Nanotechnology is an area of intense commercial development, and concerns have been raised about the possible impact of inadvertent releases to the environment. Zinc oxide nanoparticles (nanoZnO) are used in transparent sunscreens and may find their way into the environment through wastewater treatment plants and through skin contact with water. The purpose of this study was to examine the bioavailability of Zn in fathead minnows exposed to either dietary nanoZnO or ZnCl2 and to increasing concentrations of a typical chemically processed municipal effluent. Male and female fathead minnows were exposed to food spiked with 100 µg Zn/kg of either nanoZnO or ZnCl2 for 21 d at 25 °C. After the exposure period, the following biomarkers were determined: metal levels in fish carcasses, oxidative stress (lipid peroxidation), DNA damage, mitochondrial electron transport, metallothioneins, cytochrome P4501A and 3A, and glutathione S-transferase activity. The results showed that although fish did not significantly accumulate Zn from dietary nanoZnO or ZnCl2, there was a trend of higher Zn levels in females than in males, and the difference was more significant when fish were exposed to either dietary nanoZnO or ZnCl2 in the presence of municipal effluent. Levels of metallothioneins were also higher in females than in males when fish were exposed to the highest concentration of municipal effluent, to dietary ZnCl2 alone, and to the municipal effluent plus a Zn-enriched diet (nanoZnO and ZnCl2). Cytochrome P4501A activity was increased only by municipal effluent, but the increases were greater in the presence of either form of Zn in the diet. Fish expended more metabolic energy (mitochondrial activity) and sustained more DNA damage (females) at the highest tested concentration of municipal effluent (20% v/v), and this effect was exacerbated by the addition of either form of dietary Zn. Lipid peroxidation tended to be lower when dietary ZnCl2 was present but was elevated at low municipal effluent concentrations. Multivariate analysis revealed that the effects could be grouped in the following clusters: controls, dietary nanoZnO/ZnCl2, and municipal effluent with dietary Zn. Responses to dietary nanoZnO and ZnCl2 were similar, suggesting that nanoZnO toxicity involved cationic Zn. Exposure to municipal effluent led to more significant changes in metallothioneins, mitochondrial activity, Cu levels and GSI, while exposure to either form of dietary Zn led to more significant changes in lipid peroxidation, tissue Zn levels, metallothioneins and cytochrome P4501A activity. In conclusion, the effects of dietary nanoZnO and ZnCl2 appeared similar but differed from the overall effects of municipal effluent alone. In combination, different interactions were observed when the fish were exposed to nanoZnO or ZnCl2 in the presence of municipal effluent.
Fathead minnows, Municipal effluent, Zinc oxide nanoparticles, Bioavailability, Oxidative stress, Energy expenditures.
Products derived from nanotechnology are an actively growing market that involves production of new nanoscale consumer products. At this scale (1–100 nm), the surface-area-to-volume ratio is high, causing the product to have different properties from those of the components. Given the large volumes produced by this industry, the release of such materials to the environment is likely, potentially creating new toxic interactions in the environment. There is no information available about their persistence and reactivity under realistic environmental conditions, such as pH, ionic strength, and natural organic matter and carbon content [1]. Zinc oxide nanoparticles (nanoZnO) are used for a wide variety of commercial applications, including personal care products (sunscreens), optical devices and sensors. They are often included as additives in cosmetics, transparent sunscreens, ointments, pigments and lubricants [2]. They also have antibacterial properties as a result of Zn2+ release, making them even more appealing to consumers [3,4]. Nanoparticles with low surface charge density (low zeta potential) readily form aggregates that reach the microscale level (>100 nm to µm) and precipitate even in the presence of low-ionic-strength freshwater [5]. Aggregation was shown to depend on (natural) organic matter, which can coat nanoparticles and prevent aggregation [6]. The use of nanoZnO in transparent and antibacterial sunscreens has raised the possibility of contamination of surface waters in highly populated areas, including beaches, and releases via municipal effluent. The organic matter in municipal effluent differs from natural organic matter, which is mainly composed of humic and fulvic acids, substances that are proteinaceous in nature. Hence, the interactions between nanoZnO and municipal effluent are not well understood at this time.
The toxicity of municipal effluent to fish or mussels is complex and involves a series of toxic effects at different levels of biological organization. The endocrine-disrupting activity of municipal effluent is particularly noteworthy for its estrogenic effects [7]. The presence of estrogen could lead to the activation of the estrogen receptor signaling pathways, leading to vitellogenin production in males and feminization [8,9]. In fathead minnows, exposure to municipal effluent was found to have induced plasma vitellogenin and ovipositor development in male fish [10]. The secondary sexual characteristics of males were less apparent, with discoloration of the banding patterns, little development of the dorsal pad, and absence of dorsal fin dot. Exposure to municipal effluent also leads to toxicity independent of the estrogenic signaling pathways, such as serotogenicity, genotoxicity, oxidative stress, and oxidative metabolism of xenobiotics. Municipal effluent also has serotonergic properties, because it can stimulate serotonin signaling in brain tissues and induce spawning in mussels [11, 12]. In addition, exposure to municipal effluent leads to inflammation, oxidative stress and genotoxicity [7, 13]. The levels of polyunsaturated lipid peroxidation (LPO) were significantly elevated in organisms found in the vicinity of sewage pollution [14]. Continuous exposure to municipal effluent–bound xenobiotics in urban discharge could increase metabolic energy for biotransformation and elimination in aquatic organisms. In fish, exposure to municipal effluent increases cytochrome-mediated activity in the liver for the oxidative metabolism of cyclic hydrocarbons [15,16] . For example, cytochromes P4501A and 3A are involved in the hydroxylation of coplanar polyaromatic hydrocarbons and polycyclic aliphatic hydrocarbons respectively. Metabolic energy demands could be easily determined in mitochondria by measuring electron transport activity, which is coupled to O2 consumption and CO2 production in cells, which require glucose for ATP production [17]. In a previous study, mussels exposed to municipal effluent had elevated mitochondrial electron transport (MET) activity in visceral and gill tissues [18]. This suggests that aquatic organisms exposed to urban pollutants expend more energy than organisms from cleaner sites, and this increased energy expenditure may compromise their health status, growth and reproduction in the long term.
The purpose of this study was to examine the cumulative effects of dietary nanoZn and municipal effluent exposure in adult fathead minnows. Fish were exposed to three concentrations of municipal effluent diluted in freshwater and to nanoZnO through food ingestion, given the poor solubility of this nanoparticle in freshwater. For comparison purposes, fish were exposed to ZnCl2 through food ingestion as well. Zinc bioavailability was determined in the fish carcasses, and biomarkers of stress were determined in the liver. The biomarkers of stress were metallothioneins (MT), cytochrome P4501A and 3A activity, mitochondrial (MET) activity, oxidative stress (LPO), and DNA damage. An attempt was made to highlight cumulative effects of a typical chemically treated municipal effluent and dietary nanoZnO for 21 d based on sex, effluent concentration and form of dietary Zn in adult fathead minnows.
Fathead minnow reproduction assay and exposure to municipal effluent
Fish were cultured and bred at a fathead minnow facility at the Aquatic Toxicology Laboratory of the Montreal Wastewater Treatment Plant. The exposure protocol used was a 21‑d reproduction assay with a 1:2 male-to-female ratio in 12‑L glass tanks. The fish were continuously exposed to municipal effluent (5, 10 and 20% v/v) at a flow rate of 1 L/h, diluted with charcoal-filtered, UV-treated tap water from the City of Montreal. Briefly, the two males and four females were held in a 12‑L tank for seven d prior to exposure to the effluent, ZnCl2 and ZnO nanoparticles (nanoZnO). The tanks contained spawning tiles composed of 2 × 8‑cm polyvinyl pipes, 10 cm in diameter, that were cut in half lengthwise. The tiles were monitored each morning for egg fixation. Successful fertilized eggs were examined under a microscope, and the groups showing the highest egg fertilization rate were selected for the exposure experiments.
