neumatosis intestinalis (PI) has been described as a possible complication after allogeneic stem cell transplantation (HSCT) due to graft-versus-host disease (GVHD) or infections. So far, no patient with a pre-existent PI who underwent HSCT has been reported. We describe the case of a patient with Alk-negative anaplastic large B-cell lymphoma (DLBCL anaplastic) and PI who successfully received a HSCT from an unrelated donor without complications. This case suggests that this gastrointestinal condition should not be considered an absolute contraindication for an allotransplant procedure.
nhl, pneumatosis intestinalis, allogeneic hemopoietic stem cell transplantation, dlbcl, rituximab, brentuximab vedotin.
PI is a condition characterized by the infiltration of gas in the gastrointestinal (GI) tract wall, with or without free air in the abdomen and represents a spectrum of disease from benign to catastrophic. Therefore, patients can show no signs or symptoms or can present with diarrhea, tenderness, vomiting, intestinal bleeding, bloating, cramping or pain [1,2]. The diagnosis is performed by radiography or an abdomen CT scan [3]. The etiology of this disorder is unknown. It has been associated with congenital disorders, collagen vascular diseases, acquired immunodeficiency, infection, endoscopic procedures, steroid use and chemotherapy [4-8]. PI is also found in association with organ transplantation and HSCT due to pre-transplant chemotherapy, infections and GVHD after HSCT [9-13]. It is hypothesized that when a pathogenic noxa causes a GI wall disruption necrosis, an increased wall permeability can occur, leading to gas infiltration. In particular, in patients who have received chemotherapy, the rapidly proliferating epithelium of the GI tract is particularly sensitive to the cytostatic effect of drugs; indeed, nausea, vomiting and diarrhea are common side effects. Furthermore, it has been postulated that during neutropenia, microorganisms may invade the bowel wall that has been damaged by the cytostatic drugs. This may lead to necrotizing inflammatory changes of the bowel wall, such as local or diffuse irregular thickened mucosal folds, effacement and rigidity of the bowel loops. Moreover, glucocorticoid therapy is capable of inducing PI by depleting Peyer’s patches in the distal ileum and right colon, causing a mucosal defect that allows dissection of intra-luminal air gas into the sub-mucosal or sub-serosal regions; however, this relationship is not found in all studies [1,2].
On January 2012 a 35-year old woman, came to our Center because of a bilateral inguinal lymphadenopathy. An inguinal lymph node biopsy was performed and histological examination revealed a diffuse proliferation of large-size lymphoid elements with bizarre pleomorphic nuclei that were CD30+, VIM+, CD45-, ALK-, CD20+/-, CD3-, PLAP-, pan CK (MNF116)-, S100-, HMB45-, Melan A-, c-Kit-/+ phenotype. A diagnosis of diffuse large B-cell lymphoma (DLBCL), anaplastic variant, was made. She had a stage IIIB, IPI 3. She underwent 6 courses of R-CHOP 21 (rituximab, cyclophosphamide, anthracycline, vincristine and prednisone) and obtained a PET-positive partial response. She was then treated with one cycle of R-IEV (rituximab, ifosfamide, epirubicin and etoposide) and two cycles of R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin) followed by stem cell mobilization. A PET/CT performed after the last cycle of R-DHAP documented a progression of the disease; for this reason, the patient started the A1 cycle according to the methotrexate-based G-MALL scheme. The PET/CT reassessment performed after the C1 cycle of the GMALL protocol documented an increase in the number and uptake of abdominal lymph nodes. At this time, an immunotherapeutic strategy was implemented and the patient received 5 doses of Brentuximab Vedotin (anti-CD30), which was associated with a PET-positive disease stabilization. To improve the response, the P-VABEC (doxorubicin, etoposide, cyclophosphamide, vincristine, bleomycin and prednisolone) scheme was administered for 9 cycles and a partial response was obtained. On June 2013 after the 5th cycle of P-Vabec, the restaging CT scan performed to revaluate the response showed an air brim in the retroperitoneal and intraperitoneal