Abstract
Anatomical factors that narrow the upper airway predisposes for obstructive sleep apnea (OSA). Orthognathic surgery causes changes in the pharyngeal airway and a successful long-term treatment requires an understanding of all variables. This paper aims to describe the pharyngeal anatomy, factors that affects its dimension and configuration, discuss imaging of the pharynx with focus on computed tomography as well as normative volumetric data for healthy adults by 3-dimensional (3D) analysis.
The literature search was performed in PubMed with following MeSH terms: "pharynx", “diagnostic imaging", “tomography, x-ray computed”, "imaging, three-dimensional" and "adult” together with “upper airway” and “pharyngeal airway”. Articles evaluating the upper airway in relation to a surgical procedure or to specific disease were excluded. A total of 46 articles were included and analyzed.
As a fibromuscular tube, the size and shape of the pharynx is related to its mechanical intrinsic properties, surrounding anatomical structures and dynamic patient dependent factors, including breathing, gravity and postural changes, as well as technical aspects regarding imaging acquisition, processing and analysis by software. Even though 3D imaging allows cross-sectional and volumetric measurements, the current literature cannot present a consistent normative dimensional reference for the pharyngeal airway.
Airway size should be considered when planning orthognathic surgery in order to minimize negative side-effects. 3D imaging might provide a range of information concerning pharyngeal size and shapes that can be used to analyze what type and extension of surgical movement is necessary to optimize the treatment. A standard protocol for 3D assessment of the pharyngeal airway is still missing and clinical research with good quality and standardized criteria are needed.
Key words
pharyngeal airway, imaging, computed tomography, cone-beam computed tomography, obstructive sleep apnea, orthognathic surgery
Introduction
The upper airway is an anatomical structure with complex functions related to respiration, deglutition, and phonation. For decades, the orthodontists studied the relationship between upper airway, mode of breathing and dentofacial development [1]. Assessment of the size and shape of the upper airway continues to be an issue of increasing interest because its relation with craniofacial morphology and OSA [2,3].
Gold standard method for analysis of craniofacial development has been cephalometry, with linear and angular measurements made on lateral cephalograms. However, to represent the three-dimensional (3D) structures in two dimensions can overlook much of the anatomical information [4,5]. Now with the increased availability of 3D imaging, namely conventional computed tomography ("multi-slice" CT) or digital volume tomography ("cone-beam computed tomography", CBCT) data on the axial cross-sectional area and total volume can be determined and consistently contribute to a better understanding of the upper airway anatomy and physiology [1,4-7].
Recently, several studies have made a 3D analysis of the upper airway and evaluated its relationship with a surgical intervention or related to a specific disease. In the field of the maxillofacial specialty, orthognathic surgery intends to reestablish the facial harmony by correcting craniofacial anomalies and malocclusions [3]. Beside the esthetic benefit, the surgical movement of the maxilla and/or mandible also results in modifications of the surrounding soft tissues and muscles leading to positive or negative changes in the pharyngeal airway dimension [3,8-11]. Anatomical factors that narrow the upper airway predisposes to obstructive sleep apnea (OSA) and these may include mandibular or bimaxillary retrognathia [8]. The surgical maxillomandibular advancement in OSA patients has been proven to be an effective treatment by enlargement of the pharyngeal airspace, specifically at the most constricted area [8-11]. On the other hand, treatment of prognatism by means of mandibular or maxillary setback narrows the pharyngeal airway and can therefore predispose to breathing sleeping disorders in the perspective of a lifetime [3,10].
Currently, only a few studies describe normative volumetric data of the upper airway in individuals without suspected respiratory pathology. Allied with the technological development and availability of 3D imaging, the evaluation of upper airway dimension and configuration becomes important and increasingly relevant for orthognathic surgery planning and follow-up, diagnosis and treatment of OSA or other conditions that may cause reduced airflow. A successful long-term treatment within the maxillofacial and orthodontic specialty requires an understanding of all functional variables, including the upper airway [12].
The aim of this paper is to describe the anatomy of pharynx, various imaging methods and to describe the measuring process of the pharyngeal airway with focus on computed tomography. Further aims are to discuss factors that affect the size and shape of the pharynx, and finally give an overview on the scientific studies doing a 3D analysis of the upper airway in order to provide normative volumetric data for healthy adults.
Materials and Methods
The search started in PubMed database with the words “pharynx”, “upper airway”, pharyngeal airway” and "diagnostic imaging", both separately and in combination, which gave a large number of articles. Then MeSH terms were used, chosen for the purpose of literature study: "pharynx", “diagnostic imaging", “tomography, x-ray computed”, "imaging, three-dimensional" and "adult”. These MeSH terms together with “upper airway” and “pharyngeal airway” were first searched separately and then successful in combination. Filters were added, articles related to humans and written in English. The PubMed database search was performed and updated on 7th January 2018.
Of the total amount of articles, those evaluating the upper airway in relation to a surgical procedure or to specific disease were excluded by title or abstract. The search was then complemented with other articles, referred in the articles reviewed in full text, since they seemed relevant to better understand the subject in matter. Finally, the search was complemented with books, in written and electronic format. A flowchart for literature search is summarized in figure 1.
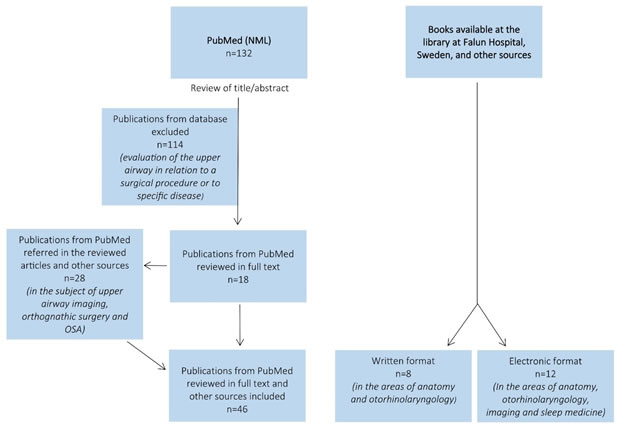
Figure 1. Flowchart for literature search.
Results
Pharynx: definition, anatomical boundaries and function
The pharynx is the connection between the nasal and oral cavity down to the larynx and esophagus [17]. It is therefore involved in the life-sustaining functions, breathing and swallowing, and belongs to both the respiratory and digestive organs [14,17]. Through the pharynx air and food has to pass separately, to avoid that air enters into the stomach and, more important, to avoid that food enters into the airway. Pharynx is active during speech, coughing, vomiting and gagging and has, therefore, a complex function which requires coordination of a variety of muscles and nerves [17].
The pharynx, forms a vertical, 12-15 cm long, funnel-shaped fibromuscular tube. It extends from the base of the skull to the level of the cricoid cartilage anteriorly and the lower limit of the sixth cervical vertebra posteriorly [14,15,18,19]. The pharynx is build-up of soft tissue: innermost mucosa, outside it a muscle membrane, and outmost a layer of connective tissue [16,17,20]. It’s anteriorly in open communication with the nasal cavity, oral cavity and larynx entrance. Laterally and posteriorly, is surrounded by connective tissue potential spaces (spatium parapharyngeum and retropharyngeum) allowing the pharynx easily to be displaced up and down by e.g. swallowing [20]. The width of the pharynx varies constantly as it is dependent on muscle tone. During sleep, muscle tone is low, which reduces the dimensions significantly. A consequence of this can be snoring and sleep apnea [19]. The muscles can be divided into two layers, the outer three circular constrictors (m. Constrictor pharyngis superior, medius and inferior) forming a ring that is incomplete anteriorly and the inner three vertically oriented muscles (m. Stylopharyngeus, m. Salpingopharyngeus and m. Palatopharyngeus) [16,21,22]. The pharynx is divided into the nasopharynx (also called epipharynx), oropharynx and hypopharynx (also called laryngopharynx) [13-19] figure 2.
