Whether bilateral lung transplantation (BLT) rather than single lung transplantation (SLT) should be preferentially performed for patients with World Health Association (WHO) Group 3 pulmonary hypertension (PH) associated with advanced lung disease has been controversial. We retrospectively examined the outcomes of 474 consecutive patients who underwent BLT or SLT at our center between 1999 and 2013. After exclusion of patients with cystic fibrosis or those undergoing retransplantation, 179 patients with PH were split into four groups based on their pulmonary artery pressure values (mild versus severe) and transplant type (BLT versus SLT). The incidence of grade 2-3 primary graft dysfunction and mechanical ventilation >48 hours was significantly higher for the BLT versus the SLT recipients. However, long-term survival via the Kaplan-Meier method and appropriate log rank tests did not differ significantly among the four cohorts. Because long-term outcomes for patients who undergo SLT for advanced lung disease associated with WHO Group 3 PH did not differ significantly from those for BLT recipients, even if PH was severe, we suggest that SLT can be performed safely in patients with Group 3 PH associated with ALD and potentially allows improved donor organ utilization.
lung transplantation, pulmonary hypertension, single lung transplantation, bilateral lung transplantation, lung allocation score
Abbreviations
ALD: Advanced Lung Disease; BLT: Bilateral Lung Transplantation; CLAD: Chronic Lung Allograft Dysfunction; COPD: Chronic Obstructive Pulmonary Disease; CPB: Cardiopulmonary Bypass; FEV1: Forced Expiratory Volume in one second; FVC: Forced Vital Capacity; ICU: Intensive Care Unit; IPF: Idiopathic Pulmonary Fibrosis; LAS: Lung Allocation Score; LOS: Length of Stay; NO: Nitric Oxide; PA: Pulmonary Artery; PAP: Pulmonary Artery Pressure; PGD: Primary Graft Dysfunction; PH: Pulmonary Hypertension; SLT: Single Lung Transplantation; WHO: World Health Organization
WHO (World Health Organization) Group 3 pulmonary hypertension (PH) is a relatively common complication of advanced lung disease (ALD), and its presence has been linked to worse survival for patients who undergo lung transplantation for ALD [1]. Due to concern that high pulmonary vascular resistance in the native lung increases the risk of significant reperfusion injury that can lead to high-grade primary graft dysfunction (PGD) and increased mortality, performing single lung transplantation (SLT) rather than bilateral lung transplantation (BLT) has been controversial in patients transplanted for ALD when Group 3 PH is present. Many centers preferentially list patients with significant Group 3 PH for BLT and avoid performing SLT for patients with ALD associated with a significant degree of PH [2-6].
Bilateral lung transplantation may not always be a viable option at centers with limited organ access, and the donor lung pool is effectively halved if only BLT is performed in patients with ALD complicated by the presence of WHO Group 3 PH. Although a number of studies have examined outcomes for Group 3 PH and SLT [2-8], these studies have been limited by small sample sizes and relatively few patients with more severely increased pulmonary artery (PA) pressures. We reviewed our clinical outcomes in patients with mild versus more severe Group 3 PH while also comparing outcomes for patients who underwent SLT versus BLT to determine if significant differences in long-term survival could be detected.
This investigation was approved by the University of Wisconsin Human Subjects Committee (approval number M-2009-1308). Outcomes of 474 consecutive patients who underwent lung transplantation between 1999 and 2013 were retrospectively reviewed (Figure 1). Of these patients, 224 were found to have WHO Group 3 PH as documented by pre-operative cardiac catheterization. A total of 39 patients were then excluded for a diagnosis of cystic fibrosis, as they were not eligible to be listed for SLT, and another 6 patients were excluded due to retransplantation as their indication for transplant. The remaining 179 patients were divided four groups according to their PA pressure values (mild versus severe) and whether they underwent SLT or BLT procedures. Our classification of PH severity was based upon criteria from the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension [9]. Mild PH was defined as having mean PA pressures of 26-40 mm Hg and severe PH as having systolic PA pressure >44 mm Hg or mean PA pressure >40 mm Hg. For patients that were transplanted prior to the implementation of the lung allocation score (LAS) system, LAS values were calculated retrospectively using historic data obtained prior to lung transplant.