The fish were exposed to 5, 10 and 20% concentrations of municipal effluent, which was constantly renewed and was preheated to 25 °C before reaching the tanks. The exposure experiments were repeated with two replicate tanks for each treatment group. In addition to real-time exposure to municipal effluent, one group of fish was exposed to either nanoZnO or ZnCl2 through food ingestion at a nominal concentration of 100 µg of total Zn per kg of food. The fish were fed daily with commercial food during the exposure experiments at a 3% fish weight ratio (based on mean fish weight of 21 g per tank, i.e. 0.6 g food per tank). The food powder was placed in a Waring blender at low speed, and zinc (ZnCl2 or nanoZnO) was added in 10 × 100‑µL volume increments to ensure homogenous distribution of the metal in the food mixture. Control fish were exposed to dechlorinated tap water. The fish were kept at 25 °C for 21 d with constant aeration and a 16 h light/8 h dark photoperiod. Water pH, dissolved oxygen and water temperatures were monitored daily, and the spawning tiles were checked for egg production. The tiles were replaced with new ones when the egg density covered a good proportion of the tile. At the end of the exposure period, the fish were anesthetized in tricaine (10 mg/L, Sigma-Aldrich, Ontario, Canada) according to animal health care guidelines. Fork length and wet body, gonad, gill and liver weights were measured. The livers were then mixed with four volumes of homogenization buffer before being stored at −85 °C. The homogenization buffer consisted of 140 mM NaCl and 25 mM Hepes-NaOH at pH 7.4, containing 1 mM dithiothreitol and 10 µg/mL apoprotin (protease inhibitor). The male and female secondary sexual characteristics (ovipositor, banding coloration in males, nuptial tubercles and fat pad appearance) were estimated by visual inspection. The fish carcasses were set aside for heavy metal analysis by ion coupled mass spectrometry, as described below.
Tissue metal analysis
To determine metal bioaccumulation in fish, tissues were acid-digested with 8 ml of concentrated HNO3, 1 ml of concentrated HCl, and 2 ml of concentrated H2O2 (Seastar Baseline). The tissues were then digested during 2 h at 170 °C using a microwave digestion system (Ethos EZ, Milestone ScientificInc, ON, Canada). The samples were completed to final volume of 12 ml with deionized water. Total metal concentrations were afterwards determined by XSERIES 2 ICP-MS (Thermo Scientific, USA) and standard solutions of these elements were used for calibration. The data were expressed as µg/g dry weight and repeatability was better than 5%. The recovery for these metals was between 90 % and 105 %.
Xenobiotic metabolism
Xenobiotic metabolism was characterized by monitoring changes in hepatic cytochrome P4501A1, P4503A-like, glutathione S-transferase (GST) activity and metallothionein levels (MT). First, the livers were thawed to 4 °C and homogenized using a Teflon pestle tissue grinder (five passes), and a portion of the homogenate was centrifuged at 15,000 × g for 20 min at 2 °C to recover the supernatant (S15 fraction). Total proteins in the homogenate and S15 fraction were determined by means of the Coomassie Brilliant Blue binding assay, using bovine serum albumin for calibration [19]. Cytochrome P4501A1 activity was determined by means of the 7-ethoxyresorufin-O-deethylase (EROD) assay as explained elsewhere [20]. Briefly, 50 µL of S15 fraction was added to 100 µL of 10 µM 7‑ethoxyresorufin and 100 µM reduced NADPH in 100 mM KH2PO4 at pH 7.4 on a dark microplate. The reaction mixture was incubated for 0, 10, 20, and 40 min and stopped by the addition of 100 µL of 0.1 M NaOH. The formation of the deethylated product, 7‑hydroxyresorufin, was measured by fluorescence at 520 nm excitation and 590 nm emission (Synergy 4, Biotek Instruments, USA). Standard solutions of 7‑hydroxyresorufin were prepared for calibration. The data were expressed as pmol of hydroxyresorufin/min/mg proteins. Cytochrome P4503A activity was determined using the dibenzylfluorescein dealkylase assay as described by [21]. Briefly, 50 µL of S15 fraction was added to 100 µL of 50 µM dibenzylfluorescein and 100 µM reduced NADPH in 125 mM NaCl containing 20 mM Hepes-NaOH at pH 7.4 on dark microplates. The reaction was incubated at 30 °C for 0, 30, 60 and 90 min, and fluorescence readings were collected at 485 nm excitation and 520 nm emission. Fluorescein standards were also prepared for quantitation, and the data were expressed as pmol fluorescein/min/mg proteins. GST activity was determined by microplate assay as described elsewhere [22] . The S15 fraction (50 µL) was mixed with 200 µL of 1mM GSH and 1mM 1-chloro-2,4-dinitrobenzene in 125 mM NaCl containing 20 mM Hepes-NaOH at pH 6.5. The increase in absorbance at 340 nm was measured at 0, 10, 20 and 30 min at 30 °C. Enzyme activity was expressed as change in absorbance/min/mg proteins.
Oxidative stress and DNA damage
Lipid peroxidation (LPO) was determined in the liver homogenates using the thiobarbituric acid-reactive substances (TBARS) method [23]. A volume of 50 µL of the homogenate was mixed with 150 µL of 10% trichloroacetic acid containing 1 mM FeSO4 and 100 µL of 0.7% thiobarbituric acid and heated to 70–80 °C for 10 min. The mixture was cooled to room temperature and centrifuged at 10,000 × g for 5 min to remove any precipitate. A volume of 200 µL was transferred to a 96-well dark microplate, and fluorescence readings were taken at 540 nm excitation and 600 nm emission. Standard solutions of tetrametoxypropane (stabilized form of malonaldehyde) were prepared for calibration in the blank (homogenization buffer). Results were expressed as µmol TBARS/mg total proteins in the homogenate. DNA damage was determined using the alkaline precipitation assay [24] which is based on potassium detergent-assisted precipitation of DNA proteins. Protein-free DNA strand breaks were measured in the supernatant using fluorescence spectroscopy. A volume of 25 µL of the homogenate was mixed with 225 µL of detergent solution (2% SDS containing 10 mM EDTA, 10 mM Tris base and 40 mM NaOH) for 1 min, followed by the addition of 250 µL of 0.12 M KCl. The mixture was mixed by inversion, heated to 60 °C for 10 min, cooled on ice for 15 min, and centrifuged at 8,000 × g for 10 min. A volume of 50 µL of supernatant was mixed with 150 µL of 100 µg/mL Hoechst in 0.1 M Tris-acetate at pH 8.5, containing 4 mM sodium cholate and 0.4 M NaCl to control for any residual detergent interference [25] and fluorescence readings were taken at 360 nm excitation and 450 nm emission. Standard solutions of salmon sperm DNA were prepared for calibration. The data were expressed as µg DNA strand/mg total protein in homogenate.
Mitochondrial electron transport activity
Mitochondrial electron transport (MET) activity was determined using a dye reduction method with spectrometric measurement [26] The assay is based on the reduction of a tetrazolium dye, which has been found to be related to cell respiration rates in organisms [16] . Briefly, mitochondria (100 μg/mL) were mixed with one volume of 0.1 M Tris-HCl at pH 8.5, containing 0.1 mM MgSO4, 0.1% Triton X-100 and 5% polyvinylpyrrolidone for 1 min, before 1 mM NADH and 0.2 mM NAPDH were added. The reaction was started by adding 1 mM of p-iodonitrotetrazolium. The reaction was allowed to proceed at 20 °C for 30 min, and absorbance readings were taken at 520 nm at 5‑min intervals. The data were expressed as increase in absorbance/min/mg mitochondrial protein content.
