Abstract
Background: The impact of donor age on survival after living donor liver transplantation (LDLT) considering clinical conditions such as hepatitis C virus (HCV) infection, Model for End-stage Liver Disease (MELD) scores and graft-to-recipient body weight ratios (GRWR) is unclear.
Objective: The present study investigated the impact of younger donors on outcomes after LDLT in clinical subgroups based on these factors.
Methods: Between November 1994 and December 2015, 874 adult patients underwent LDLT at our institution. We defined donors aged < 40 years as being younger. The recipients were assigned to eight subgroups according to presence or absence of HCV infection, MELD scores (< 25 or ≥ 25) and GRWR (< 0.8 or ≥ 0.8) (Figure 1). Overall survival rates after LDLT according to donor age (< 40 or ≥ 40 years) were compared among the sub-groups.
Results: Overall survival rates after LDLT were significantly higher in patients with grafts from younger donors than those from non-younger donors (P=0.006). Among the subgroups of recipients with HCV negative, MELD scores < 25 or ≥ 25 and GRWR < 0.8, overall survival rates were significantly higher with grafts from younger donors compared with non-younger donors (P=0.048, P=0.014, respectively).
Conclusion: Younger donors favorably impacted survival after LDLT among recipients with HCV-negative patients whose GRWR < 0.8 regardless of MELD scores.
Key words
donor age, graft size, HCV infection, liver transplantation, MELD score
Abbreviations:
DAA: direct acting antivirals; DD: deceased donor; GRWR: graft-to-recipient body weight ratio; HCV: hepatitis C virus; LD: living donor; LT: liver transplantation; MELD; model for end-stage liver disease
Introduction
Liver transplantation (LT) has become a highly promising treatment for end-stage liver diseases [1]. Living donor LT (LDLT) has prevailed in Asian countries due to religious and cultural influences resulting in a shortage of deceased donors. Indications for LDLT have now been extended based on a variety of experiences. Several reported risk factors for post-transplant patient survival include graft steatosis, livers donated after cardiac death, aged donors, hepatitis virus-positive donors, split liver grafts and extended duration of cold ischemia, that have also been referred to as marginal grafts [2,3].
Several studies have investigated the impact of elderly donors on outcomes of LDLT [4-8]. We also reported that donors aged ≥ 60 years comprise an independent risk factor for poor recipient survival after LDLT [9]. On the other hand, younger donors favorably impact survival after LDLT, especially in recipients infected with hepatitis C virus (HCV) [10,11]. However, outcomes after LDLT are affected by many factors in the clinical setting such as HCV infection, graft size, Model for End-stage Liver Disease (MELD) scores and donor age.
Nonetheless, little is known about the impact of donor age on survival considering clinical conditions such as HCV infection, MELD scores and the graft-to-recipient body weight ratio (GRWR). Therefore, the present large-scale study investigated the impact of younger donors on survival after adult-to-adult LDLT under various donor and recipient conditions.
Materials and methods
This study included 874 (male, n=435; female, n=439) consecutive patients with a median age of 51 (range, 18 – 69) years who underwent adult-to-adult LDLT between November 1994 and December 2015 at Kyoto University Hospital. The Ethics Committee of Kyoto University approved the study, which proceeded in accordance with the Declaration of Helsinki (2013).
Among the patients, 168 were ABO incompatible, and 706 were identical or compatible. The median MELD score was 18 (range, 6 - 55). The indications for LDLT were HCC (n=255), liver cirrhosis associated with hepatitis B or C virus (n=165), progressive intrahepatic cholestatic diseases including primary biliary cirrhosis and primary sclerosing cholangitis (n=149), biliary atresia (n=55), acute liver failure (n=82), alcoholic liver cirrhosis (n=25), metabolic liver diseases (n=25), Budd-Chiari syndrome (n=13) and other causes (n=105). Orthotopic LDLT proceeded using left lobe, right lobe and posterior segment grafts for 223, 628 and 17 patients. Six patients underwent domino liver transplantation using whole liver grafts from patients with familial amyloid polyneuropathy. The median GRWR was 0.98 (range, 0.45 - 2.14).
