Abstract
Purpose: The objective of this study was to examine the association between compressive cervical myeloradicular pathology and neuropathy of the median nerve at the wrist and verify the validity of the hypothesis of DCS.
Method: The study includes a retrospective analysis of 301 patients, 204 women (68.4%) and 97 men (31.6%), consecutively treated for CTS by release of the median nerve at the wrist, to detect the presence of any cervical disease able to satisfy the hypothesis of a DCH.
Results: Revisiting 301 cases of neuropathy of the median nerve at the wrist and applying all the anatomical and physiopathological restrictions requests to a DCS, about 25% of all patients with CTS have a double compression but only in 7.3 % of cases (16 patients) it was possible to find appropriate correlations between proximal and distal lesion necessary to explain a DCH.
Conclusions: The DCH has anatomic and pathophysiologic restrictions that make it not applicable in the many clinical situations of coexistence of proximal cervical compression and carpal tunnel. This hypothesis is invoked clinically far more often than is warranted and often contradictory of this often-mentioned DCS have appeared in the literature. So not always the coexistence of a cervical root lesion (CRLs) with carpal tunnel syndrome identifies a double-crush syndrome.
Keywords
Double-crush syndrome, median neuropathy, cervical radiculopathy
Introduction
In 1973 Upton and McComas, after assessing that a large group of their patients with carpal tunnel syndrome (CTS) or ulnar neuropathy at the elbow (UN-E) had a high occurrence of cervical root injury, proposed that focal compression often occurs at more than one level along the course of a single nerve fibre [1].
They assumed that in this circumstance a disturbance of axonal transport, caused by a compression in the proximal site (such as cervical roots), may impair the ability of the nerve at distal segment to withstand further focal compressive injuries. Thus, subclinical focal compressive neuropathy (e.g. carpal tunnel syndrome) can become symptomatic.
For this mechanism, in which the combination of a distal lesion and a proximal one compromise the axonal transport with death and axonal degeneration, the two authors proposed the term “double-crush syndrome” (DCS) [1].
Over the years the "double-crush hypothesis" (DCH) has been proposed in all cases of coexisting proximal and distal nerve lesions and has been supported by various studies. But considering how important clinical consequences of this phenomenon are relatively few experimental works have been published to validate this hypothesis [2–6].
The common theme in many papers published on the DCS is the explanation for some unexpected clinical results: when a surgical peripheral nerve decompression, technically satisfactory, does not relieve the patient’s symptoms, it is likely that a second compression along the nerve has not been found [5,7–13].
But not always the coexistence of a cervical root lesion (CRLs) with carpal tunnel syndrome or ulnar neuropathy at the elbow identifies a double-crush syndrome. There are, in fact, accurate anatomical and pathophysiological limitations that restrict the diagnosis.
The objective of this study was to examine the association between cervical pathology and compressive neuropathy of the median nerve at the wrist, checking the validity of the hypothesis of double-crush syndrome and to verify if and when the double-crush syndrome can be the explanation of unexpected results after a technically satisfactory surgical decompression of the carpal tunnel.
Materials and methods
Patients
The study includes a retrospective analysis of 301 patients, 204 women (68.4%) and 97 men (31.6%), consecutively treated for CTS by release of the median nerve at the wrist, to detect the presence of any cervical disease able to satisfy the hypothesis of a DCH. The mean age of patients was 59 years (23-84 years).
Inclusion criteria
All patients included in the study underwent surgical decompression of the median nerve at the wrist and had persistence or worsening of symptoms present preoperatively at the follow-up.
Nerve decompression and neurolysis were performed with regional anaesthesia in arterial ischemia. In all cases the diagnosis was confirmed during surgery.
Patients with systemic and metabolic diseases and all those who reported previous fractures of the distal radius (that could change the relationship among the anatomical structures involved in the architecture of the carpal tunnel) were excluded from the study. Moreover, 34 patients died or were not available at the follow-up. The total drop-out included 50 patients (16.6% of all the patients treated), as summarized in the table 1. Finally, 217 patients were included in the study.