Data analysis
The exposure experiment consisted of two males and four females per treatment tank, and the experiment was repeated twice. Tissue biomarkers were analyzed in N = 4 male and N = 4 female fish using a two-way (exposure group and sex) factorial analysis of variance (ANOVA) after verifying for homogeneity of variance and normality using Levene’s test and the Shapiro–Wilk test respectively. Correlation analysis was also performed using the Pearson product-moment procedure. To determine the physiological changes induced by exposure to municipal effluent and the two forms of dietary Zn, a discriminant function and factorial analyses were performed. All statistical tests were performed using Statistica software (version 8). Significance was set at α = 0.05.
The fish were exposed to a chemically processed effluent with the following characteristics: conductivity of 750–850 µScm-1, pH of 6.9, dissolved organic carbon of 80 ± 20 mg/L, ammonia of 3.8 ± 1 mg/L, and fecal coliforms of 2 ± 1 × 106/100 mL. Since pH was well below 8, ammonia is mainly ionic and its toxicity is unlikely. In fish exposed to municipal effluent and each form of dietary Zn, the condition factor was not significantly affected by the municipal effluent and/or dietary Zn treatment (Table 1). There was a significant size difference between the sexes, with males about 2.2 times larger than females. However, there was a marginal interaction between exposure treatment and Zn diet (p = 0.08). In females, no significant effects of either municipal effluent or dietary Zn treatment were observed. In males, there was no significant change, although the data were more variable, but the male versus female difference was no longer significant for fish exposed to dietary nanoZnO. This effect was lost when municipal effluent was present. The gonadosomatic index (GSI) was significantly affected by both the effluent–dietary Zn treatment and sex (two-way factorial ANOVA: municipal effluent/dietary Zn exposure and sex interaction). In the control fish, GSI in males was significantly higher than in females. In males, GSI was significantly higher with exposure to 20% municipal effluent but dropped when the fish received either nanoZnO or ZnCl2, which suggests that municipal effluent exposure increases GSI and Zn exposure through feeding reduces GSI. In females, GSI increased with the concentration of the municipal effluent, while exposure to both forms of Zn diets had little effect on GSI, although ZnCl2 increased GSI compared to female controls and females exposed to dietary nanoZnO. In the presence of municipal effluent and either form of dietary Zn, GSI of females tended to be higher than in males. Hence, males and females seem to respond differently to exposure to municipal effluent and either form of dietary Zn. GSI in males seemed to be more responsive to the reducing effects of Zn than to stimulation by municipal effluent, while in females, the effects of the municipal effluent were more evident. The hepatosomatic index (HSI) was affected by both sex and municipal/dietary Zn treatment (two-way factorial ANOVA: sex*municipal effluent/Zn form interaction). The male HSI was approximately four to five times higher than the female HSI in control fish. In both male and females, HSI was significantly higher at 20% municipal effluent, and neither dietary nanoZnO nor ZnCl2 had any effect. A significant interaction between the exposure treatments and sex was also observed. Concomitant exposure to dietary nanoZnO and ZnCl2 and 20% municipal effluent reduced HSI in males only, compared with the 20% concentration of municipal effluent alone. This effect was not observed in females. The condition factor was significantly correlated with HSI (r = 0.65; p < 0.001) (Table 2). Fish reproductive capacity was also determined by monitoring secondary sexual characteristics (ovipositor in females, nuptial/dorsal tubercles in males, male band coloration) and egg laying and production (Table 3). In control fish, egg production was two eggs per d (42 eggs after 21 d). Exposure to 10% municipal effluent stimulated egg-laying activity and egg production, causing it to rise to seven eggs per d (147 eggs after 21 d), while exposure to 20% municipal effluent inhibited egg production, causing it to fall to one egg per d (21 eggs after 21 d). Exposure to dietary nanoZnO and ZnCl2 alone also reduced egg production. However, in the presence of municipal effluent, egg production returned to control levels, reaching 11 eggs per d (231 eggs after 21 d) at 10% municipal effluent with nanoZnO. Egg hatching observations were inconclusive, so no observations on egg hatchability are provided.
Table 1 – General health status of fathead minnows exposed to municipal effluent (ME), ZnO and dissolved Zn.
|
Sex |
Fork length (mm) |
Fish weight (g) |
CF |
GSI |
HSI |
Controls |
M F |
68.3±1.7 46.25±2.1c |
5.59±0.68 1.25±0.13c |
0.082±0.009 0.027±0.002 |
0.09±0.01 0.022±0.007c |
0.0925±0.02 0.015±0.003c |
|
|
|
|
|
|
|
5% ME |
M F |
77±1 44±6 c |
8.39±1.1 1.07±0.41c |
0.10±0.02 0.02±0.006c |
0.11±0.07 0.10±0.005b, c |
0.02±0.006 a 0.02±0.006 |
10% ME |
M F |
73±22 53±15 c |
7.2±0.41 3.09±2.3c |
0.09±0.026 0.05±0.029c |
0.10±0.01 a 0.09±0.02 |
0.022±0.005 a 0.014±0.004 |
20 % ME |
M F |
75±3 45±3 c |
7.35±0.27 1.48±0.24 c |
0.098±0.007 0.03±0.003c |
0.15±0.01 a 0.013±0.001 |
0.021±0.008 a 0.02±0.008 |
|
|
|
|
|
|
|
ZnO (100 µg/g) |
M F |
58±2 50±1 |
3.41±0.05 1.54±0.05 |
0.06±0.003 0.03±0.001 |
0.045±0.005 a 0.19±0.005 |
0.065±0.005 0.01±0.003 |
|
|
|
|
|
|
|
ZnCl2 (100 µg/g) |
M F |
66.5±1.5 49.9±0.5 |
5.16±0.24 1.5±0.05 c |
0.078±0.005 0.03±0.001c |
0.07±0.005 0.09±0.005b |
0.075±0.005 0.01±0.005c |
|
|
|
|
|
|
|
5% ME-ZnO |
M F |
69±10 47.5±1.5 c |
5.44±2.2 1.43±0.15c |
0.077±0.02 0.03±0.002 c |
0.065±0.005 0.14±0.001b,c |
0.11±0.07 0.025±0.01c |
5% ME-ZnCl2 |
M F |
69.5±5.5 49.5±4.5 c |
6.13±1.6 1.45±0.32c |
0.087±0.016 0.03±0.004c |
0.08±0.02 0.11±0.001b |
0.11±0.02 0.02±0.01c |
|
|
|
|
|
|
|
10% ME-ZnO |
M F |
80±1a 46±4 c |
7.4±0.93 1.28±0.2 c |
0.092±0.01 0.028±0.002c |
0.045±0.01a 0.07±0.03 b |
0.085±0.015 0.01±0.003 |
10% ME- ZnCl2 |
M F |
68.5±6.5 50±1 |
5.0±0.8 1.45±0.14c |
0.07±0.005 0.03±0.002c |
0.086±0.02 0.085±0.02b |
0.095±0.03 0.01±0.004 c |
|
|
|
|
|
|
|
20% ME-ZnO |
M F |
76.5±7.5a 48.5±1.5 c |
6.54±0.85 1.43±0.42c |
0.085±0.003 0.03±0.008c |
0.11±0.02 0.22±0.02b,c |
0.145±0.02 a 0.065±0.01c |
20% ME- ZnCl2 |
M F |
67.5±3.5 50.5±4.5 c |
5.09±0.72 1.87±0.34 |
0.075±0.007 0.037±0.003c |
0.08±0.03 0.28±0.003b,c |
0.095±0.005a 0.06±0.01 |
a. Significantly different from controls in males (treatment groups).
b. Significantly different from controls in females (treatment groups).
c. Significantly different between males and females (in the same treatment group).
Table 2 – Reproductive activity of fathead minnows exposed to ME.