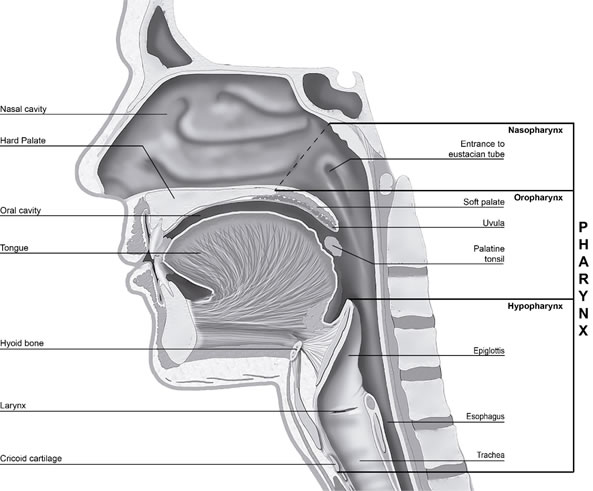
Figure 2. Image illustrating the regions of the pharynx in sagittal plane.
The nasopharynx has primarily a respiratory function. It is limited anteriorly by the both choanae (apertura nasalis posterior) and nasal septum back edge (vomer), superiorly by the base of the skull (os occipitale and sphenoidale), posteriorly by the cervical spine and inferiorly by the soft palate, that is raised and closes towards the posterior wall of the pharynx when swallowing [14,20,23,24]. The nasopharynx is quite rigid (except the floor, soft palate) and therefore can never collapse because of muscle activity, unlike the oro- and hypopharynx [19].
The oropharynx is involved in swallowing and it extends from the soft palate to the upper edge of the epiglottis. Anteriorly is oropharynx in communication with the oral cavity through the isthmus faucium at the level of the anterior tonsillar pillars [16,20,22-24].
The hypopharynx has a digestive and respiratory function. It stands at the top in connection with the oropharynx, forward with the laryngeal inlet and extends to the inferior border of the cricoid cartilage (at the level of the sixth cervical vertebra), where it narrows and becomes continuous with the esophagus [15,16,20,22-24].
Imaging modalities of pharynx
A number of different imaging methods have been used to assess the pharyngeal airway and surrounding anatomical structures [25-27]. The following is a summary of the imaging techniques used and currently available, as described by Schwab et al [25] and Sittitavornwong et al [26].
Acoustic Reflection
Acoustic reflection, also called ultrasound, is a technique where sound waves are projected down the airway and reflected back out in such a way that the software can analyze and quantify changes in the upper airway cross-sectional area in relation to the distance from the mouth. This technology has been used for comparative assessment of the pharynx size among snorers, non-snorers and patients with OSA [25-29].
Fluoroscopy
Fluoroscopy is a modality in medical imaging consisting on a sequence of x-rays. The technology enables the identification of a fixed airway disorder such as stenosis or stricture, and its distinction from a dynamic airway disorder [30].
Motion-capture techniques with intraluminal contrast allow an assessment of the functional dynamics of the pharynx using cineradiography (high resolution images obtained at a low frame rate) and video capture (low-resolution images obtained at a high frame rate) [31].
Dynamic fluoroscopic examinations during sleep have been performed as adjunct to endoscopy for evaluating OSA. It can provide information on the dynamic changes in the upper airway during the respiratory cycle and the level of obstruction during sleep [25,26,32], but it also requires that the patient is asleep. Adequate cross-sectional measurements or 3D analysis of upper airway and surrounding soft tissue structures are not possible to acquire [25,33]. Fluoroscopy has been replaced by dynamic computed tomography (CT) with low radiation, which is able to determine more precisely the position and extension of the airway abnormality and provide additional information about other intrathoracic structures [30].
Nasopharyngeal endoscopy
Nasopharyngeal endoscopy is a routine method in Otorhinolaryngology for the examination of the upper airway from the anterior part of the nasal cavity down to larynx and the entire pharynx [25,26,34]. After topical anesthesia, an endoscope is inserted into the nasal cavity at the middle nasal passage, above the inferior nasal concha [17]. This technique allows direct observation of the pharynx and has been used to evaluate its physiologic changes during wakefulness and sleep in OSA patients, as well as the effects of different treatments including mandibular advancement device (MAD), weight loss, and uvulopalato pharyngoplasty (UPPP) on airway dimension [25].
Cephalometry
Cephalometry, used mainly for analysis of craniofacial development and malocclusion, is a two-dimensional image of the patient’s facial skeleton and soft tissue profile, exposed in the lateral view. An analyzes of soft tissue and craniofacial structures related to the upper airway can also be performed. The size of the upper airways craniofacial structures can be accurately measured in a 2-dimensional perspective, and the angles between these craniofacial structures can also be identified. Standardized imaging technique is used when cephalometry is performed. The individual is seated with the head fixed to stabilize the position and the exposure should be performed at the end of expiration for measures of the pharynx [25,33].
Cephalometric studies have been used to investigate the relationship between dentofacial morphology and risk for OSA and also to evaluate the effect of intraoral appliances on airway. It can also be used for evaluation of the skeletal structure before orthognathic surgery as well as the result after orthognathic surgery [25,33].
Computed Tomography
Nowadays, Computed tomography (CT) is used frequently for imaging of the maxillofacial area. Conventional CT scans are usually made at medical radiology departments. A relatively new technology, "Cone-Beam Computed Tomography" (CBCT), is used in dental examinations and has made the 3D imaging of the maxillofacial region possible [27,35]. The advantage of this technique is a lower radiation dose compared to conventional CT, potential reduction can be about 50% to 70% [31,35]. Conventional CT "Multi-Slice Computed Tomography" is used for the examination of larger areas and is helpful in the diagnosis, for example, of facial anomalies, extensive trauma, tumors and when required detailed information about soft tissue [35]. It allows excellent imaging of the airway, bone and soft tissue from the nasopharynx to the larynx. The technology is widely available and scanning is performed in supine position [25].
Conventional CT uses a fan shaped beam to acquire images as multiple slices, obtained in the axial plane, and volume reconstructions can be performed (i.e. 3D reconstructed images of the skin, skull, facial structures, mandible, hyoid bone, spine, and airway). CBCT uses a cone shaped beam to provide direct volumetric acquisition of images in a single rotation. CBCT offers dynamic imaging of the upper airway with excellent temporal and spatial resolution [25,27]. In contrast to conventional CT, it is not possible to clearly differentiate various soft tissue structures, but CBCT can be used for evaluation of the pharyngeal airway because it shows the interface between the soft tissue and air with a high spatial resolution [27]. CBCT is usually performed in sitting position. Both CBCT and conventional CT allow accurate cross-sectional measurements of the upper airway [25,27].
CT is more expensive compared to cephalometry but has lower cost compared to magnetic resonance imaging. It emits ionizing radiation and has a limited adipose tissue resolution compared to magnetic resonance imaging [25,35]. Regardless radiation exposure, several studies have used conventional CT in normal patients and those with sleep breathing disorder leading to a better understanding of the pathogenesis of obstructive sleep apnea [25].