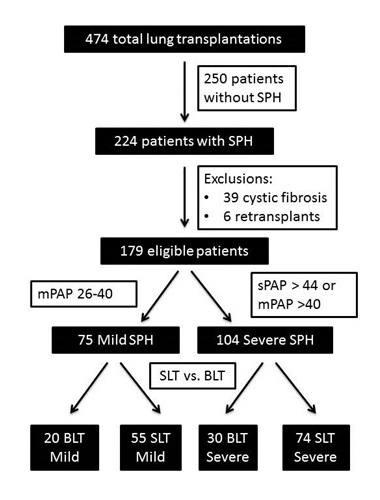
Figure 1. Flow chart depicting selection of recipients for inclusion in the study and pulmonary hypertension severity stratification
All patients met widely accepted criteria based on their primary lung disease and were determined to be suitable candidates for lung transplantation [10]. Standard surgical techniques were used, and our protocol-driven postoperative care was given to all patients and included standard induction and maintenance immunosuppressive drugs, prophylactic and preemptive antibiotics, and follow-up at specific time intervals with thoracic imaging, pulmonary function testing, and surveillance bronchoscopy.
Chronic lung allograft dysfunction (CLAD) was defined as per the ISHLT/ATS/ERS international clinical practice guideline for the diagnosis of bronchiolitis obliterans syndrome (BOS) [11]. Patients were identified as having CLAD when they had a persistent decline in FEV1 to less than 80% of the best postoperative FEV1 that could not be attributed to a reversible cause of allograft dysfunction. Graft survival was defined as either recipient death or loss of allograft function that required retransplantation.
Statistical analysis was performed using SPSS and GraphPad Prism software. Whether recipients developed CLAD and graft survival rates were compared between the four groups using Kaplan-Meier survival curves and associated log rank tests. For patient characteristics and perioperative outcomes, the chi-squared test was used to analyze categorical variables and ANOVA was used to analyze continuous variables.
Baseline characteristics
No significant differences in selected pre-operative characteristics were observed for the four cohorts (gender, prevalence of diabetes, FEV1, FVC, pre-transplant oxygen requirements, or requirement for positive pressure ventilation) (Table 1). However, BLT recipients with severe PH were, on average, younger than the other groups (52 years, p<0.005), both severe groups had higher LAS values (44.1 and 44.1, p=0.005), and SLT severe recipients had higher baseline creatinine values (0.96 mg/dl, p=0.005).
Table 1. Pre-Transplant recipient characteristics. FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; PAP: Pulmonary artery pressure; PCWP: Pulmonary capillary wedge pressure
Pre-operative Characteristics (mean values) |
Pulmonary Hypertension Severity |
p value |
BLT Mild (N=20) |
BLT Severe (N=30) |
SLT Mild (N=55) |
SLT Severe (N=74) |
Male gender (%) |
70 |
67 |
67 |
76 |
0.78 |
Mean age (years) |
55 |
52 |
57 |
58 |
<0.005 |
Diabetes (%) |
30 |
30 |
24 |
24 |
0.91 |
Race = Caucasian (%) |
90 |
97 |
98 |
82 |
0.04 |
Body mass index (kg/m2) |
25.9 |
26.4 |
26.0 |
26.7 |
0.75 |
Mechanical ventilation (%) |
10 |
20 |
11 |
11 |
0.57 |
Supplemental oxygen use (L/min) |
3.5 |
4.6 |
3.6 |
4.1 |
0.29 |
FVC (% predicted) |
49.8 |
46.5 |
46.1 |
4.67 |
0.84 |
FEV1 (% predicted) |
28.4 |
33.0 |
31.3 |
38.6 |
0.075 |
Lung allocation score (LAS) |
38.3 |
44.1 |
37.2 |
44.1 |
0.005 |
Serum creatinine (mg/dL) |
0.81 |
0.88 |
0.92 |
0.96 |
0.005 |
PCWP (mm Hg) |
19 |
39 |
54 |
70 |
0.166 |
Cardiac index (L/min/m2) |
2.77 |
3.09 |
2.94 |
2.73 |
0.073 |
Systolic PAP (mm Hg) |
39.5 |
61.2 |
39.5 |
55.5 |
<0.001 |
Diastolic PAP (mm Hg) |
23.7 |
31.0 |
21.4 |
25.9 |
<0.001 |
Mean PAP (mm Hg) |
29.9 |
42.8 |
29.3 |
