery. Considering the different stages in a transplant procedure, 12.3% of the complications occurred in the donor, while 87.7 % occurred in the SBTx recipient.
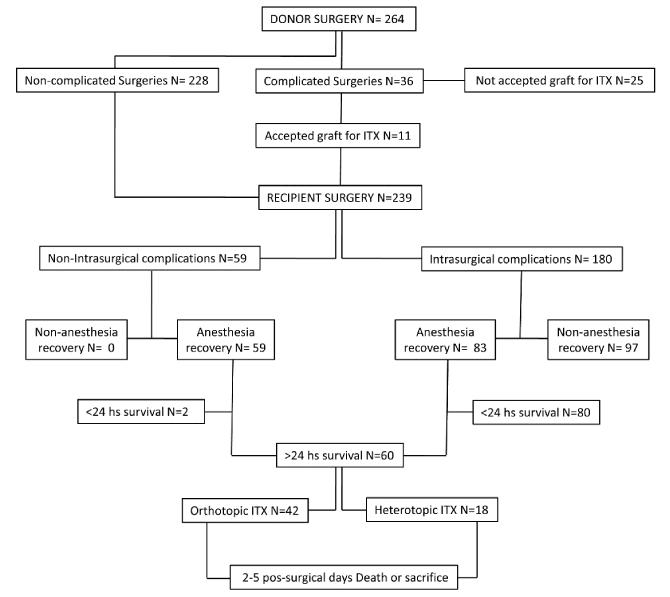
Figure 1: algorithm showing unsuccessful SBTx procedures included in the analysis.
Complications observed during the donor operation or procurement
As for the recovery procedure in the donor, excessive surgical time (more than 60 minutes) (11/36); severe hemorrhage, defined as loss of 20% of the volemia approximately, (12/36) and inappropriate infusion of the preservation solution in the intestinal graft (11/36) were the 3 most common complications (Figure 2-A).
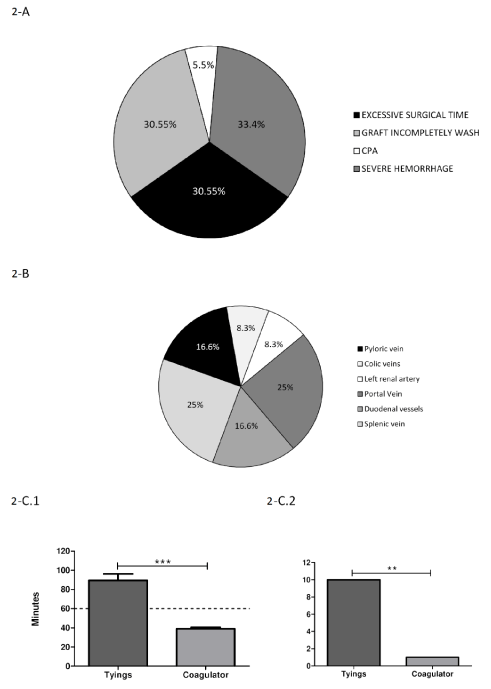
Figure 2: 2-A: Donor surgery complications. 2-B: Most frequent sites of bleeding. 2-C-1: Time used for donor surgical procedure depending on the use of tyings or coagulator. Statistically significant differences were observed (p<0.0001 T-test). 2-C-2: Number of cases of excessive surgical time depending on the use of coagulator or tyings (P<0.0005 z-test to compare sample proportion).
When severe hemorrhage occurs the transplant procedure must be stopped. A detailed study of severe donor-bleeding showed that PV and splenic vein bleeding were the most frequent cause to stop the procedure (Figure 2-B).
As for donor surgical time, significant differences regarding the use of ties or bipolar coagulator for graft dissection were observed (Figure 2-C.1). Moreover, one procedure performed with bipolar coagulator was excluded due to excessive surgical time (Figure 2-C.2). Inappropriate infusion of the preservation solution was considered as a remnant of blood in the graft after washing. In all cases, this complication was managed during cold storage by further graft perfusion, and intestinal grafts were accepted for transplant.
