Abstract
Introduction
Radiation recall dermatitis (RRD) represents the recalling of radiation exposure similar in appearance to that of an acute radiation reaction in a previously radiated field. Radiation recall dermatitis is a rare skin reaction. Several chemotherapeutic agents may trigger it. The case in the study had breast cancer, which developed RRD. Our case that is caused by 5-Flourouracil (5FU) use is the second reported one in the literature.
Case presentation
The patient was a 40-year-old female. After 6 courses epirubicin + cyclophosphamide treatment, bone metastesis was observed in lumbar 3 ( L3) vertebra and RT was applied to that area.
8 months after RT, new metastatic lesions were observed. 5-FU chemotherapic medication was administered to these new lesions and the patient was admitted to our clinic with several complaints, such as pain in the skin on the back, erythema, and edema in the previously radiated field 8 months ago. These complaints were similar in appearance to an acute radiation skin reaction. A punch biopsy taken was consistent with dermatitis. The complaints and symptoms were recovered with topical therapy.
Conclusion
Radiation Recall Dermatitis is rare. Possible reoccurrence of skin reactions in the radiotherapy area must be kept in mind in the patients applied radiotherapy when chemotherapy is applied months or years later.
Key words
radiation recall dermatitis, 5-fluorouracil chemotherapy, radiotherapy
Introduction
“Radiation recall dermatitis” (RRD) is a rare condition that occurs after the administration of chemotherapeutics or other medications at sites where radiotherapy (RT) has been previously applied. It can be also defined as an acute inflammatory reaction, which can be seen either at an early stage after RT or years later [1]. Here, we report a case with breast cancer, which developed RRD after 5-fluorouracil (5-FU) administration.
Case presentation
A segmental mastectomy was performed in a 40-year-old Turkish woman for treatment of a right breast mass in April 2010. The pathology was consistent with an invasive ductal carcinoma. The tumor had a radius of 8 mm, histological grade 2, and nuclear grade 2. The in situ component at surgery was reported to be less than 1 mm in a 3-mm area. A modified radical mastectomy was performed for a month in May 2010 because the tumor was less than 2 mm. The pathological examination showed that there was an invasive ductal carcinoma, and the tumor had a radius of 14 x 10 mm, histological grade 2, one of 31 axillary lymph nodes was metastatic and its radius was 4 mm. Fluorescent in situ hybridization was negative for HER2, and the tumor was ER(+).
In post-operative bone scintigraphy, increased activity in the bottom corner of the left acetabulum and on the left side of the lumbar 3 (L3) vertebra corpus was reported, which is consistent with metastasis. Following two doses of epirubicin + cyclophosphamide (EC) chemotherapy (CTX), palliative 300 cGy/fraction and 30 Gy external RT was applied to the localized part of the L3 vertebra (Figure 1) and the left pubic ramus + acetabulum. Subsequently, four doses of EC CTX were administered. For a total of six, there were no significant adverse effects. Biphosphonate treatment was applied. In magnetic resonance imaging (MRI) taken 8 months before RT, new metastatic lesions were detected on the right acetabulum posterior arm, and oral 5-FU was administered in March 2010. In the second month of the 5-FU treatment, the patient developed erythema, and edema which were consistent with dermatitis in the radiotherapy area on the back during physical examination. The patient with redness and pain at the waist was admitted to our clinic (Figure 2A); a punch biopsy was taken from this area because it was suspected to be RRD. Topical steroid treatment was applied. A histopathological examination revealed hyperkeratosis in the epidermis, keratin cap formation, extension in rete ridges, and irregular connection acanthosis, venous endothelium reactions, mild perivascular chronic inflammatory cell infiltration, and coarsening in the collagen of the dermal tissue (Figure 3). MRI was performed to the patient, and lesions belonging to the former metastasis in the L3 vertebra and a signal alteration consistent with inflammation in the subcutaneous soft tissue were observed. After topical corticosteroid treatment and drug cessation, the patient’s complaint eased and the lesions were seen to regress on physical examination (Figure 2B).