Table 1. Total drop-out included 50 patients
Associated Pathology | No. of patients |
Chronic Polyneuropathy | 2 |
Diabetic disease | 38 (12,6%) |
Uremic or alcoholic neuropathy- B6 vitamin deficiency | 1 |
Vascular disease | 3 |
Thoracic outlet syndrome | 1 |
DISH | 1 |
Paget disease | 1 |
Omolateral radial distal fracture | 3 |
Follow Up
Mean follow up was 36 months. Post-operative evaluation of patients included in the study was initially based on a telephone interview and the DASH evaluation score for the upper limb [5].
In patients with motor or sensory deficits of the upper limbs and the suspicion of a cervical compression after a neurological evaluation, standard radiographic examination, cervical spine MRI (axial and sagittal images, T1- and T2-weighted) and EMG / ENG were performed.
The neurophysiologic examination was performed to assess the presence of any abnormal spontaneous activity, the motor unit potential (MUP), the stability of MUPs, the recruitment of motor units and the interference pattern.
The presence of axonal loss was based on the presence of spontaneous fibrillation potentials activity and / or positive sharp-waves and increased amplitude and duration of the MUPs, as signs of reinnervation.
Evaluated elements were: the correlation between electrodiagnostic abnormalities and outcome of patients undergoing surgical decompression of the median nerve at the wrist; the presence of correlations between the level and location of the compression and the presence of motor or sensory disturbances.
Results
Patients who reported persistent peripheral symptoms after surgery were 65 (29.95% of the total): 46 patients (83,6% of the total) had mainly paresthesia, pain, reduction of fine tactile sensitivity and inability to use small objects (e.g. buttoning up shirts or holding a needle), while 9 patients (16.4%) had weakness, muscle contractures and thenar hypotrophy. In 10 cases symptoms have been attributed to tendon flexors tenosynovitis (1 case), insufficient release of the carpal tunnel (1 case), presence of post-surgical compressive perineural fibrosis (1 case), extreme severity of illness at the time of surgery with poor chance of recovery (7 cases). In 44 cases it was observed bilateral symptoms.
After the neurological examination we suspected cervical neurological compression in 55 patients. An anatomically suitable correlation between proximal and distal compression (with satisfactory pathophysiological correspondence with clinically sensory and motor evidenced abnormalities) were present in 16 patients, 7.3% of the total. The diagnostic test results were shown in the table 2.
Table 2. The diagnostic test results
| Level | Compression site | Clinical signs | EMG/ENG: motor latency | EMG/ENG: sensitive latency |
1 | C5-C6 C6-C7 | Foraminal | Sensitive | 5.8 | 3.4 dx 4.5 sx |
2 | C5-C6 / C6-T1 | Pre-foraminal- foraminal | Sensitive Motor | 6.2 dx 7 sx | 5.2 |
3 | C4-C5 C5-C7 | Foraminal Eso-foraminal | Sensitive | | 5.3 |
4 | C4-C5 | Eso-foraminal | Sensitive | | 3.65 |
5 | C6-C7 | Eso-foraminal | Sensitive | | 3.3 |
6 | C5-C6 | Intraforaminal – esoforaminal | Sensitive | | 4.8 |
7 | C4-C5 C5-C6 | Foraminal | Sensitive | | 4.3 |
8 | C4-C5 C5-C7 | Esoforaminal | Sensitive | | 4.0 |
9 | C4-C5/ C5-C7 | Intraforaminal | Sensitiva | | 4.5 |
10 | C6-C7 C7-T1 | Pre-foraminal Osteofitosis | Motor | 5.6 | |
11 | C6-C7 C7-T1 | Osteofitosi pre-foraminal | Motor sensitive | 7.8 | 4.7 |
12 | C5-C6/ C6-C7 | Foraminal | sensitive | | 4.6 |
13 | C4-C5/ C5-C7 | Foraminal-esoforaminal | sensitive | | 3.6 |
14 | C5-C6/ C6-C7 | Foraminal | sensitive | | 4.2 |
15 | C7-T1 | Pre-foraminal | Motor | 5.3 | |
16 | C4-C7 | Pre-endoforaminal | Sensitive | | 4.7 |
X-ray examination and MRI showed the following results
- Patients with motor disorders showed essentially an anterior compression, signs of C8-T1 impairment and mainly intraspinal lesion;
- Patients with sensory disturbance showed a predominantly posterior compression, signs of C5-C6-C7 impairment and ganglionic or post-ganglionic injury (Figure 1a-c, 2 a-d, 3 a-c);
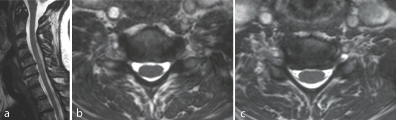
Figure 1a-c: F 69 y, persistent sensory deficit after neurolisys of median nerve at wrist. MRI shows gangliar intra-extraforaminal compression C5-C6, C6-C7.