Recipient complications observed during and after surgery
Two hundred and fifty-seven complications leading to SBTx failures were reported. Arterial anastomosis bleeding (50/257), venous anastomosis bleeding (35/257) and PV stenosis (26/257) were the most common complications (Figure 3A). AA and VA bleeding is considered when hemorrhage is not controlled by compression maneuvers with swabs. Therefore, a new vascular clamping and extra sutures are needed [10].

Figure 3: 3-A: distribution of recipient complications 3-B: Number of Intra and post-surgical complications reported (P<0.0001 z-test to compare sample proportion). 3-C: Recipient first 24 post-operative hours survival after an intra-surgical complication (NAR= no anesthetic recovery) (P<0.0001 z-test).
Considering the stage when the complication occurred, 77% were intra operative and 23% were observed in the post-surgical period (from anesthesia recovery to 5 post-transplant day) (Figure 3B). Intraoperative complications were related to recipient’s death within the first post-surgical day. Only 1.66% of the animals in 180 complicated procedures achieved more than 24 hour- survival after surgery (Figure 3C).
Other reported complications were PV thrombosis, inadequate graft reperfusion (graft abnormal and heterogeneous color), intrasurgical cardiopulmonary arrest (CPA), wrong clamping of VC and aorta, excessive for vascular anastomosis (more than 40 minutes), arterial anastomosis stenosis, PV rotation, severe hemorrhage during VC and aorta dissection, SMA irreversible damage during AA and embolism at reperfusion time (Figure 4).
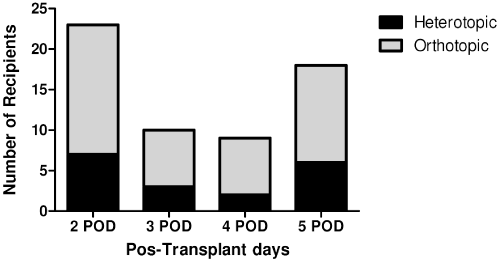
Figure 4: time distribution of OITX or HITx recipient sacrifice or post-transplant death. Most of deaths and sacrifices on both ITx types occurred in the 2nd or 5th post-operative day.
After recovery from anesthesia, several low incidence complications (less than 10 times) were reported in the recipient. In this period, HITx and OITx complications were discriminated. Regarding HITX, graft prolapse was the most common complication. Moreover, severe diarrhea with weight loss was the most common OITx observed complication. In both cases the end-point was applied and animals were sacrificed.
The cause of death (15/257) could not be determined in several cases, i.e. recipients that died during the post-surgical period without failures reported until death and no abnormalities observed during necropsy. This was reported as a common post-surgical complication, and might be associated with an inadequate post surgical intensive care of the animal.
Regarding deaths or sacrifices occurred between the 2nd and 5th pos-SBTx days, most of OITx and HITx recipients died on the 2nd (HITx 7/18 and OITx 16/42) or 5th (HITx 6/18 and OITx 12/42) post-transplant day. On the last day considered for this study, graft prolapsed in HITx recipients and severe diarrhea with weight loss (more than 20%) in OITx recipients were the most common complications reported (Table 1).