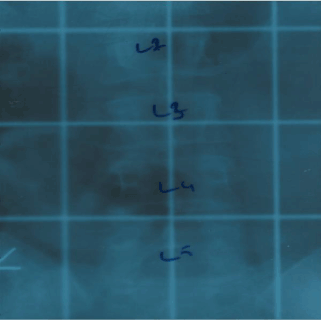
Figure 1. Radiation therapy site
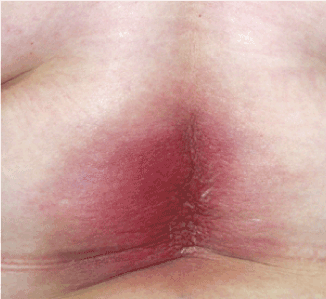
Figure 2A. Clinical findings in the lumbar area: diffuse erythema and edema are visible.
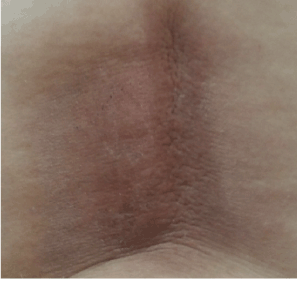
Figure 2B. Decreased erythema and edema following local steroid therapy
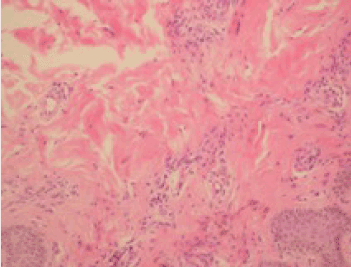
Figure 3. Histopathological examination showing hyperkeratosis in the epidermis, keratin cap formation, extension in rete ridges and irregular connection acanthosis, venous endothelium reactions, mild perivascular chronic inflammatory cell infiltration, and coarsening in the collagen of the dermal tissue (hematoxylin and eosin, ×40).
Discussion
Radiation recall dermatitis (RRD) was first defined as an unknown cause and as a dactinomycin-dependant reaction by D’Angio et al. in 1959 [2]. Radiation recall reactions are common in the skin, which comprises around two-thirds of cases. RRD is usually of mild or moderate intensity, but it can be severe in some instances (10%) [3].
Because the exact mechanism that triggers recall dermatitis has not been determined, a number of different hypotheses have been proposed, but there is little evidence. One of the hypotheses suggests a mechanism on venous damage, epithelial stem cell deficiency or oversensitivity, and increased sensitivity to the medication. Cytotoxic drugs are the most common cause. The most common chemotherapeutic agents causing RRD include docetaxel, doxorubicin, gemcitabine, and paclitaxel, but medications such as actinomycin, methotrexate, hydroxyurea, bleomycin, cyclophosphamide, 5-FU, and tamoxifen have also been reported [1] (Table 1). In our case, 5-FU use is consistent with the literature. RRD with antibiotic medication use, especially with fluoroquinolones and azythromycin, has been also reported [4].
Drugs |
Frequency |
|
(n) |
(%) |
Docetaxel |
10 |
13 |
Doxorubicin |
10 |
13 |
Gemcitabine |
8 |
11 |
Paclitaxel |
8 |
11 |
Trimetrexate |
5 |
7 |
Methotrexate |
4 |
5 |
Hydroxyurea |
4 |
5 |
Tamoxifen |
4 |
5 |
Dactinomycin |
2 |
3 |
Vinblastine |
2 |
3 |
Other |
18 |
24 |
Table 1. Drugs Reported to İnduce Radiation Recall Dermatitis (n = 75) [6]
Depending on the sever2021 Copyright OAT. All rights reserve characterized by one or more dermatological symptoms, ranging from mild rash, dry desquamation, and/or pruritus to symptoms that are increasingly painful, such as swelling/edema, vesicles, maculopapular eruptions, and papules. In the most severe cases, ulceration and skin necrosis can occur [3].