Exposure |
Egg laying sites |
Eggs /day |
Ovipositor |
Nuptial tubercles/fat pad |
Coloration |
Controls |
3 |
2.3 |
Normal |
Normal |
Normal |
|
|
|
|
|
|
5% ME |
3 |
1.8 |
Normal |
Normal |
Normal |
10% ME |
5 |
7 |
Normal |
Normal |
Normal |
20% ME |
1 |
1 |
Normal |
Normal |
Normal |
|
|
|
|
|
|
ZnO (50 µg/L) |
0 |
0 |
Small (all) |
Normal |
Normal |
|
|
|
|
|
|
ZnCl2 (50 µg/L) |
0 |
0 |
Normal to Ssmall |
Normal |
Normal |
|
|
|
|
|
|
5% ME-ZnO |
1 |
0.76 |
Normal |
Normal |
Normal |
10% ME-ZnO |
6 |
11 |
Normal |
Normal |
Normal |
20 % ME-ZnO |
0 |
0 |
Normal |
Normal |
Normal |
|
|
|
|
|
|
5% ME-ZnCl2 |
4 |
2.1 |
Normal |
Normal |
Normal |
10% ME- ZnCl2 |
4 |
2.7 |
Normal |
Normal |
Normal |
20 % ME- ZnCl2 |
3 |
1.9 |
Normal |
Normal |
Normal |
Table 3 – Correlation matrix of fish physiological endpoints, biomarkers and carcass metal levels.
| |
Fork length |
Weight |
FC |
GSI |
HSI |
DNA |
LPO |
MT |
MET |
GST |
1A1 |
3A |
Fe |
Co |
Ni |
Cu |
Zn |
Ag |
Cd |
Fork length |
1 |
0.7 p<0.001 |
0.61 p<0.001 |
−0.05 p>0.1 |
0.77 p<0.001 |
−0.3 p<0.01 |
−0.3 p<0.01 |
−0.39 p<0.001 |
−0.19 p=0.1 |
−0.23 p<0.05 |
−0.22 p<0.05 |
0.07 p>0.1 |
−0.1 p>0.1 |
−0.08 p>0.1 |
−0.25 p<0.05 |
−0.32 p=0.01 |
−0.63 p<0.001 |
−0.09 p>0.1 |
−0.07 p>0.1 |
Weight |
|
1 |
0.98 p<0.001 |
−0.06 p>0.1 |
0.76 p<0.001 |
−0.46 p<0.001 |
−0.41 p<0.001 |
−0.38 p<0.001 |
−0.19 p=0.06 |
−0.28 p<0.1 |
−0.26 p=0.01 |
0.40 p<0.001 |
−0.07 p>0.1 |
−0.10 p>0.1 |
−0.24 p<0.05 |
−0.23 p<0.05 |
−0.39 p<0.001 |
−0.12 p>0.1 |
−0.05 p>0.1 |
FC |
|
|
1 |
−0.01 p>0.1 |
0.65 p<0.001 |
−0.46 p<0.001 |
−0.45 p<0.001 |
−0.33 p=0.001 |
−0.17 p>0.1 |
−0.26 p=0.01 |
−0.26 p=0.01 |
0.43 p<0.001 |
−0.05 p>0.1 |
−0. 1 p>0.1 |
−0.21 p<0.05 |
−0.2 p=0.05 |
−0.37 p<0.001 |
−0.12 p>0.1 |
−0.02 p>0.1 |
GSI |
|
|
|
1 |
0.27 p<0.01 |
0.45 p<0.001 |
0.07 p>0.1 |
0.27 p>0.01 |
0.25 p=0.01 |
−0.15 p>0.1 |
0.26 p=0.01 |
−0.15 p>0.1 |
0.49 p>0.001 |
0.61 p<0.001 |
0.47 p>0.001 |
0.55 p<0.001 |
0.15 p>0.1 |
0. 60 p<0.001 |
0.31 p<0.01 |
HIS |
|
|
|
|
1 |
−0.23 p<0.05 |
−0.16 p>0.1 |
−0.34 p<0.001 |
−0.06 p>0.1 |
−0.33 p=0.001 |
−0.23 p<0.05 |
0.05 p>0.1 |
−0.01 p>0.1 |
0.09 p>0.1 |
−0.04 p>0.1 |
−0.08 p>0.1 |
−0.42 p=0.001 |
0.08 p>0.1 |
−0.02 p>0.1 |
DNA |
|
|
|
|
|
1 |
0.03 p>0.1 |
0.25 p<0.01 |
0.05 p>0.1 |
−0.01 p>0.1 |
0.25 p=0.01 |
−0.38 p<0.001 |
0.19 p=0.05 |
0.26 p<0.01 |
0.18 p=0.1 |
0.28 p<0.01 |
0.06 p>0.1 |
0.29 p<0.01 |
0.19 p=0.06 |
LPO |
|
|
|
|
|
|
1 |
0.04 p>0.1 |
0.09 p>0.1 |
−0.03 p>0.1 |
−0.07 p>0.1 |
−0.13 p>0.1 |
−0.20 p<0.05 |
−0.13 p>0.1 |
−0.12 p>0.1 |
−0.16 p=0.1 |
0.18 p=0.07 |
−0.07 p>0.1 |
−0.17 p=0.08 |
MT |
|
|
|
|
|
|
|
1 |
0.68 p<0.001 |
0.65 p<0.001 |
0.64 p<0.001 |
−0.09 p>0.1 |
0.23 p<0.05 |
0.23 p<0.05 |
0.26 p=0.01 |
0.31 p<0.01 |
0.47 p<0.001 |
0.23 p<0.05 |
0.15 p>0.1 |
MET |
|
|
|
|
|
|
|
|
1 |
0.65 p<0.001 |
0.32 p<0.01 |
0.06 p>0.1 |
0.20 p<0.05 |
0.23 p<0.05 |
0.19 p=0.06 |
0.30 p<0.01 |
0.26 p<0.01 |
0.26 p=0.01 |
0.23 p<0.05 |
GST |
|
|
|
|
|
|
|
|
|
1 |
0.48 p<0.001 |
0.11 p>0.1 |
−0.003 p>0.1 |
−0.07 p>0.1 |
0.06 p>0.1 |
0.02 p>0.1 |
0.23 p<0.05 |
−0.07 p>0.1 |
−0.01 p>0.1 |
1A1 |
|
|
|
|
|
|
|
|
|
|
1 |
−0.07 p>0.1 |
0.23 p<0.05 |
0.23 p<0.05 |
0.16 p>0.1 |
0.31 p<0.01 |
0.38 p<0.001 |
0.24 p<0.05 |
0.16 p>0.1 |
3A |
|
|
|
|
|
|
|
|
|
|
|
1 |
−0.22 p<0.05 |
−0.31 p<0.01 |
−0.18 p=0.09 |
−0.28 p<0.01 |
0.07 p>0.1 |
−0.28 p<0.01 |
−0.22 p<0.05 |
Fe |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.96 p<0.001 |
0.75 p<0.001 |
0.66 p<0.001 |
0.13 p>0.1 |
0.94 p<0.001 |
0.73 p<0.001 |
Co |
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.76 p<0.001 |
0.72 p<0.001 |
0.11 p>0.1 |
0.97 p<0.001 |
0.71 p<0.001 |
Ni |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.64 p<0.001 |
0.28 p<0.01 |
0.74 p<0.001 |
0.66 p<0.001 |
Cu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.2 p=0.05 |
0.72 p<0.001 |
0.63 p<0.001 |
Zn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.1 p>0.1 |
0.17 p=0.08 |
Ag |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.73 p<0.001 |
Metal levels in fish carcasses were also determined (Table 4). Zinc levels were significantly affected by sex (factorial ANOVA: sex at p < 0.001). The difference in Zn in male versus female fish carcasses was significant: females contained more Zn than males. The difference increased when dietary ZnCl2 or nanoZnO was present in conjunction with municipal effluent. However, neither the municipal effluent nor the form of dietary Zn alone influenced the difference in Zn between males and females. Levels of total Zn in fish carcasses were significantly correlated with Ni (r = 0.28; p < 0.01) and copper (r = 0.2; p = 0.05). Levels of Ni were similar for both sexes in the control fish, but Ni was significantly increased in females by exposure to 20% municipal effluent, to dietary ZnCl2, and to 10 or 20% municipal effluent with dietary ZnCl2. In males, Ni was significantly increased by exposure to 10% municipal effluent and to dietary ZnCl2 only. For Cu in fish carcasses, both the municipal effluent/form of dietary Zn exposure treatment and sex were significant (sex*exposure treatment interaction). Copper levels were similar in male and female control fish. In females exposed to municipal effluent, Cu levels were significantly higher with exposure to 20% municipal effluent alone, to 20% municipal effluent with dietary nanoZnO, to 10% municipal effluent with dietary ZnCl2, and to 20% municipal effluent with dietary ZnCl2. In males, Cu levels were significantly increased by exposure to 10% municipal effluent with dietary ZnCl2 alone.