COMPLICATION |
INCIDENCE |
TYPE OF ITX |
STAGE |
Severe diarrhea and weight loss |
8/257 (3.11%) |
OITX |
Pos-surgical |
Wrong clamping of VC and Aorta |
7/257 (2.72%) |
OITX-HITX |
Intrasurgical |
Excessive time for vascular anastomosis |
7/257 (2.72%) |
OITX-HITX |
Intrasurgical |
Graft Prolapse |
6/257 (2.33%) |
HITX |
Pos-surgical |
Graft Ischemia |
4/257 (1.55%) |
OITX-HITX |
Intrasurgical |
AA stenosis |
4/257 (1.55%) |
OITX-HITX |
Intrasurgical |
Hypovolemia |
4/257 (1.55%) |
HITX |
Pos-surgical |
Graft obstruction |
4/257 (1.55%) |
OITX |
Pos-surgical |
Volvulus |
3/257 (1.16%) |
OITX |
Pos-surgical |
Abdominal hemorrhage |
3/257 (1.16%) |
OITX-HITX |
Pos-surgical |
Dyspnea/lung edema |
3/257 (1.16%) |
OITX |
Pos-surgical |
Peritonitis |
3/257 (1.16%) |
OITX-HITX |
Pos-surgical |
Enteroanastomotic Dehiscence |
2/257 (0.76%) |
OITX |
Pos-surgical |
Donor-recipient size disproportion |
2/257 (0.76%) |
OITX-HITX |
Intrasurgical |
Hypothermia |
2/257 (0.76%) |
OITX-HITX |
Pos-surgical |
Paralysis of hind limbs |
2/257 (0.76%) |
OITX-HITX |
Pos-surgical |
VP rotation in VA |
2/257 (0.76%) |
OITX-HITX |
Intrasurgical |
Intra-abdominal abscess |
2/257 (0.76%) |
OITX-HITX |
Pos-surgical |
Graft intraluminal hemorrhage |
1/257 (0.38%) |
OITX |
Pos-surgical |
Graft intussusception |
1/257 (0.38%) |
HITX |
Intrasurgical |
Hemorrhage during VC-Aorta dissection |
1/257 (0.38%) |
HITX |
Intrasurgical |
SMA irreversible damage during AA |
HITX |
Intrasurgical |
Air embolism during graft repedusion |
1/257 (0.38%) |
HITX |
Intrasurgical |
Table 1. Low-incidence complications in the recipient.
Discussion
SBTx in rats represents a complex procedure that requires training in microsurgery and good handling of rodents to obtain prolonged survival, because many complications may lead to an unsuccessful procedure [21,22]. The learning curve of SBTx in rats was well described, and some experimental centers have reported their single experiences related to SBTx complications and long-term recipient survival [10,23]. However, this is the first multicenter study focusing on unsuccessful SBTx cases, detailing the intra and post-surgical complications that may occur during OITx and HITx procedures, accounting for 15% of the total number of SBTx performed. All centers that participate in the study follow the same technical procedures and all transplantations included were performed by specialist microsurgeons that received extensive training. The number of procedures performed in each center was equivalent; consequently we consider that no bias depending on differences on techniques, training or technical skills of the personnel participating in the study affected the data.
Donor complications are less common, and procurement is as difficult as engraftment. Among the most frequent complications, excessive surgical time was one of the most common causes leading to failure of the procedure. It has been reported that donor surgical time is important because it can directly impact on the graft quality (more time, less graft quality). For this reason it is recommended to discard the grafts obtained from prolonged surgeries. As shown in the results section (Figure 2C.1), the use of bipolar coagulator significantly improves this stage of the transplant. Donor bleeding becomes the most common complication reported during the procurement; 85 % of the cases of donor bleeding occurred during PV dissection (PV, splenic, pyloric and duodenal veins hemorrhage were reported). Considering these results, we may conclude that PV isolation is a critical step to be especially attentive during the donor surgery.
Inappropriate infusion of the preservation solution in the graft was reported as a possible complication during the second donor surgical stage. However, this is a reversible complication and the remaining graft blood may be removed with a slow and delicate additional intravascular wash with 5 ml of preservation solution administered in approximately 30 seconds during cold storage. This maneuver may be performed manually with a syringe and a catheter 24 G inserted into SMA.
In agreement with Lee et al., arterial anastomosis bleeding was the most common intra-operative complication in the recipient [5]. A successful vascular anastomosis is achieved following the microsurgery principles recommended by Guity et al. [24]. Despite hand-sewn sutures remains the most widely use, alternative methods to reduce complications associated with vascular anastomosis such as the 3-cuff technique was developed and published [12]. This technique replaces the traditional hand-sewn approach by the use of synthetic sleeves in order to reduce surgery time in the recipient [25,26].