abdominal region (Figure 1). A diagnosis of intestinal perforation was suspected. Even if the patient was asymptomatic, she was hospitalized as a precaution in a surgical division where parenteral nutrition and antibiotic therapy with meropenem were started. After the resolution of abdominal radiologic presentation, she was discharged and the 9th cycle of P-VABEC was completed obtaining a partial response. Thereafter, in August 2013, the patient was a candidate for a tandem autologous (auto)-allogeneic HSCT from an unrelated donor program [14]. The CT scan performed before starting the auto-HSCT showed a large air brim of multicystic aspect extraluminal at the level of the cecal fundus. Therefore, after this second episode, a diagnosis of pneumatosis intestinalis (PI) was made (Figure 2). Antibiotic therapy with metronidazole and ciprofloxacin was administered, parental nutrition was started and auto-HSCT was postponed. On September, with no complete resolution of PI, she underwent auto-HSCT with FEAM conditioning. The early period post-auto HSCT was complicated by febrile neutropenia, abdominal pain and vomiting, with no worsening of PI. On day +25, the patient was discharged in good clinical conditions and persistence of PI signs at CT scan. As planned, 4 months after the auto HSCT, the patient received an allogeneic HSCT from an unrelated HLA compatible donor. We employed a reduced intensity schedule of conditioning regimen [15]. With the aim of reducing the risk of intestinal complications, parenteral nutrition was started from the first day of the conditioning regimen. Platelet and granulocyte engraftment was observed on day +17 and +23 after the transplant, respectively, and with documented full donor chimerism. No intestinal complication was recorded during the follow-up. Moreover, the abdomen CT scan no longer showed the presence of intestinal pneumatosis. Currently, the patient is in good clinical conditions, in complete hematological response with total absence of radiological signs of pneumatosis, 30 months after the allo-HSCT.
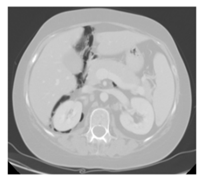
Figure 1. Abdominal CT shows an intraperitoneal and retroperitoneal air brim.
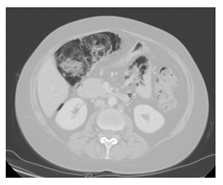
Figure 2. Abdominal CT: a large air brim of multicystic aspect extraluminal at the level of the cecal fundus.
To the best of our knowledge, this is the first case of a patient with pre-existent PI who successfully underwent a tandem auto-allogeneic HSCT. Clinical course of PI varies: most PI patients without surgical acute abdomen will improve with supportive therapy, encompassing bowel rest and antibiotics. The presence of certain CT features (mesenteric stranding, bowel wall thickening, and ascites) and the location of PI may help distinguishing more significant bowel disease and more severe cases [16]. Unfortunately, PI can be a life-threatening complication leading to bacterial peritonitis or severe sepsis in ~20% of patients with PI regardless of its aetiology [4]. Our patient showed radiologic signs of PI when she was receiving chemotherapy and steroids. Thereafter, PI worsened after FEAM conditioning for the auto-HSCT. The decision to proceed to the second transplant with an unrelated donor was of particular concern due to the risk of a further worsening of the PI, especially in the possible case of an intestinal GVHD or of infectious complications [5-7,12,13]. However, considering the poor prognostic likelihood of our patient affected by DLBCL resistant to several lines of therapy, we decided to proceed with an allo-HSCT, sharing the decision with the patient and her relatives.
Traditionally, PI has been managed surgically with high morbidity and mortality rates (33-34%) [1]. For this reason, we did not opt for a pre-transplant prophylactic colectomy. On the other hand, in patients successfully managed non-operatively was reported an acceptable mortality rate of 6% [17]. Therefore, to reduce the risk of worsening the PI, we opted for a conservative approach using early antibiotic therapy with ciprofloxacin and total parental nutrition with the aim of decreasing anaerobic bowel flora and of avoiding any mucosal stress. Following this strategy, no intestinal complications were observed during the follow-up.