Table 4 – Metal levels in fish carcasses.
Exposure conditions |
Fe µg/g dry weight |
Co ng/g dry weight |
Ni ng/g dry weight |
Cu µg/g dry weight |
Zn µg/g dry weight |
Ag ng/g dry weight |
Cd ng/g dry weight |
Controls |
M: 12.7±4 F: 10.7±1 |
M: 30±10 F: 5±0.5 |
M: 19±4 F: 27±6 |
M: 1.2±0.08 F: 1.2±0.1 |
M: 38±6 F: 53±3 |
M: 0.4±0.01 F: 0.2±0.001 |
M: 10±3 F: 6±0.5c |
5% ME |
M: nd F: 6.1±1 |
M: 2±0.5 F:7±1 |
M: nd F: 17±0.1 |
M: nd F: 1.8±0.7 |
M: 25±1 F:46±10 |
M: nd F: 1±0.03 |
M: nd F: 6±2c |
10% ME |
M: 8.2±2 F: 18±3b,c |
M:9±2 F: 81±30b |
M: 11±0.5 F: 30±0.8 |
M: 0.98±0.3 F: 2.1±0.1c |
M: 42±7 F:59±20 |
M: 1.2±0.01 F: 3±1b |
M: 4.5±0.1a F: 8±0.2 |
20% ME |
M: 12±4 F: 20±2b,c |
M: 73±20a F:136±24b |
M:20±2 F: 75±5b,c |
M: 1.85±0.4 F: 4.4±0.8b,c |
M:28±1 F:59±15c |
M: 3.4±0.1a F:7±1b,c |
M: 5.5±2a F: 8±0.1 |
ZnO |
M: 8.4±1 F: 8.5±0.5 |
M: 6±1 F:5±0.5 |
M: 13±2 F: 32±10 |
M: 1.2±0.01 F: 1.25±0.15 |
M:34±0.3 F:52±8 |
M: 0.8±0.2 F: 0.4±0.2 |
M:5.5±0.1a F: 7±1 |
ZnCl2 |
M: 9±0.1 F: 9.3±1 |
M: 4±0.4 F:3.4±0.5 |
M: 12±2 F:60±20b,c |
M:1.3±0.25 F:1.2±0.15 |
M: 24±2 F:46±1 |
M: 0.6±0.1 F: 0.3±0.1 |
M: 8.4±1 F: 7±0.5 |
5% ME-ZnO |
M: 10±0.05 F: 10±0.05 |
M: 7.6±0.2 F: 7.6±0.2 |
M: 18±3 F: 18±3 |
M: 1.3±0.02 F: 1.3±0.2 |
M:38±3 F:38±5 |
M: 0.9±0.2 F: 0.9±0.1 |
M:6±0.2 F:6±0.2 |
10% ME-ZnO |
M: 10.6±0.5 F: 8.6±0.5 |
M: 6.5±0.01a F: 6.6±1 |
M: 11±0.5 F: 11±3 |
M: 1.5±0.1 F:1.9±0.8 |
M: 40±7 F: 65±10c |
M: 1±0.05 F:0.7±0.2 |
M: 7.7±0.4 F: 7.5±1 |
20% ME-ZnO |
M: 10.6±0.1 F: 10.5±0.1 |
M: 4±0.3a F: 17±2b |
M: 8±0.5 F: 25±4c |
M: 0.7±0.03 F: 2.9±0.6b,c |
M: 38±6 F:55±10 |
M: 0.1±0.03 F:0.8±0.05 |
M:4.8±0.1a F:8.4±1 |
5% ME-ZnCl2 |
M: 10.7±2a F: 11±0.8 |
M: 9±2a F: 15±2b |
M:26±10a F: 13±0.5c |
M: 1.2±0.1 F: 1.7±0.2 |
M: 30±4 F: 67±10c |
M: 0.6±0.1 F:0.6±0.2 |
M: 5±0.4a F:5±0.3 |
10% ME-ZnCl2 |
M: 23±0.4a F: 61±4b,c |
M: 157±8 F: 442±30 |
M: 68±20a F:122±2b |
M: 2.4±0.1a F:4.9±0.08b,c |
M: 29±2 F: 68±7c |
M: 7±0.1a F:24±3b,c |
M: 13±0.1 F:25±0.5b,c |
20% ME-ZnCl2 |
M: 14.5±3a F: 18±1b |
M: 73±25 F: 135±20 |
M: 18±3 F: 58±1b |
M: 2±0.1a F:4.4±0.5b.c |
M: 25±3 F: 53±9c |
M: 3.5±1a F: 7.3±0.5bc |
M: 8±1 F:10±1 |
a. significantly different in males; b. significantly different in females; c. significantly different between males and females in the same treatment group.
Xenobiotic biotransformation activity was estimated by measuring changes in MT levels and in cytochrome P4501A1 and P4503A4 and GST activity (Figures 1 to 4). For MT, a factorial ANOVA revealed that both sex and exposure treatment were significant, with no interaction between sex and exposure treatment. MT levels in the control fish did not differ between males and females. However, females responded more to municipal effluent exposure, form of dietary Zn and the combination of municipal effluent and form of dietary Zn. MT levels were significantly higher in fish exposed to 20% municipal effluent with dietary ZnCl2 but not with dietary nanoZnO. In females, MT was also significantly increased by exposure to dietary nanoZnO or ZnCl2 and municipal effluent. Significant differences between males and females were apparent in fish exposed to 20% municipal effluent, 5% municipal effluent with dietary nanoZnO, and 5 and 10% municipal effluent with dietary ZnCl2. Correlation analysis revealed that MT was significantly correlated with the condition factor (r = −0.33; p = 0.001), GSI (r = 0.28; p < 0.01), HSI (r = −0.34; p < 0.001), Fe (r = 0.22; p < 0.05), Co (r = 0.23; p < 0.05), Ni (r = 0.26; p = 0.01), Cu (r = 0.31; p < 0.01), Zn (r = 0.47; p < 0.001) and Ag (r = 0.23; p < 0.05). Cytochrome 1A1 activity was also examined (Figure 1B). A factorial ANOVA revealed a significant sex*exposure treatment interaction. In control fish, EROD activity in males and females were similar, but females responded more strongly to municipal effluent alone and in conjunction with either dietary nanoZnO or ZnCl2. Correlation analysis revealed that EROD activity was significantly correlated with MT (r = 0.64; p < 0.001), the condition factor (r = −0.26; p = 0.01), GSI (r = 0.26; p = 0.01), HSI (r = −0.23; p = 0.02), Fe (r = 0.23; p < 0.05), Co (r = 0.23; p < 0.05), Cu (r = 0.31; p < 0.01), Zn (r = 0.38; p < 0.001) and Ag (r = 0.24; p < 0.05). Cytochrome P4503A4 activity was also determined in fish liver (Figure 1C). A factorial ANOVA also revealed a sex*exposure treatment interaction. Cytochrome P4503A4 activity in control fish was similar between males and females but was higher in males exposed to municipal effluent alone, to dietary nanoZnO, and to municipal effluent with dietary nanoZnO. Correlation analysis revealed that cytochrome P4503A4 activity was positively correlated with the condition factor (r = 0.43; p < 0.001) and negatively correlated with the following metals: Fe (r = −0.22; p < 0.05), Co (r = −0.31; p < 0.01), Cu (r = −0.28; p < 0.01), Ag (r = −0.27; p < 0.01) and Cd (r = −0.22; p < 0.05). The xenobiotic conjugating activity of GST was also determined (Figure 1D). A factorial ANOVA revealed that sex and exposure treatment were significantly influenced (sex > exposure treatment), with no interaction between the factors. GST activity in control fish was similar between males and females, but females responded more strongly to combined exposure to municipal effluent and dietary nanoZnO and ZnCl2, with the exception of 10% municipal effluent with dietary nanoZnO, to which males responded more strongly than females did. Increased GST activity in females was also observed in fish receiving dietary ZnCl2. Correlation analysis revealed that GST activity was significantly correlated with the condition factor (r = −0.26; p = 0.01), HSI (r = −0.33; p = 0.001), MT (r = 0.65; p < 0.001), cytochrome P4501A1 (r = 0.48; p < 0.001) and Zn (r = 0.23; p < 0.05).