Considering the common stages shared by OITx and HITx procedures before reperfusion, complications related to the type of SBTx were considered separately. Weight loss and diarrhea were the most characteristic post-operative complication in OITx, indicating that post-surgical feeding of the recipient is a critical aspect in this type of procedure. Our experience suggests that post-surgical feeding is very important for a good OITx recipient recovery. During the 1st 12 post-ITx hours a liquid diet (Dextrose) should be administered and no solid food should be given. Twelve hours after surgery, a waste-free, protein-rich diet was introduced. Finally, recipients return to their commercial standardized food on the 3rd pos-transplant day.
In the case of HITx, ostomy prolapse was a post-surgical complication, outlining the importance of ostomy care in HITx. Daily cleaning of stomas to remove secretions and proper pain management are good practices to avoid graft prolapse. It is also very important to check the color and appearance of ostomies periodically. Our experience suggests that the use of tramadol (20 mg/kg every 12 hours during the first 72 post-Itx hours) is appropriate for the management of post-surgical pain, avoiding stress related death.
Post-surgical and intensive care monitoring of rodents is not as frequent as in large experimental animals or humans. For this reason, some clinical signs and metabolic abnormalities in the SBTx recipient after surgery were not recorded. Therefore, as reported in the results, death from indeterminate cause was frequently observed during post-surgical stage. Implementing better care or performing basic laboratory testing during the post-transplant period might identify the causes of death.
In summary, several causes of unsuccessful SBTx were reported, that proves that SBTx in rats requires a fine surgical technique as well as careful post transplant care. A single complication may lead to failure. To obtain an acceptable survival rate, surgeons should consider the following: minimize operating time and bleeding during graft dissection in the donor surgery; a bloodless microsurgical technique to perform AA and VA is imperative considering that vascular anastomosis represents the key point to success. Finally, an adequate post-operative management of the animal is mandatory to guarantee survival after this challenging surgical procedure. To identify which complications can be early reversed without affecting the aim of the experiment are mandatory in order not to have any confounding factor in the result. We expect that results reported here may become a useful guide to maximize success rate in experimental SBTx.
All authors declare no conflicts of interest
References
- Monchik GJ, Russell PS (1971) Transplantation of small bowel in the rat: technical and immunological considerations. Surgery 70: 693-702. [Crossref]
- Pech T, von Websky M, Ohsawa I, Kitamura K, Praktiknjo M, et al. (2012) Intestinal regeneration, residual function and immunological priming following rescue therapy after rat small bowel transplantation. Am J Transplant 12 Suppl 4: S9-17. [Crossref]
- Oltean M, Joshi M, Herlenius G, Olausson M (2010) Improved intestinal preservation using an intraluminal macromolecular solution: evidence from a rat model. Transplantation 89: 285-290. [Crossref]
- Oltean M, Pullerits R, Zhu C, Blomgren K, Hallberg EC, Olausson M (2007) Donor pretreatment with FK506 reduces reperfusion injury and accelerates intestinal graft recovery in rats. Surgery 141(5): 667-77.
- Lee AD, Gama-Rodrigues J, Galvao FH, Waitzberg DL (2002) Study of morbidity in orthotopic small intestine transplantation with Wistar rats. Experimental study. Arquivos de gastroenterologia 39(1): 39-47. [Crossref]
- Zhong R, Grant D, Sutherland F, Wang PZ, Chen HF, et al. (1991) Refined technique for intestinal transplantation in the rat. Microsurgery 12: 268-274. [Crossref]
- Nakao A, Tahara K, Inoue S, Tanaka N, Kobayashi E (2002) Experimental models of small intestinal transplantation in rats: orthotopic versus heterotopic model. Acta medica Okayama 56(2):69-74.