This case report suggests that PI should not be considered an absolute contraindication to HSCT. However, a careful balance of risks and benefits must be considered together with close monitoring of the patients.
The authors have no conflict of interest to declare.
No funding was received for this research.
2021 Copyright OAT. All rights reserv
- Hepgur M, Ahluwalia MS, Anne N, Thomas J, Liu H, et al. (2011) Medical management of pneumatosis intestinalis in patients undergoing allogeneic blood and marrow transplantation. Bone Marrow Transplant 46: 876-879. [Crossref]
- St Peter SD, Abbas MA, Kelly KA (2003) The spectrum of pneumatosis intestinalis. Arch Surg 138: 68-75. [Crossref]
- Bhamidipati PK, Ghobadi A, Bauer S, DiPersio JF, Pusic I. (2014) Conservative management of pneumatosis intestinalis after allogeneic hematopoietic SCT. Bone Marrow Transplant. 49: 1436-1438. [Crossref]
- Greenstein AJ, Nguyen SQ, Berlin A, Corona J, Lee J, et al. (2007) Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg 11: 1268-1274. [Crossref]
- Ho LM, Paulson EK, Thompson WM (2007) Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol 188: 1604-1613. [Crossref]
- Lipton J, Patterson B, Mustard R, Tejpar I, Fyles G et al. (1994) Pneumatosis intestinalis with free air mimicking intestinal perforation in a bone marrow transplant patient. Bone Marrow Transplant. 14: 323-326. [Crossref]
- Zhang D, Weltman D, Baykal A (1999) Portal vein gas and colonic pneumatosis after enema, with spontaneous resolution. AJR Am J Roentgenol 173: 1140-1141. [Crossref]
- Zulke C, Ulbrich S, Graeb C, Hahn J, Strotzer M et al. (2002) Acute pneumatosis cystoides intestinalis following allogeneic transplantation -- the surgeon's dilemma. Bone Marrow Transplant. 29: 795-798. [Crossref]
- Day DL, Ramsay NK, Letourneau JG (1988) Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol 151: 85-87. [Crossref]
- Hall RR, Anagnostou A, Kanojia M, Zander A. (1984) Pneumatosis intestinalis associated with graft-versus-host disease of the intestinal tract. Transplant. Proc. 16: 1666-1668. [Crossref]
- Navari RM, Sharma P, Deeg HJ, McDonald GB, Thomas ED. (1983) Pneumatosis cystoides intestinalis following allogeneic marrow transplantation. Transplant. Proc. 15: 1720-1724. [Crossref]
- Schulenburg A, Herold C, Eisenhuber E, Oberhuber G, Volc-Platzer B et al. (1999) Pneumatosis [correction of Pneumocystis] cystoides intestinalis with pneumoperitoneum and pneumoretroperitoneum in a patient with extensive chronic graft-versus-host disease. Bone Marrow Transplant. 24: 331-333. [Crossref]
- Suzuki H, Izutsu K, Watanabe T, Oshima K, Kanda Y et al. (2008) Late-onset pneumatosis cystoides intestinalis associated with non-infectious pulmonary complications after allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 88: 116-118. [Crossref]
- Crocchiolo R, Castagna L, Fürst S, El-Cheikh J, Faucher C, et al. (2013) Tandem autologous-allo-SCT is feasible in patients with high-risk relapsed non-Hodgkin's lymphoma. Bone Marrow Transplant 48: 249-252. [Crossref]
- Corradini P, Dodero A, Farina L, Fanin R, Patriarca F et al. (2007) Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 21: 2316-2323. [Crossref]
- Lee KS, Hwang S, Hurtado Rúa SM, Janjigian YY, Gollub MJ (2013) Distinguishing benign and life-threatening pneumatosis intestinalis in patients with cancer by CT imaging features. AJR Am J Roentgenol 200: 1042-1047. [Crossref]
- Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, et al. (2008) Management and outcome of pneumatosis intestinalis. Am J Surg 195: 679-682. [Crossref]