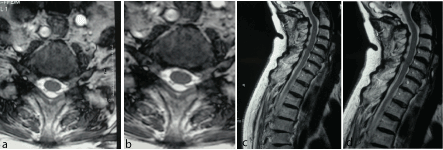
Figure 2a-d: F 71 y, persistent sensory deficit after median nerve release at wrist. MRI shows left gangliar intra-extraforaminal compression C5-C6.
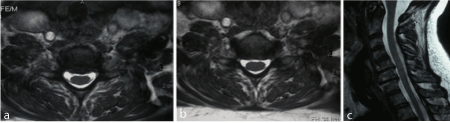
Figure 3a-c: F 64 y, persistent sensory deficit median nerve release at wrist. MRI shows C5-C6 and C6-C7 gangliar intra-extraforaminal left compression.
The EMG/ENG showed the following results
- in patients with MRI features of a pre-ganglionic (intraspinal) compression, the electrodiagnostic examination showed signs of denervation (with reduction of conduction velocity);
- in patients with a ganglionic or post-ganglionic compression, the neurophysiological examination showed a reduced axonal flow (conduction disorders);
- in patients with a probable or documented DCS, the ENG showed a mild increase in motor or sensory latency, whereas in patients without evidence of proximal compression the ENG showed a severe increase in latency;
- the neurophysiological, recorded before surgery, were improved after surgery despite the persistence of symptoms.
Discussion
The "double-crush hypothesis", proposed by Upton and McComas in 1973, suggests that more than one focal compression along the course of a single nerve fibre cause a disturbance of axoplasmic flow that, partially reduced by a proximal compression, is further reduced in the distal site of compression to the point of causing denervation [1].
The results of the present study show that even a mild compression of the median nerve at the wrist, asymptomatic as itself, can become clinically evident when a myeloradicular cervical compression coexists, as evidenced by the ENG sensory latency data.
Revisiting 301 cases of neuropathy of the median nerve at the wrist and applying all the anatomical and physiopathological restrictions requests to a DCS, about 25% of all patients with CTS have a double compression but only in 7.3 % of cases (16 patients) it was possible to find appropriate correlations between proximal and distal lesion necessary to explain a DCH.
Part of the discrepancy between our results (similar to the data of recent literature) and those of Upton and McComas (81 of 115 cases, 75% of the total) is related to the fact that, despite its acceptance, the DCH has anatomic and pathophysiologic restrictions that make it not applicable in the many clinical situations of coexistence of proximal cervical compression and carpal tunnel syndrome [1,13]. These requirements very often are ignored in the previous published data of DCS [13].