36.5 |
<0.001 |
Recipient primary disease
There was a statistically significant difference in the indication for transplantation among the stratified groups (Table 2). Both the SLT and BLT groups with mild PH were more likely to have a diagnosis of chronic obstructive pulmonary disease (COPD) (BLT 45%, SLT 51%) versus the severe PH groups (BLT 33%, SLT 24%). Furthermore, SLT severe patients were more likely to have a diagnosis of idiopathic pulmonary fibrosis (IPF) (43%) compared to all other groups (BLT mild 20%, BLT severe 27%, SLT mild 26%, p<0.001).
Table 2. Transplant Indications (Primary Disease). AATD = alpha-1-antitrypcin deficiency; COPD = chronic obstructive pulmonary disease; IPF = idiopathic pulmonary fibrosis (p value derived by χ2 analysis of all groups).
Primary Disease (transplant indication) |
Pulmonary Hypertension Severity |
p value |
BLT Mild (N=20) |
BLT Severe (N=30) |
SLT Mild (N=55) |
SLT Severe (N=74) |
COPD/emphysema – N (%) |
9 (45) |
10 (33) |
28 (51) |
18 (24) |
<0.0001
|
IPF – N (%) |
4 (20) |
8 (27) |
14 (26) |
32 (43) |
COPD with AATD – N (%) |
4 (20) |
3 (10) |
8 (15) |
2 (3) |
Sarcoidosis – N (%) |
1 (5) |
2 (7) |
1 (2) |
9 (12) |
Other (N/%) |
2 (10) |
6 (20) |
4 (7) |
13 (17) |
Intra-operative characteristics
Intra-operative characteristics included pre-incision pulmonary arterial pressure PA pressure measurements, organ ischemic time, and cardiopulmonary bypass (CPB) (Table 3). There were no statistical differences between the groups in regards to average organ ischemic time, although there was a trend toward longer times in the BLT severe and SLT mild groups (351 and 343 minutes) compared to the BLT mild and SLT severe groups (266 and 302 minutes, p=0.066). There was a significant difference in rates of CPB usage, with both BLT groups having higher rates of CPB (80% both groups) compared SLT patients (SLT mild 14.5%, SLT severe 39%, p<0.001). BLT severe recipients were found to have the longest duration of CPB (227 minutes, p<0.005).
Table 3. Intra-operative characteristics. CPB: Cardiopulmonary bypass; mPAP: Mean pulmonary artery pressure; sPAP: Systolic pulmonary artery pressure
Parameter (mean values) |
Pulmonary Hypertension Severity |
p value |
BLT Mild (N=20) |
BLT Severe (N=30) |
SLT Mild (N=55) |
SLT Severe (N=74) |
CPB required (%) |
80 |
80 |
14.5 |
39 |
<0.001 |
CPB duration (minutes) |
194 |
227 |
170 |
164 |
<0.0005 |
Ischemic time (minutes) |
266 |
351 |
343 |
302 |
0.066 |
sPAP (mm Hg) |
40 |
61 |
39 |
56 |
<0.001 |
mPAP (mm Hg) |
30 |
43 |
29 |
36 |
<0.0001 |
Immediate postoperative outcomes
Postoperatively, BLT severe patients were found to have the longest intensive care unit (ICU) length of stay (20.5 days, p=0.013), highest incidence of prolonged ventilation (67%, p<0.001), and longest overall hospital stay (41 days, p=0.026) (Table 4). Rates of higher (grade 2 or 3) primary graft dysfunction (PGD) were significantly higher in both BLT groups (mild 45%, severe 67%) compared to both SLT groups (mild 20%, severe 24%, p<0.001). Nitric oxide (NO) use was significantly increased for both the SLT and BLT high PH groups (73% both), and NO use was also higher for SLT versus BLT with mild PH (BLT mild 35%, SLT mild 49%, p<0.001). There were no significant differences in rate of readmission within 30 days or 30-day mortality among all groups.
