Rice bran is the by-product of the rice milling industries so in view of growing need and consciousness about the nutritional and functional properties the rice bran is very important co-product. In this research article the methods were described regarding different chemicals with different concentration in controlling the activity of lipase enzyme and ultimately to maximize its oil recovery from the rice bran during 60 days of storage in a room temperature. The use of hydrochloric acid at concentration of about 30 ml/Kg helps greatly in controlling the lipase enzyme activity and reduces the % of FFA while the other chemicals used in this study (Phosphoric acid, Acetic acid, Sodium metabisulphite) failed to control in the rise of free fatty acid contents. The chemicals can be applied easily by sprinkling or spraying. This operation done on small rice bran lots through manual hand mixing. This method of chemical stabilization of rice bran is really a useful method in the rice mills where there is shortage of electricity or steaming facility.
stabilization, HCL, rice bran, lipase enzyme, oil contents, inactivation
Rice is an important food as well as cash crop of Pakistan. It is also a major export commodity and earns about US $ 2.2 billion foreign exchange annually.There is more than 750 million metric tons production of rice paddy annually all around the world [1]. Rice is greatly consumed all over the world and used as a staple food product in different regions of the whole world due to its nutritional value [2].
The grain of the rice contain about 2 to 3% fat, and this fat portion is mainly concentrated in the embryo or in the germ and then in the outer layer of the seed [3]. During milling the germ and the bran powder layers detachedfrom the endosperm and this milling finally concentrate the fat portion into the residue generally said as “Rice Bran” [4]. Rice bran is massive rice milling industry discard produced during rice polishing and mainly used in animal feed. It constitutes about 7-8% of the whole paddy grainwhich contributes about more than 60 million tons per annum of the whole world production [1,5]. Rice bran has great potential to contribute to supply edible oil to the world and it ranges about 10-26% of the rice bran depending upon degree of milling, climatic conditions and variety [2,6,7].
Research in the last decades showed that rice bran is an outstanding source of vitamins, minerals, antioxidants, proteins, fats and dietary fibers [8]. Several problems of raw bran management have restricted production of edible grade rice bran oil. One of the problem is high lipase activity in the bran, which quickly hydrolyzes the fats of oil into free fatty acids [9]. To resolve this main problem the use of proper technique or deactivation method is very important.
To date, several studies have been conducted on stabilization techniques of rice bran and its oil [9,10,11]. Although a number of studies like microwave heating, ohmic heating, dry or moist heat treatment and little bit on pH lowering have been conducted for rice bran and its oil stabilization [12 ]. However, treatments with different chemicals have not been properly used for this purpose.The objective of this research work was to investigate the effect of using different chemicals for the stabilization of rice branon lipase enzyme, which ultimately leads to enhance the shelf life of rice bran and then maximize its oil contents.
Laboratory grade Hydrochloric acid, Phosphoric acid, Acetic acid and Sodium metabisulphite were purchased from Merck, Germany and Hexane (Solvent for Extraction) from Sigma-Aldrich, Germany in this present research work.Freshly milled rice bran powder samples were collected from our local rice mills situated in areas of Muridke District Sheikhupura and Kamoke, District. Gujranwala.Stabilization of rice bran was carried out by spreading rice bran in a layer of 5 cm thick and the required amount different chemicals (Hydrochloric acid used @ a rate of 20, 30 & 35 ml/Kg, Phosphoric acid used @ 0.5, 1.0 & 1.5% per Kg, Acetic acid used @ 3, 5 & 7% per Kg and Sodium metabisulphite used at 1.5, 2.0 & 2.5% per Kg) were sprinkled onbran layers with different concentrations and mixed well by hand using protective clothing.Total 12 treatments were prepared with above mentioned acids or chemicals each with 3 treatments with different concentration. After completion of the treatments, all the rice bran samples were packed in locally made polyethylene bags. Then the packed rice bran samples were stored in a room temperature and analyzed it for free fatty acid % and oil extraction % after every 10 days interval upto 60 days of storage [13].