Magnetic Resonance Imaging
Unlike CT, which relies on ionizing radiation to create images, magnetic resonance imaging (MRI) uses a powerful magnetic field and radiofrequency pulses to examine the tissues. It is particularly useful for evaluating tumors of the skull base, oral cavity, and the larynx [31]. It is also useful for imaging patients with obstructive sleep apnea, due to its excellent resolution of the upper airway and soft tissue (including adipose tissue) and possibility to be performed during wakefulness and sleep without radiation [25,33]. The absence of ionizing radiation has made MRI the imaging technique of choice for assessing children with sleep breathing disorder [26].
A summary of advantages and disadvantages of different imaging methods for the upper airway is presented in table 1.
Table 1. Summary of the advantages and disadvantages of different upper airway imaging methods.
Advantages |
Disadvantages |
Acoustic reflection [25-29] |
Inexpensive, non-invasive, fast, easily repeated
No ionizing radiation
Measures upper airway cross-sectional area in relation to the distance from the mouth
Dynamic imaging |
Cannot provide anatomical representation of the upper airway or soft tissue structures
Opening the mouth alters the anatomy
Cannot be used during sleep
Usually performed in sitting position |
Fluoroscopy [25,26,30-33] |
Dynamic imaging |
Emits ionizing radiation
2-dimensional visualization, commonly in lateral or anteroposterior view
Cannot provide adequate cross-sectional measurements or 3D analysis of upper airway and surrounding soft tissue structures |
Nasopharyngeal endoscopy [25-27,33] |
Direct visualization using endoscope
Widely available, easy to perform and minimal complications
Possible in supine or sitting position
Dynamic imaging
No ionizing radiation
State-dependent imaging (wakefulness vs sleep) |
Invasive
Sometimes hard to tolerate for the patient
Only allows the visualization of the pharyngeal lumen, not surrounding soft and hard tissue structures
“Burn-out” if the soft-tissue covers the tip of the endoscope during obstruction |
Cephalometry [25,27,33] |
Widely available, easy to perform and less expensive than CT and MRI
Allows quantification of skeletal structures with linear and angular measurements
Appropriated for the analysis of craniofacial development and malocclusion |
Cannot provide cross-sectional, 3D or volumetric data
Provides limited information about the anteroposterior structures and no information about lateral soft tissue structures
The individual must be in a standardized position, seated upright with the head fixed
Delivers magnification and positioning errors, and superimposition of bilateral structures
Unable to perform dynamic or state-dependent imaging
Cannot be performed during sleep |
CT scan [25,27,31,35] |
Widely available
Possible in supine (CT) or sitting position (CBCT usually sitting position)
Good resolution of the pharyngeal airway, soft tissue and skeletal structures
Adequate cross-sectional measurements, 3D reconstructions and volumetric data
CBCT allows upper airway imaging with excellent temporal and spatial resolution and low radiation dose
Less expensive than MRI |
Relatively expensive (compared to cephalometry)
Emits ionizing radiation
Poor resolution of adipose tissue compared to MRT
Cannot be performed during sleep
|
Magnetic resonance imaging [15,25,31,33] |
Excellent resolution on the pharyngeal airway, soft tissue and adipose tissue
Adequate cross-sectional measurements, 3D reconstructions and volumetric data
Supine position
No ionizing radiation
Dynamic imaging possible with ultrafast MRI |
Not widely available
Most expensive imaging modality
Difficult for individuals to initiate and maintain sleep in noisy MRI scanner
Exclusion of some individuals with metallic implants (i.e. pacemakers) and possible claustrophobia
Longer scanning time compared with all other examinations modalities |
|
Table 2. Range of effective dose from dental imaging techniques in mSv, adapted from tables in SEDENTEXCT Ec 2012 [66]. |
|
Effective dose (μSv) |
|
|
Panoramic radiograph |
2.7 - 24.3 |
Cephalometric radiograph |
<6 |
Dento-alveolar CBCT (FOV < 10 cm height)
Craniofacial CBCT (FOV > 10 cm height) |
11 - 674
30 - 1073 |
Conventional CT |
280 - 1410 |
Analysis methodology and measuring process with focus on computed tomography
3D imaging and evaluation of the upper airway with CT or CBCT have become popular in the field of medicine and dentistry due to its wide availability as well as improved imaging processing methods and analysis using software programs [38]. After the scan, all the related information is stored, managed and analyzed using Digital Imaging and Communications in Medicine (DICOM) files. CBCT/CT raw data is reformatted and exported in DICOM format and then imported into an image-processing software where it can be further processed to synthesize various images [5].
Guijarro-Martínez et al [2] did a systematic review of the literature on upper airway analysis using CBCT and the papers included a large variation in the software used for viewing, measuring and analyzing the upper airway. Seventeen specific DICOM viewers were reported, but according with Guijarro-Martínez et al [2] only four were properly validated (Dolphin3D, In Vivo Dental, OnDemand3D and 3dMD Vultus) [2]. Schendel et al [38] tested the accuracy and precision of the 3dMD Vultus software to calculate linear and volumetric measurements using an airway phantom as a test standard at 3 different orientations and they found the software to be accurate, reliable and fast [38]. El et al [39] studied the reliability and accuracy of 3 image-processing software, Dolphin 3D, Ondemand3D, and In Vivo Dental, to calculate upper airway volumes in the oropharynx region and nasal passage. They used preexisting CBCT data from 30 patients and the results were compared to the volumes obtained using another software, Ortho Segment (OS), which was considered the gold standard. They found that the three software were highly reliable but had poor accuracy, differences were found among them and compared with the OS program, suggesting systematic errors [39].
More recently the study of Weissheimer et al [40] investigated the precision and accuracy of six software programs in measuring oropharynx volumes: Dolphin 3D, Ondemand 3D, and In Vivo Dental popular within the orthodontic and maxillofacial specialty in the USA; Mimics often used in biomedical engineering; OsiriX and ITK-Snap free, open-source software frequently used in medicine. Their study sample consisted on pretreatment CBCT data from 33 growing patients with transverse maxillary deficiency and no congenital malformations, and as the gold standard CBCT data from an oropharynx acrylic phantom. They found that all six programs were reliable but presented significant differences compared to the gold standard in oropharynx evaluation. Dolphin 3D, Mimics, OsiriX and ITK-Snap were nevertheless the more accurate [40].
The assessment of the volume and configuration of the upper airway begins with segmentation, which is the selection of the area in interest by delineating and separating the airway from all the anatomical surrounding structures. This permits a clear visualization and analysis of upper airway in a 3D model [3,39,40]. The segmentation of the upper airway can be either manual or semiautomatic. In the manual approach, the segmentation is performed slice by slice by the user, every region of interest has to be selected individually, and then the software creates a 3D model. In the semiautomatic segmentation, the software distinguishes the different anatomical structures (for example the airway from the surrounding soft tissue) based on their different density values (Hounsfield Units, HU) allowing this method to be significantly faster and subsequently more interesting for clinical use [3,39,40]. As described by Weissheimer et al [40] the semiautomatic segmentation includes in some software the placement of initial seed regions in the 3 planar slices, and the selection of an initial threshold [40]. Subsequently, the limits of the upper airway and regions of interest are outlined by the user and the software calculate the total airway volume (mm3) and minimum cross-sectional area (mm2) [6].