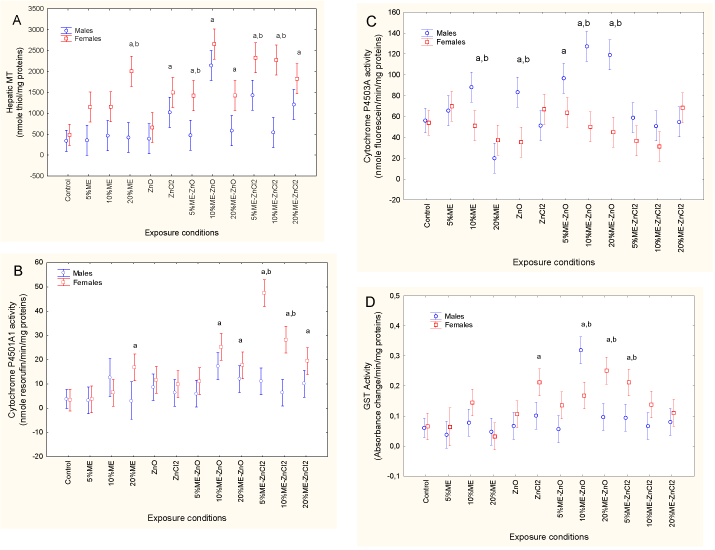
Figure 1. Xenobiotic metabolism in fish exposed to dietary nanoZn and ZnCl2 in the presence of municipal effluent.
The fish were analyzed for metallothionein (A), cytochrome P4501A (B) and 3A (C) activity and glutathione S-transferase activity (D). The letters a and b indicate a significant difference from the controls and between males and females.
Mitochondrial activity was determined in the livers of fish exposed to municipal effluent and fish receiving dietary nanoZnO and ZnCl2 (Figure 2). The fish were analyzed for mitochondrial electron transport activity. The letters a and b indicate a significant difference from the controls and between males and females.
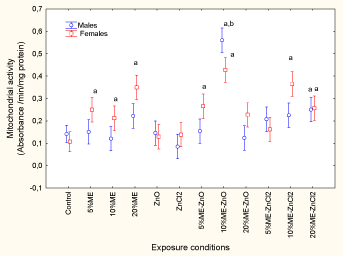
Figure 2. Change in mitochondrial electron transport activity in fish exposed to dietary nanoZnO or ZnCl2 in the presence of municipal effluent.
The results revealed that only the exposure concentration was significant, while sex-related effects were marginal (p = 0.06). MET activity was significantly increased by exposure to municipal effluent, whereas neither form of dietary Zn had an effect. MET activity was also elevated in fish exposed to municipal effluent and in those receiving dietary nanoZnO and ZnCl2. The strongest response was observed for 10% municipal effluent with dietary ZnCl2, which caused a 5.5- fold increase compared to the controls. Correlation analysis revealed that MET activity was significantly correlated with GSI (r = 0.26; p = 0.01), MT (r = 0.68; p < 0.001), GST (r = 0.65;p < 0.001), cytochrome P4501A1 activity (r = 0.32; p < 0.01), Fe (r = 0.2; p < 0.05), Co (r = 0.23; p < 0.05), Cu (r = 0.30; p < 0.01), Zn (r = 0.26; p < 0.01), Ag (r = 0.25; p = 0.01) and Cd (r = 0.23; p < 0.05). Tissue damage was monitored by determining LPO and DNA strand breaks (Figures 3A and 3B).
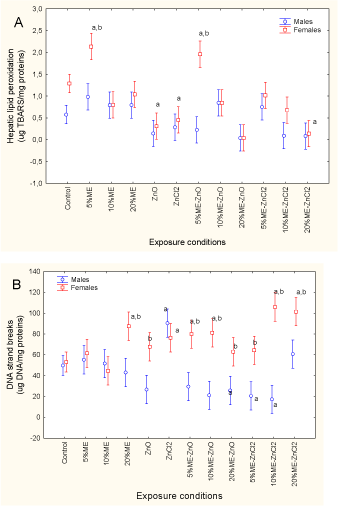
Figure 3. Oxidative damage and DNA strand breaks in fish exposed to dietary nanoZnO and ZnCl2 in the presence of municipal effluent.
The fish were analyzed for lipid peroxidation (A) and DNA strand breaks (B) in the liver. The letters a and b indicate a significant difference from the controls and between males and females.
LPO levels were significantly influenced by sex and exposure concentration, with no significant interactions between the two factors (sex > exposure treatment). In control fish, hepatic LPO was significantly higher in females than in males. Exposure to municipal effluent and both forms of dietary Zn reduced LPO levels in females, and this effect was also present in fish exposed to municipal effluent with either form of dietary Zn. Correlation analysis revealed that LPO was significantly correlated with the condition factor (r = −0.45; p < 0.001) and Fe (r = −0.2; p < 0.05). DNA strand breaks were significantly influenced by a sex*exposure treatment interaction. DNA strand breaks were identical in control fish, whereas exposure to municipal effluent for fish receiving both forms of dietary Zn increased DNA strand breaks in females. The strongest effects were observed with municipal effluent on fish receiving dietary ZnCl2, reaching a twofold increase in DNA strand breaks. Levels of DNA strand breaks in males did not differ from the control fish, except in fish receiving dietary ZnCl2 alone, for which levels were significantly increased. Correlation analysis revealed that DNA strand breaks were significantly correlated with the condition factor (r = −0.46; p < 0.001), GSI (r = 0.45; p < 0.001), HSI (r = −0.23; pp < 0.05), MT (r = 0.25; p < 0.01), cytochrome P4501A1 activity (r = 0.25; p = 0.01), cytochrome P4503A4 activity (r = −0.38; p < 0.001), Co (r = 0.26; p=0.01), Cu (r = 0.28; p < 0.01) and Ag (r = 0.29; p < 0.01).
To obtain an overall view of the cumulative impact of municipal effluent and dietary Zn forms on fathead minnows, factorial and discriminant function analyses were performed (Figure 4).
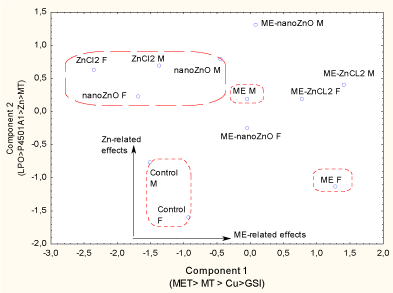
Figure 4. Discriminant function and factorial analysis of fish responses to dietary nanoZnO and ZnCl2 in the presence of municipal effluent.