Table 4. Post-operative recipient characteristics. ECMO: Extracorporeal membrane oxygenation; ICU: Intensive care unit; PGD: Primary graft dysfunction
Parameter (mean values) |
Pulmonary Hypertension Severity |
p value |
BLT Mild (N=20) |
BLT Severe (N=30) |
SLT Mild (N=55) |
SLT Severe (N=74) |
ICU length of stay (days) |
5.5 |
20.5 |
10.7 |
7.0 |
0.013 |
Prolonged ventilation >48 hrs (%) |
45 |
67 |
20 |
24 |
<0.001 |
PGD Grade 0-1 (%) |
55 |
33 |
80 |
76 |
<0.001 |
PGD Grade 2-3 (%) |
45 |
67 |
20 |
24 |
Nitric oxide use (%) |
35 |
73 |
49 |
73 |
<0.001 |
Hospital length of stay (days) |
24 |
41 |
23 |
23 |
0.026 |
Readmission within 30 days (%) |
15 |
27 |
35 |
19 |
0.082 |
30-day mortality (%) |
5 |
7 |
2 |
4 |
0.84 |
Long-term survival
There were no differences in long-term survival among the four groups (Figure 2). Unadjusted survival for BLT recipients with mild PH was 85.0%, 70.8%, and 56.7% at 1, 3, and 5 years respectively, compared to 83.3%, 68.9%, and 64.8% for the BLT group with severe PH. Unadjusted survival to the SLT cohort with mild PH was 87.3%, 77.9%, and 68.1% compared to 83.8%, 73.7%, and 62.5% in the SLT with mild PH cohort (p=0.748).
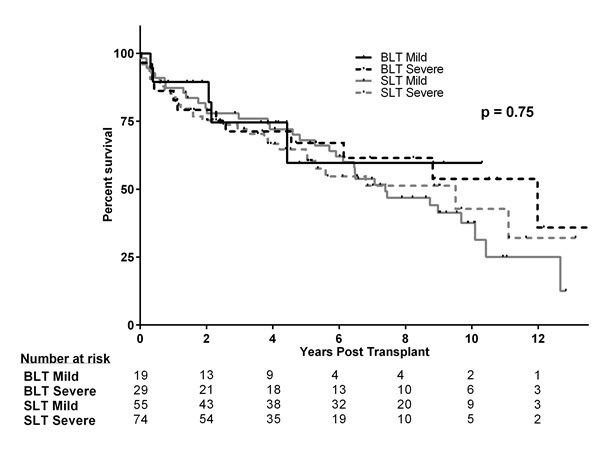
Figure 2. Long-term survival by Kaplan-Meier analysis
Freedom from chronic lung allograft dysfunction
Rates of CLAD were similar in all groups (Figure 3). Freedom from rejection for BLT patients with mild SPH was 93.3%, 60.6%, and 51.5% at 1, 3, and 5 years respectively compared to 92.4%, 76.9%, and 65.1% in the BLT severe group, 95.9%, 72.8%, and 48.2% in the SLT mild group, and 95.5%, 81.1%, and 68.6% in the SLT severe group (p=0.415).