Extraction of the oil contents of the samples were conducted on a laboratory scale Soxhlet extractor (Sayre et al., 1985). Analytical grade hexane was used and hexane temperature during extraction was about 60°C. Determination of free fatty acid (FFA) was done by alkalimetric titration (AOAC, 2006). The proximate analysis of chemically stabilized rice bran for moisture, protein, fats, crude fiber and ash contents were determined by [14-17].
Fatty acid composition of the treated rice bran samples
First from the four chemicals, we select the best treatment from each chemical on the basis of rice bran oil FFA% and then covert this crude oil into refined form. The best treatments selected for the fatty acid composition analysis are as follows:
- Hydrochloric Acid (HCL) @ 30 ml/Kg
- Phosphoric Acid @ 1.5% W/W
- Acetic Acid @ 7% W/W
- Sodium Metabisulphite @ 2% W/W
All the above treatments were analyzed for fatty acid analysis by using the method of [18] in which the methyl esters of fatty acids were separated with a GC-17A (Shimadzu Co., Japan) column equipped with HP-20M (25 m × 0.32 mm, 0.3 µm) at the following temperature program: initial temperature of 180°C (10 min hold) to 200°C at 4/min (2 min hold) and to 220°C at 4/min (12 min hold). Identification of fatty acid methyl esters was made by comparing their relatives retention times with that of known standard samples (linoleic acid, oleic acid, palmitic acid, stearic acid, arachidic acid, linolenic acid,eicosenoic acid, myristic acid and behanic acid) (Figure1-5).
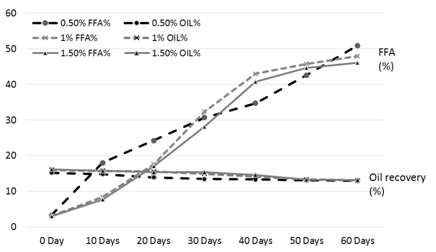
Figure 1. FFA and oil % of the rice bran treated with phosphoric acid during storage
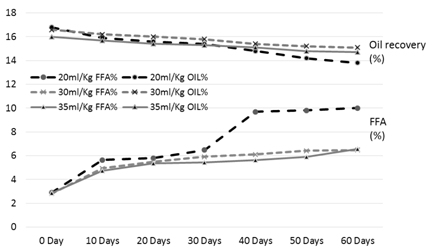
Figure 2. FFA and oil % of the rice bran treated with hydrochloric acid (HCL) during storage
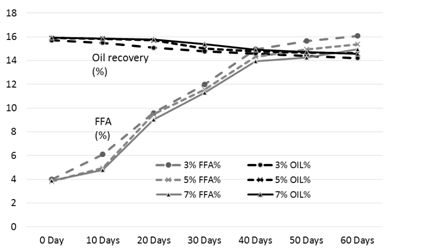
Figure 3. FFA and oil % of the rice bran treated with acetic acid during storage
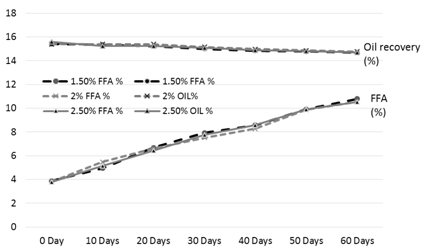
Figure 4. FFA and oil % of the rice bran treated with sodium metabisulphite during storage
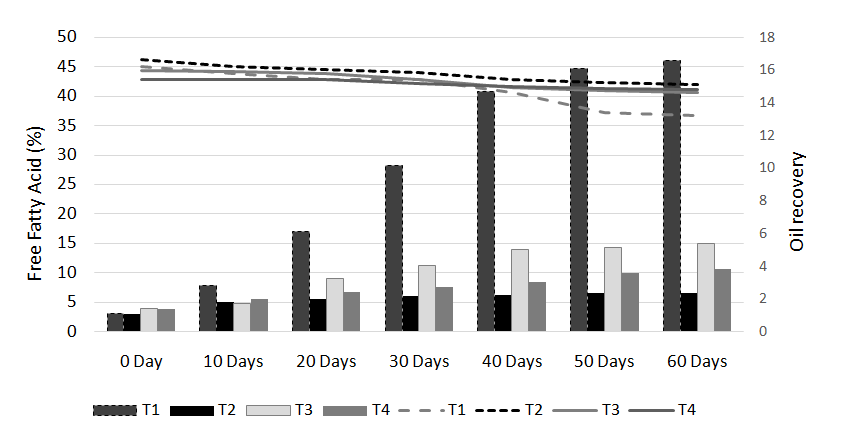
Figure 5:
Statistical analysis
Analysis of variance of the data was computed using the Statistica computer program. The Least Significance Difference test at 5% level of significance was used to test the differences among mean values [19].