Standard selection criteria for living donors have been described in detail elsewhere [12-14]. The donor age for LDLT at our institute is limited in principle to < 65 years. Donors aged from 65 to 67 years were evaluated by our institutional review board. Graft and remnant liver volumes in donors were preoperatively estimated using three-dimensional reconstructed images of the hepatic vascular anatomy generated by a software package based on reconstructed, multi-detector CT images of livers. We gradually decreased the lower limit of GRWR to preferentially select a left-, instead of a right-lobe graft (from ≥ 0.8% until early December 2007, to ≥ 0.7% starting in December 2007, and to ≥ 0.6% starting in April 2009).
The selection criteria for the recipients and the surgical techniques for both donor and recipient operations have been described in detail [12-14]. The standard immunosuppression protocol consisting of tacrolimus and low-dose steroid was applied until January 2011 [15,16]. After February 2011, a steroid-free protocol using mycophenolate mofetil was applied as described elsewhere excluding patients with ABO-incompatible transplants or who had been administered with steroid before LT [17].
We defined donors aged < 40 years as being younger. The median donor age was 45 (range, 19 – 67) years. Four donors aged 19 years were included, although the current definition of minimum donor age is ≥ 20 years. The overall survival rates after LDLT according to donor age were assessed. The recipients were then assigned to eight subgroups according to presence or absence of HCV infection, MELD scores (< 25 or ≥ 25) and GRWR (< 0.8 or ≥ 0.8) (Figure 1). Overall survival rates after LDLT according to donor age (< 40 or ≥ 40 years) were compared among the sub-groups.
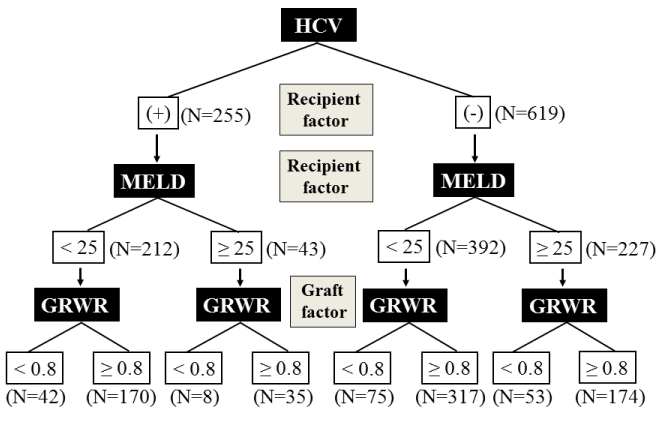
Figure 1. Sheme of subgroups according to HCV infection, MELD score and GRWR.
Cumulative overall survival rates were calculated using the Kaplan-Meier method, and differences between curves were evaluated using the log-rank test. Values with P < 0.05 were considered significant. All data were statistically analyzed using SPSS software (SPSS Inc., Chicago, IL, USA).
Results
Analysis of overall survival after LDLT
In all recipients, overall survival rates after LDLT were significantly higher in patients with grafts from younger donors than those from non-younger donors (P=0.006; Figure 2).
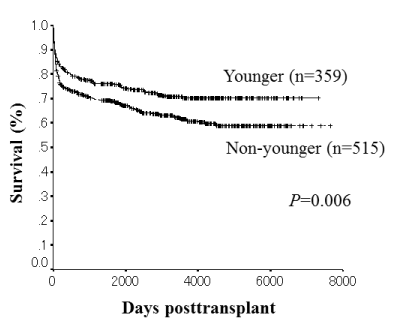
Figure 2. Overall survival rates after LDLT in all patients.