The threshold value or interval consists of a selection of all voxels with density values (grey levels) representative for the area of interest and it can be manually modified by the user, e.g. to comprise all voxels containing air in the 3D volume [2,40]. For the airway, the lower threshold level is approximately – 1000 HU [41]. Nakano et al [41] aimed to determine the optimal and most suitable upper threshold level for airway evaluation using a phantom model and ten patients and concluded that it should range from -460 HU to -470 HU [41]. The threshold interval varies among studies, some includes also the fat tissue (-30 HU to -70 HU) extending the upper threshold level [41]. Both Guijarro-Martínez et al [2] and Weissheimer et al [40] argued that threshold selection is a critical issue in the segmentation accuracy and consequently in the evaluation of upper airway configuration and dimension.
A schematic image of analysis methodology and measuring process with focus on computed tomography is presented in figure 3.
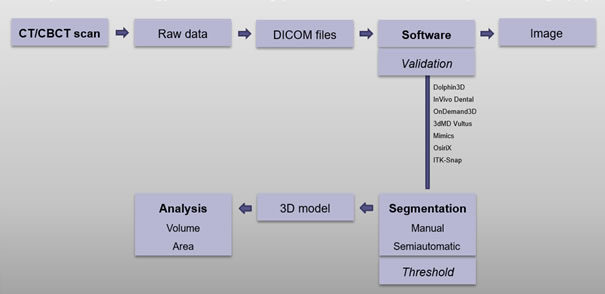
Figure 3. Analysis methodology and measuring process with focus on computed tomography - schematic image.
Factors that affect size and shape
The pharynx is a fibromuscular tube with skeletal and soft tissue support. Its configuration and dimension is related to the surrounding anatomical structures but also to dynamic patient dependent factors. A correct assessment of the upper airway should include all the variables [42].
The study of Schendel et al [12] using CBCT data on a large study population showed that as the airway grows, the total volume, length, area, and volume-to-length index all increase until age 20, then remain relatively stable, and after age 50 they begin to decrease profoundly [12]. Abramson et al [42] using 3D-CT found that the growth of the upper airway occurred predominantly during primary and permanent stages of dentition, corresponding to periods of significant somatic growth. Additionally, they found that changes occurred also in shape and that adults had a larger and more elliptical airway [42].
Castro-Silva et al [43] used preoperative CBCTs from patients with skeletal class I, II, and III malocclusions for a 3D evaluation of the pharyngeal airspace (PAS). They concluded that the mean volume and area were statistically greater in class III malocclusions. Also, individuals with class I had a larger volume compared to class II [43]. The study of Hong et al [44] investigated the pharyngeal airway volume and cross-sectional area in patients with skeletal class I and III malocclusions using CBCT. They concluded that patients with class III had a larger upper pharyngeal airway volume compared with class I malocclusions, which correlated to the anterior position of the mandible. Additionally, patients with class III had a larger cross-sectional area at the lower part of the pharyngeal airway compared with class I malocclusions [44]. Dalmau et al [45] assessed upper airway measurements among different sagittal (skeletal class I, II, II) and vertical (brachyfacial, mesofacial, dolichofacial) craniofacial morphologies using CBCT data. Patients with Class III presented a tendency to have larger measures at the upper edge of the hyoid bone compared with patients with Class I and II. Regarding the vertical pattern, the dolichofacial type presented a tendency to have lower linear measures and smaller area measures at the level of the hard palate and the upper edge of the hyoid bone compared with the brachyfacial and mesofacial pattern [45]. Brasil el al [46] studied the association of facial profile, vertical patterns (brachyfacial, mesofacial, dolichofacial) and sagittal (class II and III) skeletal types with upper airway dimensions using CBCT imaging. They found that the cross-sectional area at the soft palate was larger in patients with class III and that the facial profile based on proportions of the upper/medial/lower thirds allowed inferences on the pharyngeal airway volume. However, no association could be made between upper airway volume and the sagittal skeletal type and vertical pattern [46]. Grauer et al [47] assessed the differences in airway volume and configuration among patients with different facial morphologies using CBCT. They concluded that the anteroposterior skeletal type (Class I, II or III) is related to variation in upper airway size and shape and that vertical skeletal patterns only influences the upper airway shape [47]. Celikoglu et al [4] studied pharyngeal airway volumes among adult patients with different vertical skeletal patterns and a clinically normal sagittal skeletal pattern (dental and skeletal class I relation) using CBCT. They concluded that significant differences exist: nasopharyngeal, oropharyngeal, and total airway volumes were lowest in the high-angle group; oropharyngeal and total airway volumes were the highest in the low angle group [4]. Wang et al [48] aimed to study skeletal class II patients with different vertical patterns (low, normal and high angle) and its relation with upper airway dimensions. Using CBCT imaging, they concluded that the upper airway dimension was smaller in high angle patients comparing to the other vertical skeletal patterns [48].
According to Gonçales et al [49] maxillomandibular deformities are expected to affect the pharyngeal airway space as well as the treatment of these deformities with orthognathic surgery [49]. The authors used pre- and postoperative CBCT data from patients submitted to orthognathic surgery (divided in 4 groups: only maxillary advancement; only mandibular advancement; maxillomandibular advancement; maxillary advancement and mandibular setback) to assess the pharyngeal airway and concluded its size follows the performed surgical maxillomandibular movements and that bimaxillary advancements increased the sagittal and lateral upper and middle pharyngeal airway [49]. Recently, Lee et al [50] did a prospective clinical study using CBCT and sleep parameters to access the changes in the pharyngeal airway on patients with skeletal class III malocclusions undergoing bimaxillary surgery (in form of a simultaneous maxillomandibular setback) and to correlate the prevalence of snoring and OSA as a degree of skeletal movement. They found that the performed maxillomandibular movement narrowed the pharyngeal airway at the oropharynx, specifically at retropalatal and retroglossal level, decreasing the total volume, and causing snoring or sleep apnea in some previous healthy patients with class III malocclusions [50]. Kim et al [51] used CBCT data from patients with mandibular prognathism who underwent bimaxillary surgery (in form of maxilla advancement with clockwise rotation and mandible setback) to study positional changes of the hyoid bone and evaluate the pharyngeal airway at different times, pre-operative, 2 and 6 months postoperative. They found that the surgery induced a more inferoposterior position of the hyoid bone and a decreasing of the total pharyngeal airway volume up to 6 months postoperative. The amount of reduction was associated with the changes in palatal plane inclination and position of the hyoid bone [51].
The hyoid bone is a highly movable and strong bony anchor for a number of muscles and soft tissue structures in the head and neck [52]. Because it is not articulated with other bones, the position of the hyoid bone changes with head posture, body position, and other physiologic states and moves during various oral functions in close conjunction with tongue activity [53]. An inferior hyoid bone is indicative of tongue shape, position and tone, which can lead to upper airway obstruction [54]. The vertical position of the hyoid bone is believed to be a predictor of OSA [53].
Generally, the following factors nasal obstruction, a long palate, a micro- and retrognathic mandible, a large tongue, thick lateral pharyngeal walls, and/or pharyngeal fat, all predispose to upper airway collapse during sleep [55]. Airway narrowing or increased collapsibility at oropharynx or tongue base is most common in OSA patients [50]. Also, the configuration of the upper airway is suggested to be different in OSA patients, being narrower laterally compared with normal individuals [55]. A laterally elliptical pharynx displays increased volume when pulled anteriorly by muscle action, compared to an airway that is originally rounded or elongated in the anteroposterior direction [56]. The lack of muscles on the lateral walls of the pharynx requires the internal pressure on the airway to maintain patency. It is the posterior and lateral walls that collapse in apneic individuals [56].