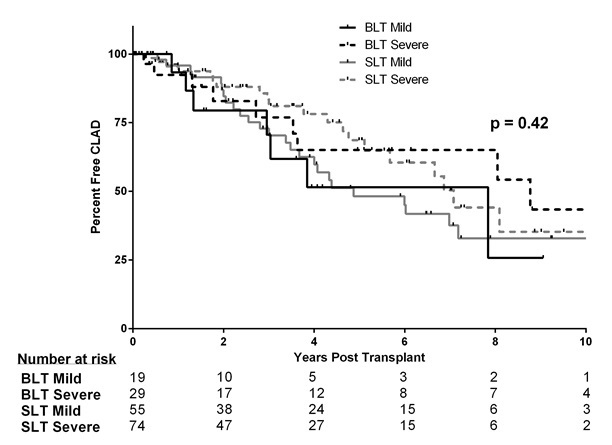
Figure 3. Freedom from chronic lung allograft dysfunction (CLAD by Kaplan-Meier analysis
Our findings suggest that long-term outcomes for lung transplant recipients with ALD and significant Group 3 PH who undergo SLT are not significantly different from patients with PH who undergo BLT, nor did we identify significant differences among the recipient groups for mild versus severe PH. This was demonstrated by both equivalent long-term survival (Figure 2) and freedom from CLAD (Figure 3). Additionally, patients with PH who underwent SLT had lower rates of CPB usage and severe PGD (Tables 3 and 4), although this lower use of CPB may only reflect our program’s tendency to use CPB preventively for recipients perceived as being at increased risk for significant PGD following transplant. For recipients with severe PH, patients who underwent BLT had increased ICU length of stay (LOS), prolonged ventilation, and overall hospital LOS compared to those who underwent SLT.
2021 Copyright OAT. All rights reserv
Our results support previous studies that did not identify the presence of Group 3 PH as a risk factor for poorer survival after lung transplantation [2,7,8,12]. Additionally, our study supports our recently reported findings that the presence of Group 3 PH, even when severe, does not have a significant impact on outcomes for patients who receive SLT [13]. Our results suggest that SLT can be an appropriate consideration for patients with Group 3 PH in the setting of limited organ availability, and our study also has a much longer patient follow-up and larger patient population compared to prior studies of the effect of PH on lung transplant outcomes published by other transplant centers.
Reluctance to perform a SLT procedure in patients with significant Group 3 PH mostly stems from concern that the transplanted lung will be exposed to higher pulmonary pressures that will increase reperfusion injury and primary graft dysfunction. However, we found that our cohorts with mild or severe PH who underwent BLT had higher rates of severe PGD than SLT recipients (Table 4). This may be explained, at least in part, by the increased use of CPB for BLT recipients, who were more likely to be placed on CPB (80%) compared to SLT patients (14.5% mild, 39% severe). A recent meta-analysis examined recipient-related risk factors for PGD and found that CPB carried a significant risk of PGD, with an odds ratio of 2.29 [14], but this study did not find any correlation between SLT and increased risk for PGD. Taken together with our results, the theoretical risk of increased reperfusion injury does not appear to translate clinically to increased PGD risk when SLT is performed for patients with Group 3 PH.
Increasing the number of organs available for transplant has been a primary focus in the field of lung transplantation over the past decade, and a number of methods have been proposed or implemented to achieve this goal. A point-based system has been initiated in Israel that rewards potential donors by increasing the likelihood of receiving an organ themselves should they develop such need, and this policy has led to an increase in registered donors [15]. An “opt out” instead of the current “opt in” system (which theoretically will increase the number of registered donors) has been under examination in the US [16], and other measures to increase the donor pool include utilizing living donors for lobar lung transplants, increased use of donation after cardiac death donors, using donors with a significant history of smoking, using donors older than 55 years, and rehabilitation of marginal lungs with ex vivo perfusion [17-22]. Our study suggests that using SLT for patients with ALD accompanied by PH (a population that historically has been treated with BLT) can increase organ availability without compromising outcomes. This approach would have the benefit of not only allowing organ block splitting from a double lung to two single lungs, but it would also allow centers to accept single lung offers that they may have historically declined. Indeed, in COPD, there is evidence to suggest that a policy of using SLT improves access to organs for other potential recipients without significantly increasing post-transplant mortality [23,24].