Analysis of overall survival after adult-to-adult LDLT based on HCV infection,MELD scores and GRWR
In patients with HCV positive, overall survival rates were not significantly different in the subgroup of recipients with MELD scores < 25 and GRWR < 0.8 or GRWR ≥ 0.8 who received grafts from younger and non-younger donors (P=0.671; Figure 3A and P=0.149; Figure 3B, respectively). Overall survival rates did not significantly differ in the subgroup of recipients with MELD scores ≥ 25 and GRWR ≥ 0.8 who received grafts from younger and non-younger donors (P=0.388; Figure 3C). In the subgroup of recipients with MELD scores ≥ 25 and GRWR < 0.8 (n=8), the analysis could not be statistically possible due to small numbers of recipients.
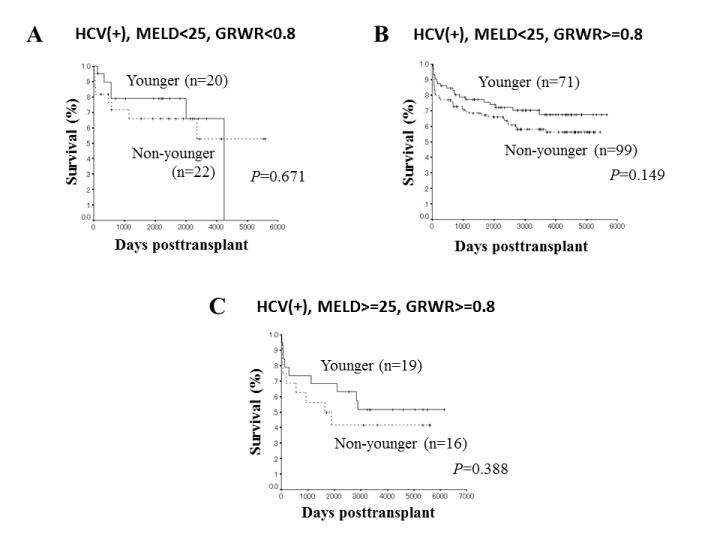
Figure 3. Overall survival rates among recipients with HCV positive, MELD scores < 25 and GRWR < 0.8 (A) and GRWR ≥ 0.8 (B) and HCV positive, MELD scores ≥ 25 and GRWR ≥ 0.8 (C) who underwent LDLT with grafts from younger and non-younger donors.
In recipients with HCV negative, overall survival rates were significantly higher in the subgroups of recipients with MELD scores < 25 and GRWR < 0.8 and MELD scores ≥ 25 and GRWR < 0.8 who received grafts from younger, compared with non-younger donors (P=0.048; Figure 4A, P=0.014; Figure 4C, respectively). However, overall survival rates did not significantly differ in the subgroups of recipients with MELD scores < 25 and GRWR ≥ 0.8 and MELD scores ≥ 25 and GRWR ≥ 0.8 who received grafts from younger and non-younger donors (P=0.246; Figure 4B and P=0.864; Figure 4D, respectively).
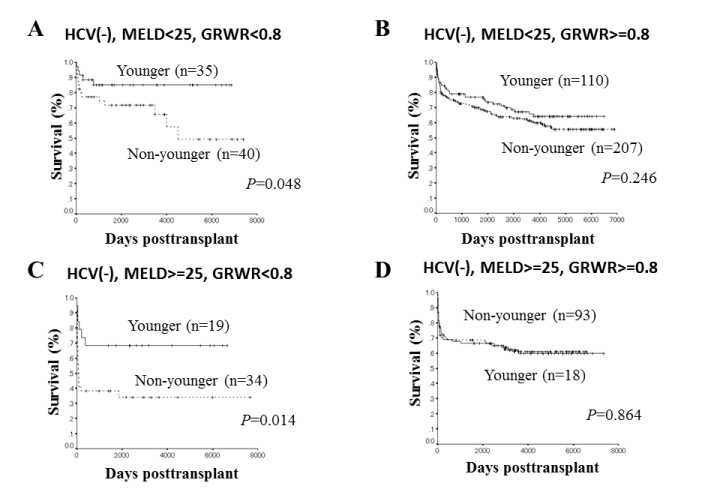
Figure 4. Overall survival rates among recipients with HCV negative, MELD scores < 25 and GRWR < 0.8 (A) and GRWR ≥ 0.8 (B) and HCV negative, MELD scores ≥ 25 and GRWR < 0.8 (C) and GRWR ≥ 0.8 (D) who underwent LDLT with grafts from younger and non-younger donors.