Upper airway patency is determined by several factors acting during awake and sleep, but during sleep these are compromised which cause changes in upper airway function possibly leading to sleep-related breathing disorders. The main factors are neuromuscular activity, craniofacial morphology, surrounding tissues, and the intrinsic properties of the airway [54]. Mechanical influences on airway dimension include dynamic factors such as upstream resistance in the nasal cavity and pharynx, tissue compliance and pharyngeal muscle activity. Also, the pharyngeal airway patency is strongly influenced by static factors such as neck and jaw position, and gravity [57].
The study of Sutthiprapaporn et al [58] aimed to analyze the effect of gravidity on the oropharyngeal morphology in relation to postural changes. The population sample was evaluated in the upright position using CBCT and in the supine position using conventional CT showing that gravity produces movements in the oropharyngeal structures. The authors also found that the smallest area in the oropharynx was larger in the upright position [58]. The natural head position (NHP) varies among individuals as well in physiologic posture at different points in time [3,59]. Muto et al [59] studied the relation between cranio-cervical inclination and the pharyngeal airspace at different head postures in the same subjects and found a correlation between them [59]. The study of Glupker et al [60] assessed the volumetric variations in the upper airway between open and closed jaws using 3D imaging with CBCT. They found that the oropharynx volume and soft palate area decreased with jaw opening, on the other hand the nasopharynx volume increased [60]. Guijarro-Martínez et al [6] argued the importance to instruct the patient during data acquisition to avoid deglutition and movements, maintain the mandible in a reproducible position and breathe lightly [6].
Discussion
The upper airway presents both a respiratory and a digestive function, having two entrances, the nasal and the oral cavity, which combine in the pharynx [14,17,60]. Imaging of the upper airway using CT/CBCT can be visualized and analyzed by software programs allowing 3D volumetric reconstructions. It permits the assessment of anatomic and pathologic changes that alter upper airway morphology and to infer increased airway resistance and potential obstruction or collapse of the airway [57]. The major advantage compared with cephalometry is the 3D imaging and with MRI the lower costs and widely availability. In CBCT, like in cephalometry, data acquisition is made with the patient usually in upright position [3], and according to Guijarro-Martínez et al [2] this position should be used for standard evaluation of size and shape of the upper airway since its closer to NHP [2]. On the other hand, in conventional CT, like in MRI, the patient is in a supine position [3] which is more suitable for OSA research in agreement to Guijarro-Martínez et al [2].
This insight indicates that only a few, recently published studies [6,12,42,61], have tried to establish normative volumetric data for the pharyngeal airway in healthy adults using 3D imaging with conventional CT or CBCT. The study of Schendel et al [12] aimed to investigate the changes of the upper airway during growth and development using CBCT data to provide a normative reference. The study population were 1300 individuals, aged between 6 and 90 years, with no history of obstructive breathing problems, a normal body mass index and no need for orthognathic surgery in presence of a dental malocclusion. They examined the total airway volume, smallest airway cross-sectional area, length of the airway from the PNS to the top of C3, and airway index (volume/length) using the 3dMD Vultus software. They found that the airway size and length decrease in the middle age, at first more slowly and after age 50 more rapidly. Also, the total airway volume correlated highly with the smallest airway area and only mildly with the length, which leads to believe that the airway area at different segments is more important [12].
Abramson et al [42] did a retrospective study with the objective of develop normative data for airway size and shape and to evaluate the changes associated with age and sex using conventional CT data. The population sample was 46 patients (67% men), age range 4 months to 46 years, without evidence of upper airway pathology, and without reported sleep-related symptoms/ diagnosis of OSA. The study sample was divided into 4 different age groups where an adult was defined older than 16 years. They used an in-house software, 3-D Slicer, to measure airway size and shape (from the hard palate to base of the epiglottis) and to obtain skeletal relationships. They didn’t find significant gender differences in airway parameters and their conclusion was that adults had a larger airway size, with a more elliptical shape. Partially this agrees with Schendel et al [12] who found that the airway volume in individuals with 45 years was slightly larger than in those with age 15, however they also found that afterwards the volume decreased profoundly at the later middle age [12]. Abramson et al [42] used a limited study sample in which 46 years old was the maximum age included and the definition of adult, older than 16 years, is controversial.
The randomized retrospective study of Tso et al [61] had the purpose to define and measure the human airway using CBCT data. The population sample included 10 adults, 50% male and female, with normal class I occlusion, no craniofacial anomalies and no history of sleep apnea and other airway abnormalities. They used the CB Works software to analyze cross-sectional areas and determine the most constricted site in the upper airway, concluding it was located most often at the oropharynx level and varied widely (90-360 mm2). There were no analyses on gender differences and the study sample used was small.
The study of Guijarro-Martínez et al [6] aimed to establish and translate clinical 3D anatomical boundaries for the pharyngeal subregions (nasopharynx, oropharynx and hypopharynx) into precise and reproducible cephalometric landmarks in CBCT, and validate the measuring method [6]. CBCT images were prospectively taken in 40 healthy subjects (20 women and 20 men) who were Caucasian, aged between 23 to 35 years, with dental and skeletal class I and no facial asymmetry, normal body mass index (BMI) and no previous surgery in the head and neck area. Individuals at risk of sleep-disordered breathing, and with congenital or acquired craniofacial malformations were excluded. Data was processed using Dolphin software and the total volume and smallest cross-sectional area of the pharynx subregions were calculated after defining their technical boundaries. Unlike Abramson et al [42] they found gender differences, males had a significantly greater volume of the oropharynx, hypopharynx and minimum cross-sectional area at the oropharyngeal level. Surprisingly the most constricted site in the upper airway was found at the nasopharynx which contradicts Tso et al [61]. This fact might be explained by the different population sample, different anatomical and technical boundaries of the different pharyngeal subregions, different CBCT machines and settings, patient positioning, different software and imaging processing method considered in the studies. Schendel et al [12] analyzed the pharyngeal airway as whole, not the subregions, so they can’t add clarity in this matter. Neither can Abramson et al [42], the study only considered the oropharynx and part of hypopharynx. Guijarro-Martínez et al [6] reported a preliminary normative reference for volumetric data in the pharyngeal subregions of healthy young Caucasian adults and concluded that the anatomical landmarks and technical limits proposed were reasonable, convenient, and reliable [6]. The study is based on their previous work in a systematic review [2] where they summarized different anatomical limits of the upper airway (in the sagittal plane) reported in the literature. In the context of evaluating the pharyngeal airway after orthognathic surgery raises the question if their superior and anterior limit of the nasopharynx, and by consequence superior limit of the pharyngeal airway as a whole, is the most appropriate considering that the posterior nasal spine (PNS) can change significantly with maxillary surgical movements. Large anterior movements of the maxilla might implicate a representation of part of the nasal cavity in the total volume and nasopharyngeal volume in particular. Chang et al [62] studied the volume changes of the upper airway related to maxillomandibular movements using CBCT data and established as superior limit the line connecting the sella and PNS in agreement with the study of Sears et al [63]. Guijarro-Martínez et al [6] argued that the exact 3D definition of the anatomical limits of the pharyngeal airway subregions is a critical issue for upper airway analysis and that their standardization should homogenize upper airway assessment and allow comparisons between scientific studies [6].