Proximate analysis of rice bran
The results of analysis regarding moisture, protein, fats, crude fiber and ash contents/percentage of procured rice bran from rice processing mills and local Sheller’swere shown in Table 1. The rice bran used in this research work selected from local rice Sheller’s having good nutritional properties regarding proximate analysis with 11.2% moisture content, 16.87% protein contents, 20.20% rice bran fats, 8.29% crude fiber and 9.21% of ash contents. All these values were significantly different from proximate analysis of rice bran procured from rice processing mills. The oil contents of the rice bran vary according to the different chemical treatments and shown in Table 2 to 5. Acid stabilization appears to facilitate extraction of the crude oil from the rice bran. Outof these chemical treatments hydrochloric acid (HCL, Table 3) gave the best results in achieving maximum oil recovery that were in the range of (16.8-13.8%) in T1, (16.6-15.1%) & (16-14.7%) in T2 and T3 in 60 days storage respectively. The treatment with concentration of 30 ml/Kg of HCL gives the best optimum results in % of Crude oil recovery. While in other chemical treatments it ranges from (16.2-12.96%) in phosphoric acid, (15.94-14.2%) in acetic acid and (15.6-14.68%) in sodium metabisulphitein all three treatments respectively. Statistical analysis showed significant differences in the oil contents. The ranges of oil contents in the present work match the results of [9,20,21].
Table 1. Proximate analysis of oils of rice bran collected from different sources
Parameters |
T1 (From Local Sheller’s) |
T2 (From Rice Processing mills) |
Moisture |
11.22 |
11.60 |
Protein |
16.87 |
16.60 |
Fats |
20.20 |
19.15 |
Crude Fiber |
8.29 |
7.99 |
Ash Contents |
9.21 |
8.75 |
Values presented are averages of duplicate samples
Table 2. FFA and oil % of the rice bran treated with phosphoric acid during storage
Treatments |
T1 |
T2 |
T3 |
Concentrations (%) |
0.50% |
1% |
1.50% |
Parameters |
FFA% |
OIL% |
FFA% |
OIL% |
FFA% |
OIL% |
|
Storage days |
0 Day |
3.38g |
15.24a |
3.13g |
16a |
3.09g |
16.2a |
10 Days |
18.05f |
14.88b |
8.46f |
15.8b |
7.76f |
15.8b |
20 Days |
24.25e |
13.94c |
17.62e |
15.6b |
17.06e |
15.4c |
30 Days |
30.69d |
13.5d |
32.4d |
15c |
28.2d |
15.4c |
40 Days |
34.72c |
13.4d |
43c |
14.2d |
40.8c |
14.6d |
50 Days |
42.58b |
13.12e |
45.8b |
13.4e |
44.69b |
13.4e |
60 Days |
50.9a |
12.96f |
47.9a |
13.1f |
46.1a |
13.2f |
Table 3. FFA and oil % of the rice bran treated with hydrochloric acid (HCL) during storage
Treatments |
T1 |
T2 |
T3 |
Concentrations (%) |
20ml/Kg |
30ml/Kg |
35ml/Kg |
Parameters |
FFA% |
OIL% |
FFA% |
OIL% |
FFA% |
OIL% |
|
Storage days |
0 Day |
2.9f |
16.8a |
2.81f |
16.6ab |
2.88f |
16a |
10 Days |
5.64e |
15.9b |
4.94e |
16.2a |
4.72e |
15.7b |