Discussion
2021 Copyright OAT. All rights reserv
We previously clarified that elderly donors comprised an independent risk factor for poor survival after adult-to-adult LDLT [9]. Although grafts from younger donor are supposed to be more favorable for long-term survival, the effects of other factors are not clear. Here, we focused on HCV infection, the “disease” severity of recipients and graft size to clarify the significance of younger donor age on overall survival after LDLT. Yoshizumi et al. reported predictive score about prognosis based on graft weight, donor age, MELD scores and other factors [18]. Other investigators have also proposed using donor-recipient matching using D-MELD (donor age × recipient MELD score) to predict early and long-term surgical outcomes in HCV-positive recipients with smaller grafts [19]. Jin et al. proposed the following predictors of post-transplant survival in patients after acute liver failure: vasopressor requirement, estimated glomerular filtration rate, serum sodium concentration, recipient age and donor age at the time of transplant [20]. However, HCV infection, MELD scores and GRWR have not been analyzed using algorithms. In the present study, we clarified that younger donor age favorably impacted survival after LDLT among recipients without HCV infection whose GRWR < 0.8 regardless of MELD scores.
Younger donors have been reported to comprise a favorable factor for survival after LDLT, especially among recipients with HCV [21-25]. We also reported that the overall survival rates of recipients with HCV were significantly lower after receiving grafts from elderly, ≥ 60 years old, compared with younger donors [9]. Berenguer et al. reported that outcomes among HCV-positive recipients were worsened by more advanced donor age and more powerful immunosuppression [22]. Furthermore, Busquets et al. also reported that the effects of advanced donor age on early liver function comprised decreased graft and recipient survival [23]. Wali et al. showed that donor age is a powerful determinant of the rate of the progression of fibrosis in recipient grafts. They determined that the progression rate was 0.6 (median, 0.78) units/year and that the interval to cirrhosis was 10 (median, 7.7) years when the liver donor was aged < 40 years [24]. On the other hand, these values significantly increased when grafts were donated by individuals aged ≥ 50 years. Accelerated progression might be modulated by the age of the liver at the time of HCV infection. Selzner et al. analyzed LT for HCV-related end-stage liver disease to elucidate whether the progression of HCV recurrence differs between LDLT and deceased donor liver transplantation (DDLT) [25]. Their univariate analysis significantly associated donor age, cold ischemic time and DDLT with fibrosis stage ≥ 1 after one year and fibrosis stages of 3 or 4 at two years post-LT. In multivariate analysis, donor age was the only independent variable associated with both outcomes, indicating that LDLT with grafts from younger donors might particularly provide beneficial outcomes for patients with hepatitis C.
In the present study, however, overall survival rates were significantly higher in the subgroups of recipients with HCV negative, GRWR < 0.8, regardless of MELD scores who received grafts from younger, compared with non-younger donors. On the other hand, overall survival rates did not differ in all subgroups of HCV-positive recipients who received grafts from younger and non-younger donors. For this reason, we defined donors aged < 40 years as being younger in this study, while our previous report compared overall survivals of recipients between elderly (≥ 60 years old) and non-elderly donors [9]. In fact, moreover, overall survival rates of adult-to-adult LDLT in and after April 2006 did not differ significantly between the elderly group and the younger group [9]. The improvement of treatments for HCV recurrence after LT might influence the finding. Treatment for HCV recurrence in liver transplant recipients has recently dramatically changed after the introduction of interferon-free therapy with direct-acting antivirals (DAA) [26]. Such therapy is apparently safe and effective for patients with HCV before and after LT as well as for those with severe cholestatic hepatitis C, although problems such as drug-drug interactions, direct-acting antiviral-resistant HCV, and other issues affecting patients with decompensated cirrhosis and renal failure must be overcome before its widespread adoption [27]. The impact of younger donors on HCV-positive LT recipients in the DAA era might be disappeared.