The first problem concerning the application of the DCS hypothesis is related to the type of nerve pathology at the distal lesion site. In fact, the impaired axoplasmic flow and axonal degeneration causes conduction failure as the DCH requires, which has specific appearance on electrodiagnostic examination. In contrast, most of the experimental studies and several entrapment neuropathies show conduction slowing or conduction block (pathophysiologic changes attributed to focal demyelination) [1,11,13].
The applicability of the DCS hypothesis is also compromised by the type of proximal lesion. The median motor and sensory fibres compressed at the wrist with CTS derive from C6 to T1 roots, all three trunks and two of the three cords of the brachial plexus before forming the median nerve in the axilla [12]. So, a widespread lesion is required to damage all the axons proximally with severe axonal loss and diffuse substantial deficits in the limb.
All patients with DCS showed a multi-radicular compromission. On the other hand, it is highly unlikely that a single cervical root impairment can lead to distal axon block of a nerve composed of the confluence of 5 spinal roots as the median nerve is.
Furthermore, the DCH requires a direct axonal continuity from the proximal to the distal lesion sites. The anatomical organization of sensory nerve elements is represented by two separated groups of nervous elements associated with any dorsal root ganglion (DRG) with separate axonal transport systems and distinct axoplasmic outflows: the peripherally directed sensory nerve branch and the centrally directed dorsal root branch (Figure 4) [14,15].
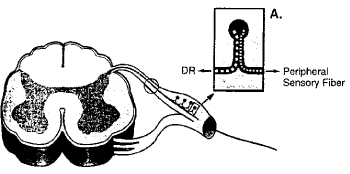
Figure 4: Anatomical organization of sensory nerve elements.
Injury to one of them has no material effect on the other unless there is a concomitant damage to their shared DRG. CRLs, often cited as the proximal lesions in double-crush syndromes (DCS), characteristically affect the sensory axons composing the dorsal root branch proximal to the DRG and they have no effect on the peripherally directed sensory branch or on its axoplasmic flow. If the sensory NCS responses are unelicitable, the lesion is within the plexus (i.e., post-ganglionic) and involves the peripheral sensory fibres [12,15]. For this reason, lesions within the intraspinal canal are in compliance with the DCS hypothesis whenever they involve motor fibres while they are inconsistent when involving sensory fibres [13].
When all of these restrictions on the DCS are considered, it is apparent that they were not taken into consideration in the many publications that have linked CRLs and CTS via a DCH mechanism. In fact, this hypothesis is invoked clinically far more often than is warranted and often contradictory of this often-mentioned DCS have appeared in the literature [16–23].
Conclusions
The objective of this study was to examine the association between compressive cervical myeloradicular pathology and neuropathy of the median nerve at the wrist, and verify the validity of the hypothesis of DCS. We have tried to answer some questions: does double crush 'explain' many diagnostic errors of CTS and may be the explanation of an unexpected surgical outcome? Can double crush mimic the symptoms and test results of CTS making many patients treat for CTS in absence of a real disease?
The results of this study demonstrate that symptoms may be masked, but the plexus or radicular cervical lesion does not produce the typical results of nerve conduction of the STC at the wrist, while the same individual studies may produce results that can be misinterpreted as STC when considered by themselves.
The present study also demonstrates that a lesion of a peripheral nerve can coexist with a concomitant root injury. Considering the extreme rarity of the DCS in the literature (3% on average in the various works), we observed that the association between proximal compression and STC supports the notion that cervical radiculopathy may predispose the nerve to two lesions along its course.
From a practical point of view, there are several clinical findings that can be emphasized.
First of all, double compression syndrome of the median nerve exists as a clinical entity. The neurophysiological results indicate that in carpal tunnel syndromes without proximal compression, EMG shows significant signs of focal demyelination, while distal lesions with DCS produce denervation with axonal loss.
As demonstrated by neuroradiological examinations, patients with movement disorders showed essentially an anterior compression and signs of compression mainly at C8-T1, essentially intraspinal. Patients with predominantly sensory disturbances showed had a posterior compression and ganglionic or post-ganglionic compression localized at C5-C6-C7 levels.