Our study has numerous limitations. This is a descriptive, retrospective analysis of non-randomized patients, and it is, therefore, subject to selection bias for patients chosen for SLT versus BLT procedures. Additionally, our recipients represent a heterogeneous population in terms of lung disease indication for transplantation, and relatively more patients received SLT versus BLT, which reflects our center’s policy of listing nearly all patients to receive either SLT or BLT to optimize organ utilization and decrease the risk of death on the waiting list if patients are listed for BLT only. Another limitation is the duration of the study period (approximately 14 years). Although this time period has allowed us to analyze outcomes for a large number of patients with a long period of follow-up, many changes in lung transplantation techniques and post-transplant management have occurred, and use of the LAS system was implemented in 2005, which has changed the primary indication for transplantation from COPD to idiopathic pulmonary fibrosis (IPF) at our institution. This trend of a change from COPD to IPF has been observed around the country in general after implementing the LAS [25]. Additionally, we have observed that following the implementation of the LAS at our institution, patients with the transplant indication of IPF were significantly older, had increased supplemental oxygen requirements, a lower cardiac index, and more comorbidities [26]. However, despite the increased disease severity and strong association of significant Group 3 PH with IPF, long-term recipient outcomes have not been significantly compromised for our patients with a pre-transplant diagnosis of pulmonary fibrosis [27].
We conclude that patients with WHO Group 3 PH associated with ALD, including those with more severely increased PA pressure values, can safely undergo a SLT procedure and have long-term survival that is not significantly different from candidates who receive a BLT procedure. Furthermore, our SLT recipients had lower rates of CPB use, lower incidence of PGD, and shorter post-transplant hospital LOS. We suggest that the presence of Group 3 PH should not be a contraindication to SLT, and the expanded use of SLT has the added benefit of functionally expanding a limited pool of donor lungs, especially for specific candidate populations, (e.g. older patients with IPF or COPD who have Group 3 PH). Future studies should target the validation of these results in a prospective fashion.
Supported in part by the George and Julie Mosher Pulmonary Research Fund
Dr. Meyer has been an investigator in clinical trials sponsored by Abbott, Actelion, Altana, Amgen, Asthmatx, Bayer, Boehringer-Ingelheim, Bristol Meyers Squibb, Chiron, Discovery Labs, DuPont Merck, Fibrogen, Genentech, Gilead, GlaxoSmithKline, Inspire. InterMune, Johnson & Johnson, Nivalis, Novartis, Nycomed, Parion, Pfizer, Pharmaxis, PreAnalytiX, Roche, Ross, Vertex, and Wyeth. Dr. Meyer has also previously served on a Clinical Advisory Board for InterMune and a clinical trial adjudication committee for Medimmune. All authors do not report any other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
- Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, et al. (2013) Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 187: 527-534. [Crossref]
- Conte JV, Borja MJ, Patel CB, Yang SC, Jhaveri RM, et al. (2001) Lung transplantation for primary and secondary pulmonary hypertension. Ann Thorac Surg 72: 1673-1679. [Crossref]
- Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS (2009) Survival after single versus bilateral lung transplantation for high-risk patients with pulmonary fibrosis. Ann Thorac Surg 88: 1616-1625. [Crossref]
- Rinaldi M, Sansone F, Boffini M, El Qarra S, Solidoro P, et al. (2008) Single versus double lung transplantation in pulmonary fibrosis: a debated topic. Transplant Proc 40: 2010-2012. [Crossref]
- Neurohr C, Huppmann P, Thum D, Leuschner W, von Wulffen W, et al. (2010) Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int 23: 887-896. [Crossref]