A large variation in the software used for viewing, measuring and analyzing the upper airway is found in the scientific literature [2] but to the best of my knowledge only a few have been validated in previous studies [38-40]: Dolphin3D, InVivo Dental, OnDemand3D, 3dMD Vultus, Mimics, OsiriX and ITK-Snap. Both studies Guijarro-Martínez et al [6] and Schendel et al [12] used software properly validated and the segmentation of the upper airway was achieved with a semi-automatic approach. This approach is considered the most relevant for clinical use compared with the manual segmentation, which is significantly time-consuming [38-40]. Guijarro-Martínez et al [6] used a single predefined threshold level as reference, then manually adjusted before 2D and 3D calculations by the software in an attempt to minimize the potential error associated to different threshold intervals. Lenza et al [5] considered that a fixed threshold is more reproducible than a dynamic one, although it might produce errors in airway morphology and volume analysis. As Weissheimer et al [40] argues a fixed threshold minimizes investigator subjectivity in boundary selection, a dynamic threshold is based on the human visual capacity to outline the airway limits and it is affected by multiple factors. Schendel et al [12] do not make any reference to the threshold value used. Abramson et al [42] used an in-house software, 3-D Slicer, but no reference was made to the validation of the software, neither to the protocol used in segmentation. Tso et al [61] used the CB Works software, also no reference was made to its validation and the segmentation process was not clearly clarified. According to Weissheimer et al [40] the final linear and volumetric measurements of the upper airway are influenced by the software's imaging processing methods and segmentation techniques and these differs between the different softwares that are available on the market, no standard protocol exists [40].
Several studies [4,43-48] assess the upper airway dimension in relation to the different sagittal and vertical skeletal patterns using mainly CBCT data. Even though all of them do not show statistically significant results, a common finding is that skeletal class III malocclusions, where the mandible have an anterior position, is associated with a larger dimension of PAS compared to skeletal class I and II, respectively in decreasing order [43,44]. Also, vertical skeletal patterns seem to influence the airway dimension and shape, larger volumes were found in low angle subjects, i.e. with a horizontal growth pattern [4,48,45].
As maxillomandibular deformities affects the PAS, also the treatment of these deformities with orthognathic surgery is expected to affect the PAS [49]. Orthognathic surgery is performed in patients where good occlusion and facial aesthetics cannot be achieved with orthodontic treatment alone [50,63]. The most common deformities that requires combined orthodontic-surgical treatment are severe class II and III malocclusions and vertical skeletal discrepancies [50]. Several studies using 2D imaging and more recently 3D imaging evaluated the pharyngeal airway in the context of orthognathic surgery and concluded pharynx size follows the surgical maxillomandibular movements performed, bimaxillary advancements increases the PAS whereas mandibular setback narrows of the pharyngeal airway [49,50,63]. Recently, Lee et al [50] treated skeletal class III malocclusions with a simultaneous maxillomandibular setback and found that the surgery narrowed the pharyngeal airway at the oropharynx, specifically at retropalatal and retroglossal level, decreasing the total volume, and causing snoring or sleep apnea in some patients who were previously healthy. The population sample was 22 patients (77% women) with mean age of 22 years, normal body mass index (BMI), no sleep-related symptoms and normal sleep study preceding surgery [50]. Kim et al [51] enhanced the importance of the palatal plane angle (rotational movement of the maxilla) and vertical movements of the hyoid bone in jaw surgery and how they change the pharyngeal airway. As Lee et al [50] argues, sleep breathing disorders including OSA can be seen as late complication of orthognathic surgery which causes structural modifications of the surrounding bone, muscle and soft tissues supporting the pharynx. OSA is characterized by upper airway collapse creating episodes of breathing reduction or cessation during inspiration while sleeping [48,50,62]. The diagnosis is objectively established by polysomnography. OSA is a common medical disorder that can be life-threatening, causing adverse cardiovascular and metabolic outcomes, neurocognitive dysfunction and poor quality of life [9,11,50]. It is associated with increased morbidity and mortality without proper treatment [9]. Beside conservative treatments, like lifestyle modifications in case of high BMI and high alcohol consumption, positional therapy, positive airway pressure therapy, oral appliances providing an anterior position of the mandible, the treatment can also include surgical procedures [9,11], among them orthognathic surgery. Li et al [64] studied the relation between pharyngeal cross-sectional measurements and polysomnography data of patients with sleep-disordered breathing using conventional CT. They found that a cross-sectional area smaller than 55 mm2 at retropalatal level implicates a high respiratory disturbance index (over 30 total apnea and hypopnea episodes/hour) and that a cross-sectional area larger than 110 mm2 is associated with a low respiratory disturbance index [64,43]. Bimaxillary advancement leads to an enlargement of the pharyngeal airway by pulling forward all tissues attached to the maxilla, mandible and hyoid bone and enhances the neuromuscular tone of pharyngeal walls preventing them from collapse or reducing the number of events [8,11,62]. Traditionally, bimaxillary advancement has been seen as the final approach for treatment of OSA, but in certain patients with severe disorder and craniofacial deformities it may be considered as the first choice or sole surgical approach when conservative therapies are ineffective or not tolerated [8-11]. As Prinsell et al argues [11] surgery can provide definitive treatment with long-term success if performed proficiently in terms of technical skills and planned correctly to address the site of obstruction. Assessment of the pharyngeal airway is therefore argued to be a crucial diagnostic step in OSA patients [6]. A definitive treatment implicates a reduction in OSA-related health risks that will result in economic benefits for the whole health care system [11].
3D imaging is an invaluable technique for accurate visualization of the pharyngeal airway and characterization of the complex anatomy [48]. The scientific literature shows an increasing number of publications using CBCT for evaluation of the upper airway in the context of orthodontics and maxillofacial research due to its easy access and low radiation dose, with maintained high spatial resolution that allows to distinguish the air-filled PAS from surrounding anatomical structures [62,43]. CBCT imaging combined with the continuous technological developments in software give an unprecedented tool for virtual planning in combined orthodontic-surgical treatment of craniofacial deformities allowing to explore and visualize different maxillomandibular movements and understand their implications in the related anatomical structures, as well as facial esthetics. Airway size should be considered in planning orthognathic surgery [44], in agreement with Castro-Silva et al [43] that state PAS is an important anatomic feature for surgeons who intend to change the maxillofacial with osteotomies [43]. CBCT might provide a range of information concerning pharyngeal size and shapes that can be used to analyze what type and extension of surgical movement is necessary to optimize the treatment [8,65]. A limitation of CBCT imaging in upper airway characterization is that it is a static examination representing a dynamic structure [49] which is continuously influenced in its size and shape by breathing, gravity and postural changes. Another important limitation is the use of ionizing radiation, even in small doses. All ionizing radiation can cause a permanently damage in DNA which can lead to a development of a tumor during a lifetime. The tissues of younger patients are more radiosensitive and a long-life span implicates a bigger risk to injuries [66]. The radiation dose is dependent on equipment type and exposure parameters, especially the field of view. Table 2 presents the reported effective doses for CBCT in comparison with other dental imaging techniques. Like any X-ray, CBCT involves a risk to the patients so it must be used with responsibility, only with justified potential benefits to the patient and using the minimum radiation dose that is reasonably achievable [66].