The analyses were performed on male and female fish because significant sex-related effects were observed, especially for municipal effluent. The mean classification efficiency was 75%, and three clusters were identified: the controls, the municipal effluent group and the dietary Zn groups. The biomarkers shown in each axis were the biomarkers with the highest factorial weights.
The analysis considered the effects on males versus females, since sex often influenced responses. The control males and females formed a separate cluster (75% classification accuracy) from those of the exposure treatments with municipal effluent alone, either form of dietary Zn, and dietary Zn in conjunction with municipal effluent. The first component was municipal effluent–related effects and a factorial analysis revealed that the following endpoints had the highest factorial weights: MET > MT > Cu > GSI. The second (y‑axis) component was more closely related to the forms of dietary Zn , and factorial analysis revealed that the following endpoints had the highest factorial weights: LPO > P4501A1 > Zn > MT. The forms of Zn in the food formed a distinct cluster, suggesting a common mode of action although sex-related differences were more significant with nanoZnO-supplemented food. Sex-related effects were also more apparent in the group exposed to municipal effluent alone: responses seen in females were more pronounced than those seen in males. The combination of municipal effluent and either dietary nanoZnO or ZnCl2 formed an intermediate cluster between the municipal effluent and form of dietary Zn clusters, though it was closer to the municipal effluent group, suggesting that municipal effluent had a stronger influence than the form of dietary Zn.
In this study, fish received a daily intake of 3.3 µg of dietary Zn, which resulted in Zn tissue levels that did not differ from control fish (38 ± 6 and 53 ± 5 µg/g tissue in males and females respectively) when presented alone. However, hepatic MT levels increased threefold in fish receiving dietary ZnCl2, though not in those receiving dietary nanoZnO, suggesting that no significant release of Zn from nanoparticles occurred during ingestion and that the low concentration of Zn intake was processed by the normal metabolism of the fish. The reported lethal concentration (LC50) was approximately 3.1 ± 0.4 mg/L for nanoZnO and 1.2 ± 0.5 mg/L for ZnCl2 [27,28]. In another study, the Zn concentration that could block population growth was approximately 80 µg/L (5% of the lethal concentration) in fathead minnows [29]. This concentration is in the same order of magnitude as reported Zn concentrations in municipal effluent: between 3 and 72 µg/L of total Zn in municipal effluent [30]. The presence of dissolved organic matter (DOM) has been found to reduce the toxicity of Zn: DOM concentrations greater than 11 mg/L significantly reduced toxicity in fathead minnows [28]. The highest municipal effluent concentration contained approximately 16 mg DOM/L, which suggests that this fraction could likely modulate Zn availability in fish, though the 5 and 15% v/v concentrations could not. However, the fish had received Zn-spiked food, which was independent of the surface water contact route. NanoZnO can adsorb to organic matter and reduce the microbial toxicity of sludge leachates, suggesting a possible interaction between nanoparticles and exposure to municipal effluent in fish [31]. However, this was not the case with titanium dioxide nanoparticles, which increased the toxicity of sludge leachates.
The nature of the interactions of nanoZnO and ZnCl2 with the organic matter in municipal effluent and food matrices is not well understood at this time. In the present study, the following biomarker responses were enhanced by dietary nanoZnO in the presence of municipal effluent: increased cytochrome P4503A activity (males), increased GST and MET activity. For fish exposed to dietary ZnCl2 in the presence of municipal effluent, the following biomarkers were influenced: decreased LPO and GST, increased MT (females) and cytochrome P4501A activity. These biomarkers were therefore more sensitive to either dietary nanoZnO or ZnCl2 in the presence of municipal effluent. The interaction between ZnCl2 and the organic matrix is expected to be ionic in nature, while the interaction of nanoZnO with the organic matrix would, in addition to the ionic binding of positively charged Zn at the surface, occur via hydrogen bonding or hydrophobic interactions through the neutral oxides at the surface of the nanoparticles. Indeed, nanoZnO treated with oleic acids, poly(methacrylic acid) or other components from nutritional cell culture media significantly reduced the production of reactive oxygen species and reduced cytotoxicity in vitro [32]. This is consistent with the observation that cationic surfaces are more toxic than anionic or neutral ones [33]. The ionic interaction of ZnCl2 with organic matter/colloid could displace other metals, which can contribute to the observed elevation of MT in the presence of municipal effluent. The antioxidant properties of released Zn2+ could also explain the reduced levels of LPO and GST in fish exposed to municipal effluent with dietary ZnCl2 [34]. In the case of municipal effluent with dietary nanoZnO, nanoZnO is difficult to degrade in freshwater at pH 7.6 [35] and bulk ZnO microparticles and ZnCl2 are toxic to freshwater algae (60 µg/L). This is consistent with multivariate statistical analysis, which shows that the observed responses between dietary ZnCl2 and nanoZnO were similar, at the least in part. Because of other nanoZnO interactions besides ionic binding of Zn, the release of polar compounds by hydrogen bonding could be related to increased cytochrome P4503A activity, which is involved in the metabolism of polar cyclic aliphatic hydrocarbons [36]. Although nanoZnO toxicity is mitigated by antioxidants such as vitamins C and E [27] no oxidative damage (LPO) was found in the present study in fish that received either dietary nanoZnO or ZnCl2 with or without exposure to municipal effluent. LPO was increased only 1.8-fold in females at the lowest municipal effluent concentration alone and in conjunction with dietary nanoZnO. This indicates that the applied concentration of Zn in fish food either was not bioavailable or was low enough to be processed by normal fish physiology. NanoZnO has been shown to be transferable through diet in fish fed with contaminated Daphnia magna [37] Another explanation could be that Zn was localized in other organs, such as the gills, liver and digestive system, instead of the fish carcass. Combined exposure to municipal effluent and the forms of Zn increased Zn tissue levels in females compared to males. The accumulation of Zn during egg production in female squirrelfish has been previously demonstrated, with Zn mobilization in the liver and oocytes [38] Vitellogenin, the egg yolk protein precursor, is a Zn-binding protein and could represent one means of Zn transport to and accumulation in the ovary. Hence, a positive interaction exists between exposure to Zn as either nanoZnO and ZnCl2 in the presence of an estrogenic effluent, where females exposed to municipal effluent would have higher levels of Zn than males would. The increasing levels of Zn in female fish can be enhanced by the estrogenic properties of the municipal effluent, which could induce vitellogenin, a protein that binds Zn, without necessarily leading to egg production. This is consistent with a previous study in which the same municipal effluent caused induction of estrogen-signaling pathways in fathead minnows [9].
In conclusion, the toxicity of dietary nanoZnO 2021 Copyright OAT. All rights reserved by the presence of municipal effluent. On the whole, nanoZnO and ZnCl2 were best characterized by LPO, cytochrome P4501A1 activity, tissue Zn and MT levels, suggesting that some of the nanoZnO effects were mediated by the release of Zn cations. The effects of municipal effluent were more dependent on sex, with females responding more strongly to MET activity, MT, Cu tissue levels and GSI. The cumulative effects of municipal effluent and dietary nanoZnO were best explained by changes in MT, cytochrome P4503A activity, GST and MET, while the cumulative effects of municipal effluent and dietary ZnCl2 were best explained by decreased LPO and GST activity, increased MT in females and cytochrome P4501A activity. It appears that municipal effluent lead to more significant sex-dependent impacts, while the effects of dietary ZnCl2 and nanoZn were least related to sex-related impacts.
This project was funded under the St. Lawrence Action Plan and Chemical Management Plan of Environment Canada. We would like to thank Patrick Cejka (in memoriam) of the Montreal Wastewater Treatment Plant for his contribution in relation to the fathead minnow exposure laboratory. We would also like to thank Nicholas Siron for his technical assistance.