- Gammie JS, Keenan RJ, Pham SM, McGrath MF, Hattler BG, et al. (1998) Single- versus double-lung transplantation for pulmonary hypertension. J Thorac Cardiovasc Surg 115: 397-402. [Crossref]
- Huerd SS, Hodges TN, Grover FL, Mault JR, Mitchell MB, et al. (2000) Secondary pulmonary hypertension does not adversely affect outcome after single lung transplantation. J Thorac Cardiovasc Surg 119: 458-65. [Crossref]
- Fitton TP, Kosowski TR, Barreiro CJ, Chan V, Patel ND, et al. (2005) Impact of secondary pulmonary hypertension on lung transplant outcome. J Heart Lung Transplant 24: 1254-1259. [Crossref]
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493-537. [Crossref]
- Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, et al. (2006) International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 25: 745-55. [Crossref]
- Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, et al. (2014) An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 44: 1479-503. [Crossref]
- Brown CR, Mason DP, Pettersson GB, Murthy SC (2013) Outcomes after single lung transplantation in older patients with secondary pulmonary arterial hypertension. J Heart Lung Transplant 32: 134-6. [Crossref]
- Julliard WA, Meyer KC, De Oliveira NC, Osaki S, Cornwell RC, et al. (2016) The presence or severity of pulmonary hypertension does not affect outcomes for single-lung transplantation. Thorax 71: 478-480. [Crossref]
- Liu Y, Liu Y, Su L, Jiang SJ (2014) Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One 9: e92773. [Crossref]
- Cronin AJ (2014) Points mean prizes: priority points, preferential status and directed organ donation in Israel. Isr J Health Policy Res 3: 8. [Crossref]
- Li D, Hawley Z, Schnier K (2013) Increasing organ donation via changes in the default choice or allocation rule. J Health Econ 32: 1117-1129. [Crossref]
- Bittle GJ, Sanchez PG, Kon ZN, Claire Watkins A, Rajagopal K, et al. (2013) The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. J Heart Lung Transplant 32: 760-768. [Crossref]
- Shigemura N, D'Cunha J, Bhama JK, Shiose A, Abou El Ela A, et al. (2013) Lobar lung transplantation: a relevant surgical option in the current era of lung allocation score. Ann Thorac Surg 96: 451-456. [Crossref]
- Sabashnikov A, Patil NP, Mohite PN, García Sáez D, Zych B, et al. (2014) Influence of donor smoking on midterm outcomes after lung transplantation. Ann Thorac Surg 97: 1015-1021. [Crossref]
- Morrissey PE, Monaco AP. (2014) Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation 97: 258-64. [Crossref]
- Boffini M, Ricci D, Barbero C, Bonato R, Ribezzo M, et al. (2013) Ex vivo lung perfusion increases the pool of lung grafts: analysis of its potential and real impact on a lung transplant program. Transplant Proc 45: 2624-2626. [Crossref]
- Wallinder A, Ricksten SE, Silverborn M, Hansson C, Riise GC, et al. (2014) Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg 45: 40-44. [Crossref]
- Munson JC, Christie JD, Halpern SD (2011) The societal impact of single versus bilateral lung transplantation for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184: 1282-1288. [Crossref]
- Anyanwu AC, Rogers CA, Murday AJ (2013) Does splitting the lung block into two single lung grafts equate to doubling the societal benefit from bilateral lung donors? Comparisons between two single versus one bilateral lung transplant. UK Cardiothoracic Transplant Audit Steering Group. Transpl Int 13 Suppl 1: S201-2. [Crossref]
- Kozower BD, Meyers BF, Smith MA, De Oliveira NC, Cassivi SD, et al. (2008) The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. J Thorac Cardiovasc Surg 135: 166-171. [Crossref]
- De Oliveira NC, Osaki S, Maloney J, Cornwell RD, Meyer KC (2012) Lung transplant for interstitial lung disease: outcomes before and after implementation of the united network for organ sharing lung allocation scoring system. Eur J Cardiothorac Surg 41: 680-5. [Crossref]
- De Oliveira NC, Osaki S, Maloney J, Cornwell RD, Meyer KC (2012) Lung transplant for interstitial lung disease: outcomes for single versus bilateral lung transplantation. Interact Cardiovasc Thorac Surg 14: 263-267. [Crossref]