Conclusion
The major limitation of this literature study is that it is not a systematic review and it only provides an insight into the complex subject that is the 3D analysis of the upper airways as a dynamic structure. One way to evaluate the pharyngeal airway is to calculate the cross-sectional area and volume, and these vary widely depending on patient and head positioning, respiratory phase and craniofacial morphology [5]. Technical aspects regarding data acquisition, software processing and analysis method, significantly influence the results obtained. Many authors don’t report this information and when they do a great variation is found. This supports Guijarro-Martínez et al [6] who argued that a systematic comparison between scientific studies is often impossible and the combination of data to obtain a normative volumetric reference for the pharyngeal airway is really difficult [6]. An understanding of the pharyngeal airway is important and should be considered in planning and monitoring combined orthodontic-surgical treatment. It is important to identify risk patients and consider the different surgical alternatives with the purpose of preventing restriction of the pharyngeal airspace that may lead to snoring and OSA in a short- or long-term perspective. Further, it is important to underline that when assessing outcomes of treatment with bimaxillary advancement in OSA patients, overnight sleep apnea registration (e.g. polysomnography) is the gold standard method for objective assessment of OSA. A systematic protocol for 3D assessment of the pharyngeal airway is still missing in the clinical routine [6] and significant clinical research with good quality and standardized criteria are needed.
Declaration
Funding: Umeå University
Conflicts of interest/Competing interests: All authors declare no conflicts of interest.
Authors’ contributions: Conceptualization: D.F.; methodology: M.S. and D.F.; investigation: DF; resources: M.S.; data curation: D.F.; writing–original draft preparation: M.S. and D.F.; project administration: M.S. All authors have read and agreed to the published version of the manuscript.
Statement of clinical relevance
Airway size should be considered when planning orthognathic surgery. Sleep breathing disorders including OSA can be seen as late complication of orthognathic surgery in healthy patients. Maxillomandibular advancement can provide definitive treatment for OSA if planned correctly to address the site obstruction.
References
- Di Carlo G, Polimeni A, Melsen B, Cattaneo PM (2015) The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod Craniofac Res 18:1-11. [Crossref]
- Guijarro-Martínez R, Swennen GR (2011) Cone-beam computerized tomography imaging and analysis of the upper airway: a systematic review of the literature. Int J Oral Maxillofac Surg 40:1227-1237. [Crossref]
- Burkhard JP, Dietrich AD, Jacobsen C, Roos M, Lübbers HT, et al (2014) Cephalometric and three-dimensional assessment of the posterior airway space and imaging software reliability analysis before and after orthognathic surgery. J Craniomaxillofac Surg 42:1428-1436. [Crossref]
- Celikoglu M, Bayram M, Sekerci AE, Buyuk SK, Toy E (2014) Comparison of pharyngeal airway volume among different vertical skeletal patterns: a cone-beam computed tomography study. Angle Orthod 84:782-787. [Crossref]
- Lenza MG, Lenza MM, Dalstra M, Melsen B, Cattaneo PM (2010) An analysis of different approaches to the assessment of upper airway morphology: a CBCT study. Orthod Craniofac Res 13:96-105. [Crossref]
- Guijarro-Martínez R, Swennen GR (2013) Three-dimensional cone beam computed tomography definition of the anatomical subregions of the upper airway: a validation study. Int J Oral Maxillofac Surg 42:1140-1149. [Crossref]
- Kaur S, Rai S, Kaur M (2014) Comparison of reliability of lateral cephalogram and computed tomography for assessment of airway space. Niger J Clin Pract 17:629-636. [Crossref]
- Ronchi P, Novelli G, Colombo L, Valsecchi S, Oldani A, et al (2010) Effectiveness of maxillo-mandibular advancement in obstructive sleep apnea patients with and without skeletal anomalies. Int J Oral Maxillofac Surg 39:541-547. [Crossref]
- Aurora RN, Casey KR, Kristo D, Auerbach S, Bista SR, et al (2010) Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 33:1408-1413. [Crossref]
- Li KK, Powell NB, Riley RW, Troell RJ, Guilleminault C (2000) Long-Term Results of Maxillomandibular Advancement Surgery. Sleep Breath 4:137-140. [Crossref]
- Prinsell JR (2002) Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 133:1489-1497. [Crossref]
- Schendel SA, Jacobson R, Khalessi S (2012) Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg 70:2174. [Crossref]
- Fasting U, Hougaard J (2009) Fysiologi och anatomi. Den levande människan. Stockholm: Nordstedts; 11:282-283.
- Sonesson B, Sonesson G (2006) Anatomi och fysiologi. 4:e uppl. Stockholm: Liber. 11:288.
- Hagberg CA (2013) Benumof and Hagberg’s Airway Management 3rd ed. Philadelphia, PA. Elsevier/Saunders 1:3-8.
- Petrén T, Carlsöö S (2006) Anatomi för tandläkarstuderande och tandläkare. Stockholm: Norstedts Akademiska Förlag. 16:194-198.
- Levring Jäghagen E (2009) Videografisk undersökning av svalgfunktion under tal och sväljning. Tandläkartidningen 101: 68-78.
- Moses KP, Banks JC, Nava PB, Petersen DK (2013) Atlas of Clinical Gross Anatomy [Electronic resource]. 2nd ed. Philadelphia, PA. Elsevier/Saunders 10:108-109.
- Standring S (2016) Gray's Anatomy: The Anatomical Basis of Clinical Practice [Electronic resource]. 41st ed. Philadelphia. Elsevier 34:571-585.
- Rundcrantz H (1991) Öron-, näs- och halssjukdomar. 4:e. uppl. Lund: Student litteratur 3:69-73.
- Haaga JR, Boll D (2017) CT and MRI of the Whole Body [Electronic resource]. 6th ed. Philadelphia, PA. Elsevier 22:651.
- Gore RM, Levine MS (2015) Textbook of Gastrointestinal Radiology [Electronic resource]. 4th ed. Philadelphia, PA. Elsevier/Saunders 14:207-209.
- Friis-Liby J, Groth A (2010) ÖNH-handboken. Lund: Student litteratur 4:154.
- Matti A (2012) Öron- näs- och halssjukdomar, huvud- och halskirurgi. 4:e uppl. Stockholm: Liber; 2:102.
- Schwab RJ, Goldberg AN (1998) Upper airway assessment: radiographic and other imaging techniques. Otolaryngol Clin North Am 31:931-968. [Crossref]
- Sittitavornwong S, Waite PD (2009) Imaging the upper airway in patients with sleep disordered breathing. Oral Maxillofac Surg Clin North Am 21:389402. [Crossref]
- Jayaratne YS, Zwahlen RA (2016) The Oropharyngeal Airway in Young Adults with Skeletal Class II and Class III Deformit ies: A 3-D Morphometric Analysis. PLoS One 11:e0148086. [Crossref]
- Viviano JS (2002) Acoustic reflection: review and clinical applications for sleepdisordered breathing. Sleep Breath 6:129-149. [Crossref]
- Kumar S, Jayan B, Bansal AK (2015) Acoustic pharyngometry: An objective assessment tool for determining pharyngeal airway. J Dent Specialities 3:102-108.
- Coley BD (2013) Caffey's pediatric diagnostic imaging [Electronic resource]. 12th ed. Philadelphia, PA. Elsevier/Saunders 49:507.
- Flint PW (2015) Cummings otolaryngology--head & neck surgery [Electronic resource]. 6th ed. Philadelphia, PA. Elsevier/Saunders 101:1508-1511.
- Som PM, Curtin HD (2011) Head and Neck Imaging [Electronic resource]. 5th ed. St. Louis: Mosby. 30:1819.