- Kort WJ, Westbroek DL, MacDicken I, Lameijer LD (1973) Orthotopic total small bowel transplantation in the rat. Eur Surg Res 5(2): 81-9. [Crossref]
- Schroeder P, Deltz E, Seifert J, Sandforth F, Thiede A (1987) Absorptive capacity of the transplanted small bowel. Gut 28 Suppl: 275-279. [Crossref]
- Lopez-Fernandez S, Hernandez F, Hernandez-Martin S, Barrena S, Wang Z, et al. (2012) Technical aspects of experimental intestinal transplant. Cirugia pediatrica 25(2): 103-8.
- Grant D, Zhong R, Hurlbut D, Garcia B, Chen HF, et al. (1991) A comparison of heterotopic and orthotopic intestinal transplantation in rats. Transplantation 51: 948-954. [Crossref]
- Wallander J, Holtz A, Larsson E, Gerdin B, Lackgren G, et al. (1988) Small-bowel transplantation in the rat with a nonsuture cuff technique. Technical and immunological considerations. Transplant International 1(3):135-9.
- Waisberg DR, Galvao FH, De Castro Galvao R, Chaib E, D'Albuquerque LA (2011) Intestinal transplantation using cuff-glue sutureless technique for microanastomosis in rats. Microsurgery 31(7):584-5.
- Camprodon RA, Bowles MJ (2006) Perioperative analgesia in experimental small bowel transplantation. Transplantation proceedings 38(6): 1857-1858.
- Lausada N, Stringa P, Cabanne A, Ramisch D, Machuca M, Galvao F, et al. (2011) Impact of ishemia-reperfusion injury on long survival rate in intestinal transplantation in rats. Acta gastroenterologica Latinoamericana 41(2): 129-136.
- Stringa P, Romanin D, Lausada N, Machuca M, Raimondi JC, et al. (2013) Ischemic preconditioning and tacrolimus pretreatment as strategies to attenuate intestinal ischemia-reperfusion injury in mice. Transplantation proceedings 45(6): 2480-2485.
- Wang Z, Hernandez F, Pederiva F, Andres AM, Leal N, et al. (2011) Ischemic preconditioning of the graft for intestinal transplantation in rats. Pediatric transplantation 15(1):65-69.
- Hernandez F, Zou Y, Lopez G, Romero M, Martinez L, et al. (2005) Is portal venous outflow better than systemic venous outflow in small bowel transplantation? Experimental study in syngeneic rats. Journal of pediatric surgery 40(2): 336-340.
- Navarro-Zorraquino M, Guemes A, Pastor C, Soria J, Sousa R, et al. (2002) Apoptosis and CD8 and CD54 cell expression in rat small bowel transplantation. The Journal of surgical research. 103(1): 37-40.
- Andres AM, Santamaria M, Hernandez Oliveros F, Guerra L, Lopez S, et al. (2016) Difficulties, guidelines and review of developing an acute rejection model after rat intestinal transplantation. Transplant Immunology 36(1): 32-41.
- Squiers EC, Kelley SE, West JC (1992) Small bowel transplantation in the mouse: development of a model. Microsurgery 13: 345-347. [Crossref]
- Liu X, Song P, Tian J, Zhou S, Yan S, et al. (2013) A simple novel technique for heterotopic intestinal transplantation in mice. Transplant Proc 45: 654-658. [Crossref]
- Galvão FH, Bacchella T, Cerqueira Machado M (2007) Teaching intestinal transplantation in the rat for medical student. Microsurgery 27: 277-281. [Crossref]
- Guity A, Young PH, Fischer VW (1990) In search of the "perfect" anastomosis. Microsurgery 11: 5-11. [Crossref]
- Xue L, Lu Y, Qiu W, Zhou H, Zhang G, Jin Z, et al. (2009) Surgical experience of refined 3-cuff technique for orthotopic small-bowel transplantation in rat: a report of 270 cases. American journal of surgery 198(1):110-21.
- Nakao A, Mitsuoka N, Shen SD, Tanaka N, Kobayashi E (2000) Rat small intestinal transplantation: a comparison of the cuff and hand-suture methods. Acta Med Okayama 54: 259-264. [Crossref]