20 Days |
5.8d |
15.6c |
5.5d |
16ab |
5.36d |
15.4c |
30 Days |
6.48c |
15.4c |
5.92c |
15.8a |
5.43d |
15.3d |
40 Days |
9.7b |
14.8d |
6.1b |
15.4ab |
5.64c |
15.1e |
50 Days |
9.8b |
14.2e |
6.42a |
15.2b |
5.92b |
14.8f |
60 Days |
10.01a |
13.8f |
6.47a |
15.1b |
6.56a |
14.7f |
Free fatty acid (FFA) status of rice bran
The free fatty acids contents (FFA %) of rice bran is shown in Table 2 to 5. From all the treatments again HCL gave the good results in controlling the activity of lipases enzyme and from the three concentrations of HCL, concentration of 30 ml/Kg appears to give the best results in enzyme deactivation that is in the range of (2.81-6.47%) in 60 days.While the other two concentration of HCL also give satisfactory performance regarding enzymes deactivation that is (2.9-10.01%) in 20 ml/Kg and (2.88-6.56%) in 35 ml/Kg respectively in 60 days.The other chemical treatments failed in controlling lipase activity these treatments lose their control right after 10 and 20 days of storage and breaks the recommended limit of less than 10% FFA. All the results shown in the table gave significant differences in the FFA contents of rice bran and its oil contents. Prabhakar and Venkatesh [9] finding of FFA% depicts the results of the present study of FFA%.
Table 4. Free fatty acids and oil % of the rice bran treated with acetic acid during storage
Treatments |
T1 |
T2 |
T3 |
Concentrations (%) |
3% |
5% |
7% |
Parameters |
FFA% |
OIL% |
FFA% |
OIL% |
FFA% |
OIL% |
|
Storage days |
0 Day |
4g |
15.7a |
3.81g |
15.91a |
3.88g |
15.94a |
10 Days |
6.1f |
15.5a |
4.94f |
15.85a |
4.79f |
15.86a |
20 Days |
9.59e |
15.09b |
9.47e |
15.71b |
9.02e |
15.78a |
30 Days |
11.99d |
14.78c |
11.56d |
15c |
11.28d |
15.4b |
40 Days |
14.95c |
14.6cd |
14.32c |
14.8d |
13.96c |
14.9c |
50 Days |
15.65b |
14.41de |
14.95b |
14.65e |
14.24b |
14.73cd |
60 Days |
16.07a |
14.2e |
15.37a |
14.62e |
14.95a |
14.58d |
Table 5. FFA and oil % of the rice bran treated with sodium metabisulphite during storage
Treatments |
T1 |
T2 |
T3 |
Concentrations (%) |
1.50% |
2% |
2.50% |
Parameters |
FFA % |
FFA % |
FFA % |
OIL% |
FFA % |
OIL % |
|
Storage days |
0 Day |
3.88g |
15.4a |
3.81g |
15.4a |
3.8g |
15.6a |
10 Days |
4.94f |
15.35a |
5.47f |
15.39a |
5.16f |
15.26ab |
20 Days |
6.67e |
15.23a |
6.61e |
15.39a |
6.42e |
15.26ab |
30 Days |
7.93d |
15b |
7.52d |
15.15b |
7.77d |
15.1ab |
40 Days |
8.57c |
14.84bc |
8.27c |
14.98bc |
8.56c |
14.9b |
50 Days |
9.92b |
14.78c |
9.87b |
14.86c |
9.9b |
14.8b |
60 Days |
10.78a |
14.68c |
10.53a |
14.77c |
10.54a |
14.68ab |