- Hadioui M, Leclerc S, Wilkinson KJ (2013) Multimethod quantification of Ag+ release from nanosilver. Talanta 105: 15-19. [Crossref]
- Kundu P, Anumol EA, Ravishankar N (2013) Pristine nanomaterials: synthesis, stability and applications. Nanoscale 5: 5215-5224. [Crossref]
- Shi LE, Li ZH, Zheng W, Zhao YF, Jin YF, et al. (2014) Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31: 173-186. [Crossref]
- Li M, Pokhrel S, Jin X, Mädler L, Damoiseaux R, et al. (2011) Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nanoparticles in aquatic media. Environ Sci Technol 45: 755-761. [Crossref]
- Gagné F, Auclair J, Peyrot C, Wilkinson KJ (2015) The influence of zinc chloride and zinc oxide nanoparticles on air-time survival in freshwater mussels. Comp Biochem Physiol C Toxicol Pharmacol 172-173: 36-44. [Crossref]
- Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, et al. (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44: 1962-1967. [Crossref]
- Holeton C, Chambers P A, Grace L (2011) Wastewater release and its impacts on Canadian waters. Can J Fish Aquat Sci 68:1836-1859.
- Leet JK, Sassman S, Amberg JJ, Olmstead AW, Lee LS, et al. (2015) Environmental hormones and their impacts on sex differentiation in fathead minnows. Aquat Toxicol 158: 98-107. [Crossref]
- Arstikaitis J, Gagné F, Cyr DG (2014) Exposure of fathead minnows to municipal wastewater effluent affects intracellular signaling pathways in the liver. Comp Biochem Physiol C Toxicol Pharmacol 164: 1-10. [Crossref]
- Tetreault GR, Bennett CJ, Cheng C, Servos MR, McMaster ME (2012) Reproductive and histopathological effects in wild fish inhabiting an effluent-dominated stream, Wascana Creek, SK, Canada. Aquat Toxicol 110-111: 149-61. [Crossref]
- Lajeunesse A, Gagnon C, Gagné F, Louis S, Cejka P, et al. (2011) Distribution of antidepressants and their metabolites in brook trout exposed to municipal wastewaters before and after ozone treatment--evidence of biological effects. Chemosphere 83: 564-571. [Crossref]
- Gagné F, Fournier M, Blaise C (2004) Serotonergic effects of municipal effluents: Induced spawning activity in freshwater mussels. Fresen Environ Bull 13:1099-1103.
- Lacaze E, Devaux A, Bony S, Bruneau A, André C, et al. (2013) Genotoxic impact of a municipal effluent dispersion plume in the freshwater mussel Elliptio complanata: an in situ study. J Xenobiotics 3(s1):14-16.
- Bianchi V A, Rocchetta I, Luquet C (2014) Biomarker responses to sewage pollution in freshwater mussels (Diplodon chilensis) transplanted to a Patagonian river. J Environ Sci Health Part A, 49:1276-1285.
- Liu J, Lu G, Zhang Z, Bao Y, Liu F, et al. (2015) Biological effects and bioaccumulation of pharmaceutically active compounds in crucian carp caged near the outfall of a sewage treatment plant. Environ Sci Process Impacts 17: 54-61. [Crossref]
- Gagné F, Smyth SA, André C, Douville M, Gélinas M, et al. (2013) Stress-related gene expression changes in rainbow trout hepatocytes exposed to various municipal wastewater treatment influents and effluents. Environ Sci Pollut Res Int 20: 1706-1718. [Crossref]
- King F, Packard T T (1975) Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol Oceanog 20:849-854.
- Gagné F, Blaise C, André C, Salazar M (2006) Effects of pharmaceutical products and municipal wastewaters on temperature-dependent mitochondrial electron transport activity in Elliptio complanata mussels. Comp Biochem Physiol C Toxicol Pharmacol 143: 388-393. [Crossref]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254. [Crossref]
- Martín-Díaz M L, Gagné F, Blaise C (2008) The use of biochemical responses to assess ecotoxicological effects of Pharmaceutical and Personal Care Products (PPCPs) after injection in the mussel Elliptio complanata. Environ Toxicol Pharmacol 28:237-242.
- Quinn B, Gagné F, Blaise C (2004) Oxidative metabolism activity in Hydra attenuata exposed to carbamazepine. Fresen Environ Bull 13:783-788.
- Boryslawskyj M, Garrood A C, Pearson JT (1988) Elevation of glutathione-S-transferase activity as a stress response to organochlorine compounds, in the freshwater mussel, Sphaerium corneum. Mar Environ Res 24:101-104.
- Wills ED (1987) Evaluation of Lipid Peroxidation in Lipids and Biological Membranes. In: Snell K, Mullock B (Eds.), Biochemical Toxicology: A Practical Approach. Washington, USA: IRL Press: 127.
- Olive PL (1988) DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen 11: 487-495. [Crossref]
- Bester MJ, Potgieter HC, Vermaak WJ (1994) Cholate and pH reduce interference by sodium dodecyl sulfate in the determination of DNA with Hoechst. Anal Biochem 223: 299-305. [Crossref]
- Gagné F (2014) Chapter 8: Cellular Energy Allocation. In Biochemical Ecotoxicology:- Principles and Methods. 1st Edition. USA: Elsevier Inc :131-144.
- Alkaladi A, El-Deen NA, Afifi M, Zinadah OA (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22: 556-563. [Crossref]
- Bringolf RB, Morris BA, Boese CJ, Santore RC, Allen HE, et al. (2006) Influence of dissolved organic matter on acute toxicity of zinc to larval fathead minnows (Pimephales promelas). Arch Environ Contam Toxicol 51: 438-444. [Crossref]
- Iwasaki Y, Hayashi TI, Kamo M (2010) Comparison of population-level effects of heavy metals on fathead minnow (Pimephales promelas). Ecotoxicol Environ Saf 73: 465-471. [Crossref]
- Clara M, Windhofer G, Weilgony P, Gans O, Denner M, et al. (2012) Identification of relevant micropollutants in Austrian municipal wastewater and their behaviour during wastewater treatment. Chemosphere 87: 1265-1272. [Crossref]
- Jośko I, Oleszczuk P (2013) The influence of ZnO and TiO2 nanoparticles on the toxicity of sewage sludges. Environ Sci Process Impacts 15: 296-306. [Crossref]
- Yin H, Casey P S, McCall M J, Fenech M (2010) Effects of surface chemistry on cytotoxicity, genotoxicity, and the generation of reactive oxygen species induced by ZnO nanoparticles. Langmuir, 26:15399-15408.
- Zheng H, Mortensen LJ, DeLouise LA (2013) Thiol antioxidant-functionalized CdSe/ZnS quantum dots: synthesis, characterization, cytotoxicity. J Biomed Nanotechnol 9: 382-392. [Crossref]
- Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol 146: 443-459. [Crossref]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, et al. (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41: 8484-8490. [Crossref]
- Stresser D M, Blanchard A P, Turner S D, Erve J C, Dandenau AA, et al.(2000) Substrate-dependent modulation of CYP3A4 catalytic activity: analysis of 27 test compound with four fluorometric substrates. Drug Metab Dispos 28:1440-1448
- Skjolding LM, Winther-Nielsen M, Baun A3 (2014) Trophic transfer of differently functionalized zinc oxide nanoparticles from crustaceans (Daphnia magna) to zebrafish (Danio rerio). Aquat Toxicol 157: 101-108. [Crossref]
- Thompson ED, Mayer GD, Balesaria S, Glover CN, Walsh PJ, et al. (2003) Physiology and endocrinology of zinc accumulation during the female squirrelfish reproductive cycle. Comp Biochem Physiol A Mol Integr Physiol 134: 819-828. [Crossref]