- Lee-Chiong TL (2006) Sleep: a comprehensive handbook. 39:293-301.
- Andersson M (2010) Undersökningstekniker inom öron, näsa, hals: en fotoillustrerad handbok för läkare. [Sollentuna]: MSD. 42.
- Petersson A, Gröndahl HG, Suomalainen A (2009) Datortomografi inom odontologisk radiologi. Tandläkartidningen 101:42-50.
- Ciscar MA, Juan G (2001) Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J 17:7986. [Crossref]
- Wenig BM (2016) Atlas of Head and Neck Pathology [Electronic resource], 3rd ed. Philadelphia, PA. Elsevier 8:399.
- Schendel SA, Hatcher D (2010) Automated 3-dimensional airway analysis from cone-beam computed tomography data. J Oral Maxillofac Surg 68:696701. [Crossref]
- El H, Palomo JM (2010) Measuring the airway in 3 dimensions: a reliability and accuracy study. Am J Orthod Dentofacial Orthop 137:S50.e1-9. [Crossref]
- Weissheimer A, Menezes LM, Sameshima GT, Enciso R, Pham J, et al (2012) Imaging software accuracy for 3-dimensional analysis of the upper airway. Am J Orthod Dentofacial Orthop 142:801-813. [Crossref]
- Nakano H, Mishima K, Ueda Y, Matsushita A, Suga H, et al (2013) A new method for determining the optimal CT threshold for extracting the upper airway. Dentomaxillofac Radiol 42:26397438. [Crossref]
- Abramson Z, Susarla S, Troulis M, Kaban L (2009) Age-related changes of the upper airway assessed by 3-dimensional computed tomography. J Craniofac Surg 20:657-663. [Crossref]
- Castro-Silva L, Monnazzi MS, Spin-Neto R, Moraes M, Miranda S, et al (2015) Cone-beam evaluation of pharyngeal airway space in class I, II, and III patients. Oral Surg Oral Med Oral Pathol Oral Radiol 120:679-683. [Crossref]
- Hong JS, Oh KM, Kim BR, Kim YJ, Park YH (2011) Three-dimensional analysis of pharyngeal airway volume in adults with anterior position of the mandible. Am J Orthod Dentofacial Orthop 140:e161-169. [Crossref]
- Dalmau E, Zamora N, Tarazona B, Gandia JL, Paredes V (2015) A comparative study of the pharyngeal airway space, measured with cone beam computed tomography, between patients with different craniofacial morphologies. J Craniomaxillofac Surg 43:1438-1446. [Crossref]
- Brasil DM, Kurita LM, Groppo FC, Haiter-Neto F (2016) Relationship of craniofacial morphology in 3-dimensional analysis of the pharynx. Am J Orthod Dentofacial Orthop 149:683-691. [Crossref]
- Grauer D, Cevidanes LS, Styner MA, Ackerman JL, Proffit WR (2009) Pharyngeal airway volume and shape from cone-beam computed tomography: relationship to facial morphology. Am J Orthod Dentofacial Orthop 136:805-814. [Crossref]
- Wang T, Yang Z, Yang F, Zhang M, Zhao J, et al (2014) A three dimensional study of upper airway in adult skeletal Class II patients with different vertical growth patterns. PLoS One 9:e95544. [Crossref]
- Gonçales ES, Duarte MA, Palmieri C Jr, Zakhary GM, Ghali (2014) Retrospective analysis of the effects of orthognathic surgery on the pharyngeal airway space. J Oral Maxillofac Surg 72:2227-2240. [Crossref]
- Lee UL, Oh H, Min SK, Shin JH, Kang YS, et al (2017) The structural changes of upper airway and newly developed sleep breathing disorders after surgical treatment in class III malocclusion subjects. Medicine 96:e6873. [Crossref]
- Kim MA, Kim BR, Choi JY, Youn JK, Kim YJ, et al (2013) Three-dimensional changes of the hyoid bone and airway volumes related to its relationship with horizontal anatomic planes after bimaxillary surgery in skeletal Class III patients. Angle Orthod 83:623-629. [Crossref]
- Drake RL, Vogl AW, Mitchell A (2010) Gray's Anatomy for Students. 2nd ed. Philadelphia, PA. Churchill Livingstone/Elsevier 8:803.
- Pae EK, Blasius JJ, Nanda (2004) Heterogeneity in vertical positioning of the hyoid bone in relation to genioglossal activity in men. Angle Orthod 74:343-348. [Crossref]
- Kryger M, Roth T, Dement WC (2017) Principles and Practice of Sleep Medicine [Electronic resource]. 6th ed. Philadelphia, PA. Elsevier 111:1081-1082.
- Berry RB (2012) Fundamentals of Sleep Medicine [Electronic resource]. Philadelphia, PA. Elsevier/Saunders 16:263.
- Haskell JA, Haskell BS, Spoon ME, Feng C (2014) The relationship of vertical skeletofacial morphology to oropharyngeal airway shape using conebeam computed tomography: possible implications for airway restriction. Angle Orthod 84:548-554. [Crossref]
- White SM, Huang CJ, Huang SC, Sun Z, Eldredge JD, et al (2015) Evaluation of the Upper Airway Morphology: The Role of Cone Beam Computed Tomography. J Calif Dent Assoc 43:531-539. [Crossref]
- Sutthiprapaporn P, Tanimoto K, Ohtsuka M, Nagasaki T, Iida Y, et al (2008) Positional changes of oropharyngeal structures due to gravity in the upright and supinepositions. Dentomaxillofac Radiol 37:130-135. [Crossref]
- Muto T, Takeda S, Kanazawa M, Yamazaki A, Fujiwara Y, et al (2002) The effect of head posture on the pharyngeal airway space (PAS). Int J Oral Maxillofac Surg 31:579-583. [Crossref]
- Glupker L, Kula K, Parks E, Babler W, Stewart K, et al (2015) Three-dimensional computed tomography analysis of airway volume changes between open and closed jaw positions. Am J Orthod Dentofacial Orthop 147:426-434. [Crossref]
- Tso HH, Lee JS, Huang JC, Maki K, Hatcher D, et al (2009) Evaluation of the human airway using cone-beam computerized tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:768-776. [Crossref]
- Chang MK, Sears C, Huang JC, Miller AJ, Kushner HW, et al (2015) Correlation of Airway Volume With Orthognathic Surgical Movement Using Cone-Beam Computed Tomography. J Oral Maxillofac Surg 73:67-676. [Crossref]
- Sears CR, Miller AJ, Chang MK, Huang JC, Lee JS (2011) Comparison of pharyngeal airway changes on plain radiography and cone-beam computed tomography after orthognathic surgery. J Oral Maxillofac Surg 69:e385-394. [Crossref]
- Li HY, Chen NH, Wang CR, Shu YH, Wang PC (2003) Use of 3-dimensional computed tomography scan to evaluate upper airway patency for patients undergoing sleep-disordered breathing surgery. Otolaryngol Head Neck Surg 129:336-342. [Crossref]
- Stratemann S, Huang JC, Maki K, Hatcher D, Miller AJ (2011) Three-dimensional analysis of the airway with cone-beam computed tomography. Am J Orthod Dentofacial Orthop 14:607-615. [Crossref]
- SEDENTEXCT Ec (2012) Radiation protection N° 172: Cone beam CT for dental and maxillofacial radiology. In: Directorate-General for Energy DDNE, Unit D4-Radiation Protection editor. [Crossref]