Table 6 shows the fatty acid (FA) composition results of treated rice bran samples.The contents of total saturated fatty acids of all the treatments were in the range of 0.27-0.35 Myristic acid (C 14:0), 18.95-22.82 Palmitic acid (C 16:0), 1.43-1.55 Stearic (C 18:0),0.62-0.76 arachidic (20:0), 0.71-1.24 Ecosanoic (20:1) and 0.43-0.76 Behanic acid (22:0) respectively. The content of total saturated fatty acids of rice bran oil appeared more in samples treated with phosphoric acid @ 1.5% W/W that is about about (32.19%) after that in acetic acid @ 7% W/W (29%), then in sodium metabisulphite @ 2% W/W (26%) and atlast in HCL treated sample @ 30 ml/Kg (22%). While in case of unsaturated fatty acid, the percentage of unsaturated fatty acids of all the treatments were in the range of 43.10-45.00 Oleic acid (C 18:1), 23.81-31.67 Linoleic acid (C 18:2) and0.90-1.15 Linolenicacid (C 18:3) respectively. The content of unsaturated fatty acids of rice bran oil appeared more in samples treated with Hydrochloric acid @ 30 ml/Kg that is about (77%) after that in sodium metabisulphite @ 2% W/W (74%), acetic acid @ 7% W/W (70%) and phosphoric acid @ 1.5% W/W (68%) respectively. So the conclusion is that the sample of rice bran treated with HCL gives the best results regarding unsaturated fatty acids. The above results reveals the findings of [22].
Table 6. Fatty acid composition of rice bran samples treated with four different chemicals
Fatty Acids |
HCL @ 30ml/Kg |
Phosphoric Acid @ 1.5% W/W |
Acetic Acid @ 7% W/W |
Sodium Metabisulphite @ 2% W/W |
C 14:0 |
0.27 |
0.35 |
0.35 |
0.33 |
C 16:0 |
18.95 |
22.82 |
21.04 |
19.02 |
C 18:0 |
1.43 |
1.55 |
1.54 |
1.46 |
C 18:1 |
45.00 |
43.10 |
43.20 |
44.25 |
C 18:2 |
31.67 |
23.81 |
26.82 |
29.19 |
C 18:3 |
1.15 |
0.90 |
0.92 |
1.05 |
C 20:0 |
0.72 |
0.62 |
0.76 |
0.73 |
C 20:1 |
0.71 |
1.24 |
1.08 |
0.74 |
C 22:0 |
0.44 |
0.76 |
0.43 |
0.47 |
∑SAFFA |
21.81 |
26.1 |
24.12 |
22.01 |
∑USFFA |
78.53 |
69.05 |
72.02 |
75.23 |
2021 Copyright OAT. All rights reserv
C 14:0 Myristic Acid, C 16:0 Palmitic Acid, C 18:0 Stearic Acid, C 18:1 Oleic Acid, C 18:2 Linoleic Acid, C 18:3 Linolenic Acid, C 20:0 Arachidic Acid, C 20:1 Eicosanoic Acid, C 22:0 Behenic Acid.
The chemical methods for stabilization of the rice bran provides an answer to the problem of handling raw bran deterioration mainly in the developing countries with numerous small rice mills which lack adequate electricity. Out of all the chemicals used in this experiment hydrochloric acid @ 30 ml/Kg gives the best results in the stabilization of rice bran from lipase enzyme during the storage of 60 days at room temperature.
This study was supported by a grant from the Punjab Agriculture Research Board and awarded to Rice Research Institute, Kala Shah Kaku. (From Year 2010-2013).
- 1. Iqbal S, Bhanger MI, Anwar F ( 2005) Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem 93: 265-272.
- 2. Anwar FM, Ashraf, Bhanber MI (2005) Interprovence variation in the composition of Moringa oliefera oil seeds from Pakistan. J Am Oil Chem Soc 82: 45-50.