As for the impact of donor age as prognostic factor, we also reported that elderly donor, ABO incompatibility and preoperative intensive care unit stay were independent risk factors for poor patient survival in adult-to-adult LDLT according to a multivariate analysis. Therefore, we omit the multivariate analysis for younger donors in this study.
As for the benefit of liver from younger donors, Ono et al. found significantly higher rates of liver regeneration in younger than in aged donors, and revealed that liver regeneration becomes more impaired with age in donors after hepatectomy [28]. One reason for this might be the influence of declining populations of hepatic progenitor cells. Various biological changes during aging also cause the liver to lose the proliferative response and regeneration [29]. Taking this into consideration, the present outcomes of recipients without liver impairment seem to support our findings; overall survival rates in the present study were significantly higher among recipients of grafts from younger than from non-younger donors.
Some limitations of this retrospective, single-center study must be considered. A nation-wide study is needed to confirm the present findings despite the fact that they were derived from the largest LT center in Japan. One subgroup with HCV positive, MELD scores ≥ 25 and GRWR < 0.8 was quite small (n=8) with respect to statistical analysis. Second, we defined younger donors as being aged < 40 years and elderly recipients as being aged ≥ 60 years, which would be appropriate to examine the effects of donor and recipient ages. However, a nation-wide study is also needed in this context. Third, this study included 874 consecutive recipients who underwent LDLT at our institute over a period of > 20 years, during which surgical procedures, perioperative management and HCV treatment considerably changed. Such bias might affect the findings of this study.
In conclusion, younger donors favorably impacted survival after LDLT among recipients with HCV-negative patients whose GRWR < 0.8 regardless of MELD scores. The results of this study stratified by 3 critical factors, HCV infection, MELD scores and GRWR, might offer the choices for donor selection.
Acknowledgements
The authors would like to thank Ms. Mayumi Kawashima for her help with collecting the data for this study.
Financial support
None of the authors have any grants to declare.
Conflict of interest
None of the authors have any conflicts of interest.
References
- Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, et al. (1982) Evolution of liver transplantation. Hepatology 2: 614-636. [Crossref]
- Busuttil RW, Tanaka K (2003) The utility of marginal donors in liver transplantation. Liver Transpl 9: 651-663. [Crossref]
- Attia M, Silva MA, Mirza DF (2008) The marginal liver donor--an update. Transpl Int 21: 713-724. [Crossref]
- Tokodai K, Kawagishi N, Miyagi S, Nakanishi C, Hara Y, et al. (2016) Poor long-term outcomes of adult liver transplantation involving elderly living donors. Transplant Proc 48: 1130-1133. [Crossref]
- Akamatsu N, Sugawara Y, Tamura S, Kaneko J, Matsui Y, et al. (2007) Impact of live donor age (?50) on liver transplantation. Transplant Proc 39: 3189-3193. [Crossref]
- Morioka D, Egawa H, Kasahara M, Ito T, Haga H, et al. (2007) Outcomes of adult-to-adult living donor liver transplantation: a single institution's experience with 335 consecutive cases. Ann Surg 245: 315-325. [Crossref]
- Yoshizumi T, Shirabe K, Taketomi A, Uchiyama H, Harada N, et al. (2012) Risk factors that increase mortality after living donor liver transplantation. Transplantation 93: 93-98. [Crossref]
- Iwamoto T, Yagi T, Umeda Y, Sato D, Matsukawa H, et al. (2008) The impact of donor age on the outcome of adult living donor liver transplantation. Transplantation 85: 1240-1245. [Crossref]
- Kamo N, Kaido T, Hammad A, Ogawa K, Fujimoto Y, et al. (2015) Impact of elderly donors for liver transplantation: A single-center experience. Liver Transpl 21: 591-598. [Crossref]
- Selzner M, Kashfi A, Selzner N, McCluskey S, Greig PD, et al. (2009) Recipient age affects long-term outcome and hepatitis C recurrence in old donor livers following transplantation. Liver Transpl 10: 1288-1295. [Crossref]
- Sandy F and ohn R (2005) An older liver in the hand, or a (possibly) younger liver in the bush? Am J Transplant 5: 425-427.
- Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, et al. (2013) Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 154: 1053-1060. [Crossref]
- Inomata Y, Uemoto S, Asonuma K, Egawa H (2000) Right lobe graft in living donor liver transplantation. Transplantation 69: 258-264.
- Ito T, Kiuchi T, Egawa H, Kaihara S, Oike F, et al. (2003) Surgery-related morbidity in living donors of right-lobe liver graft: lessons from the first 200 cases. Transplantation 76: 158-163. [Crossref]
- Inomata Y, Tanaka K, Egawa H, Uemoto S, Ozaki N, et al. (1996) The evolution of immunosuppression with FK 506 in pediatric living related liver transplantation. Transplantation 61: 247-252. [Crossref]
- Takada Y, Ueda M, Ito T, Sakamoto S, Haga H, et al. (2006) Living donor liver transplantation as a second-line therapeutic strategy for patients with hepatocellular carcinoma. Liver Transpl 12: 912-919. [Crossref]
- Fukumitsu K, Hammad A, Kaido T, Ogawa K, Fujimoto Y, et al. (2015) Validation of steroid-free immunosuppression regimen after liver transplantation. J Clin Gastroenterol Treat 1: 1-4.
- Yoshizumi T, Ikegami T, Bekki Y, Ninomiya M, Uchiyama H, et al. (2014) Re-Evaluation of the Predictive Score for 6-Month Graft Survival in Living Donor Liver Transplantation in the Modern Era. Liver Transpl 20: 323-332. [Crossref]
- Tanemura A, Mizuno S, Kato H, Murata Y, Kuriyama N, et al. (2016) D-MELD, the product of donor age and preoperative MELD, predicts surgical outcomes after living donor liver transplantation, especially in the recipients with HCV-positive and smaller grafts. Transplant Proc 48: 1025-1031. [Crossref]
- Jin YJ, Lim YS, Han S, Lee HC, Hwang S, et al. (2012) Predicting survival after living and deceased donor liver transplantation in adult patients with acute liver failure. J Gastroenterol 47: 1115-1124. [Crossref]
- Mutimer DJ, Gunson B, Chen J, Berenguer J, Neuhaus P, et al. (2006) Impact of donor age and year of transplantation on graft and patient survival following liver transplantation for hepatitis C virus. Transplantation 81: 7-14. [Crossref]
- Berenguer M, Prieto M, San Juan F, Rayón JM, Martinez F, et al. (2002) Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology 36: 202-210. [Crossref]
- Busquets J, Xiol X, Figueras J, Jaurrieta E, Torras J, et al. (2001) The impact of donor age on liver transplantation: influence of donor age on early liver function and on subsequent patient and graft survival. Transplantation 71: 1765-1771. [Crossref]
- Wali M, Harrison RF, Gow PJ, Mutimer D (2002) Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut 51: 248-252. [Crossref]
- Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, et al. (2008) The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl 14: 1778-1786. [Crossref]
- Ueda Y, Kaido T, Hatano E, Ohtsuru S, Uemoto S (2015) Safe and effective treatment with daclatasvir and asunaprevir in a liver transplant recipient with severe cholestatic hepatitis C. Hepatol Res 45: 1360-1362. [Crossref]
- Ueda Y, Uemoto S (2016) Interferon-Free Therapy for Hepatitis C in Liver Transplant Recipients. Transplantation 100: 54-60. [Crossref]
- Ono Y, Kawachi S, Hayashida T, Wakui M, Tanabe M, et al. (2011) The Influence of Donor Age on Liver Regeneration and Hepatic Progenitor Cells Population. Surgery 150: 154-161. [Crossref]
- Alberto L, Estela S, Pedro B, Sara L, Juan JA, et al. (2016) How important is donor age in liver transplantation? World J Gastroenterol 22: 4966-4976. [Crossref]