The severity of RRD can be graded according to the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). Radiation recall was previously listed separately in the CTCAE, but in the most recent version (v. 3), it was merged into the category “Rash: dermatitis associated with radiation”. According to the criteria, our case is grade 2.
RRD caused by the use of 5-FU was first reported by Von Essen et al. in 1963. First, a dose of 15 mg/kg/day of 5-FU was administered for 4 days, and then 30 Gy RT was applied. Medication was initially administered 7 weeks after RT, and then a skin reaction was observed 2 weeks later [5].
Our case is apparently the second reported case of RRD caused by use of the oral 5-FU analog, capecitabine. Most recall reactions occur when RT and CTX treatment periods are less than 2 months [6]. The ideal difference in the period of time between RT and CTX is unknown; however, it is accepted that generally more severe symptoms are experienced in cases where the period of time between RT and the initiation of medication is short [1]. The time period between RT and CTX is indeed short in many cases but has been reported as longer than 7 weeks in 27% of cases. The median period of time between RT and CT has been estimated to be 39 days [6,7]. The period in our case was 8 months. In one case, it was reported that recall dermatitis developed 15 years after medication, and the longest period of recall dermatitis formation was 7 years [8,9].
Steroids and non-steroidal anti‑inflammatory drugs (NSAIDs) are administered in therapy although the mechanism is not known well. The response to this therapy is generally good [10]. In another case study, an antioxidant ointment (Mapisal(®) was reported to be used for treatment successfully [11]. 5-FU was stopped in our case because another agent may be used after stopping the chemotherapeutic agent causing skin reaction. Also, in our case, corticosteriods and NSAIDs were administered in the treatment and the reactions were observed to regress.
Conclusion
Our case is the first RRD one, after the case in 1963, triggered by 5-FU chemotherapeutic medication. If all the patients treated with RT previously are given CTX, RRD must be kept in mind in the skin lesions occurring in RT area. In the treatment, local steroids can be used. The literature needs more studies including more cases in order to make treatment and mechanism clear.
A written consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Camidge R, Price A (2001) Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol 59: 237-245. [Crossref]
- D'Angio GJ, Farber S, Maddock CL (1959) Potentiation of x-ray effects by actinomycin D. Radiology 73: 175-177. [Crossref]
- Burris HA 3rd, Hurtig J (2010) Radiation recall with anticancer agents. Oncologist 15: 1227-1237. [Crossref]
- Vujovic O (2010) Radiation recall dermatitis with azithromycin. Curr Oncol 17: 119-121. [Crossref]
- Vonessen CF, Kligerman MM, Calabresi P (1963) Radiation and 5-Fluorouracil: A Controlled Clinical Study. Radiology81: 1018-1027. [Crossref]
- Hird AE, Wilson J, Symons S, Sinclair E, Davis M, et al. (2008) Radiation recall dermatitis: case report and review of the literature. Curr Oncol 15: 53-62. [Crossref]
- Putnik K, Stadler P, Schäfer C, Koelbl O (2006) Enhanced radiation sensitivity and radiation recall dermatitis (RRD) after hypericin therapy -- case report and review of literature. Radiat Oncol 1: 32. [Crossref]
- Burdon J, Bell R, Sullivan J, Henderson M (1978) Adriamycin-induced recall phenomenon 15 years after radiotherapy. JAMA 239: 931. [Crossref]
- Mayer EG, Poulter CA, Aristizabal SA (1976) Complications of irradiation related to apparent drug potentiation by adriamycin. Int J Radiat Oncol Biol Phys1: 1179-1188. [Crossref]
- Mizumoto M, Harada H, Asakura H, Zenda S, Fuji H, et al. (2006) Frequency and characteristics of docetaxel-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys 66: 1187-1191. [Crossref]
- Duncker-Rohr V, Freund U, Momm F (2014) Radiation recall dermatitis after docetaxel chemotherapy. Treatment by antioxidant ointment. Strahlenther Onkol 190: 491-493. [Crossref]