- 3. Sastry BS, Ramakrishna M, Raghavendra Rao MR (1974) J Food Sci Technol 14: 273.
- 4. Yokochi K (1977) In proceedings of the international conference on rice by-products utilization, Valencia, Spain, Vol. III.
- 5. Renuka Devi R, Arumughan C (2007) Phytochemical characterization of defatted rice bran and optimization of a process for their extraction and enrichment. Bioresour Technol 98: 3037-3043. [Crossref]
-
- 6. Chatha SAS, Anwar F, Manzoor M, Bajwa JR (2006) Evaluation of antioxidant activity of rice bran extracts using different antioxidant assays. Grasasy Acetics 57: 328-335.
- 7. Saunders RM (1985) Rice Bran: composition and potential food uses. Food Rev Int 1: 465-495.
- 8. Moldenhauer KA, Champagne ET, McCaskill DR, Guraya H (2003) Functional products from rice. In: Functional foods (Ed. G. Mazza), Technomic Publishing Co., Inc. Lancaster, Base: 71-89.
- 9. Prabhakar JV, Venkatesh KVL (1986) A simple method for stabilization of rice bran. JAOCS 63.
- 10. Lakkakula NR, Lima M, Walker T (2004) Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresour Technol 92: 157-161. [Crossref]
- 11. Tao J, Rao R, Liuuzo J (1993) Microwave heating for rice bran stabilization. J Microw Power Electromagn. Energy 28: 156-161.
- 12. Pourali O, Salak Asghari F, Yoshida H (2009) A rapid and ecofriendly treatment technique for rice bran stabilization and extraction under sub-critical water condition. Proceedings of the world congress on engineering and computer science, Vol. I, WCECS: ISBN: 978-988-17012-6-8.
- 13. Malekian F, Rao RM, Prinyawiwatkul W, Marshall WE, Windhauser M, et al. (2000) ‘‘Lipase and lipoxygenase activity, functionality, and nutrient losses in rice bran during storage’’, Bulletin No. 870, Baton Rouge: LSU Agricultural Center, Louisiana Agricultural Experiment Station: 1–68.
- 14 AOAC (1984) Official Methods of Analysis, (14th Edn.), In: S.Williams (Edr.), AOACpub!, Assoc.Off.Anal.Chem., Washington, DC.
- 15. AACC 1976. Approved Methods. Am Assoc Cereal Chem. Inc., St. Paul, MN.
- 16. ISO (1981) Animal Feeding Stuff. Determination of nitrogen and calculation of crude protein contents. Geneva, Switzerland. Standard No. 5983.
- 17. Randall JM, Sayre RN, Schultz WG, Fong RY, Mossman AP, et al. (1985) Rice bran stabilization by extrusion cooking for extraction of edible oil. J Food Sci. 50: 361-368.
- 18. Young-Hee Hwang, Young-Su Jang, Moo-Key Kim, Hoi-Seon Lee (2002) Fatty acid composition of rice bran oil and growth-promoting effect of rice bran extract and rice bran oil on Bifidobacterium and Lactobacillus. Agric Chem Biotechnol 45: 121-124.
- 19. Steel RGD, Torrie JH Dickey DA (1997) Principles and procedures of statistics: A biometrical approach, (3rd Edn.), McGraw Hill Book Co., New York, USA.
- 20. Lee BL, New AL, Ong CN (2002) Simultaneous determination of tocotrienol, tocopherol, retinol and major carotenoids in human plasma. Am Assoc Clinical Chem 49: 2056-2066. [Crossref]
- 21. Absar N, Ali MM, Rahman MM (1998) A comparative study on characteristics of boiled and non-boiled types of rice bran and their oils. Bangladesh J Biochem 4: 43-54.
- 22. Rossell JB (1991) Purity criteria for edible vegetable oils and fats. Fat Sci Technol 93: 526-531.