So, a "double-crush" of the median nerve can be suspected in female patients, elderly, with paresthesia symptoms prevalent than motor deficits, with proximal symptoms (neck pain, stiff neck, etc..), and with an increase in distal sensory latency at electrodynamic examination. In fact, in patients with DCS the values of the sensory and motor latency are lower than in patients with simple carpal tunnel syndrome.
Ultimately, in patients with DCS the surgical release of the median nerve at the wrist has a poor prognosis, and it is very important to anticipate to the patient the eventuality of a persistence of symptoms postoperatively.
Despite this, the presence of a double compression doesn’t contraindicate the operation, because even in these patients there was an improvement of neurophysiological data after surgery.
References
- Wilbourn AJ, Aminoff MJ (1988) AAEM minimonograph #32: the electrophysiologic examination in patients with radiculopathies. Muscle Nerve 11: 1099-1114. [Crossref]
- Dahlin LB, Lundborg G (1990) The neurone and its response to peripheral nerve compression. J Hand Surg Br 15: 5-10. [Crossref]
- Dellon AL, Mackinnon SE (1991) Chronic nerve compression model for the double crush hypothesis. Ann Plast Surg 26: 259-264. [Crossref]
- Mackinnon SE, Dellon AL (1988) Surgery of the peripheral nerve. New York: Thieme-Stratton.
- Mackinnon SE (1992) Double and multiple "crush" syndromes. Double and multiple entrapment neuropathies. Hand Clin 8: 369-390. [Crossref]
- Nemoto K, Matsumoto N, Tazaki K, Horiuchi Y, Uchinishi K, et al. (1987) An experimental study on the "double crush" hypothesis. J Hand Surg Am 12: 552-559. [Crossref]
- Kuntzer T (1994) Carpal tunnel syndrome in 100 patients: sensitivity, specificity of multi-neurophysiological procedures and estimation of axonal loss of motor, sensory and sympa-thetic median nerve fibers. J Neurol Sci 127: 221-229. [Crossref]
- Nakano KK, Lundergan C, Okihiro MM (1977) Anterior interosseous nerve syndromes. Diagnostic methods and alternative treatments. Arch Neurol 34: 477-480. [Crossref]
- Nakano KK (1978) The entrapment neuropathies. Muscle Nerve 1: 264-279. [Crossref]
- Niakan E, Harati Y, Ashizawa T (1998) Double-crush syndrome in patients with spasmodic torticollis. Neurology 38: 204-205.
- Osterman AL (1991) Double crush and multiple compression neuropathy. In: Gelberman RH, ed. Operative nerve repair and reconstruction. Philadelphia: JB Lippincott, 2:1211-1229.
- Wilbourn AJ, Gilliatt RW (1997) Double-crush syndrome: a critical analysis. Neurology 49: 21-29. [Crossref]
- Wilbourn AJ, Porter J (1988) Thoracic outlet syndrome. Spine: State of the Art Reviews 2:597-626.
- Clemete CM (1985) Gray's anatomy of the human body. 30th ed. (Amer). Philadelphia: Lea & Febiger.
- Ochs S, Erdman J, Jersild RA Jr, McAdoo V (1978) Routing of transported materials in the dorsal root and nerve fiber branches of the dorsal root ganglion. J Neurobiol 9: 465-481. [Crossref]
- Frith RW, Litchy WJ (1985) Electrophysiologic abnormalities of peripheral nerves in patients with cervical radiculopathy (abstract). Muscle Nerve 8: 613.
- Hudak PL, Amadio PC, Bombardier C (1996) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand). The Upper Extremity Collaborative Group (UECG). Am J Ind Med 29: 602-8. [Crossref]
- Massey EW, Riley TL, Pleet AB (1981) Coexistent carpal tunnel syndrome and cervical radiculopathy (double crush syndrome). South Med J 74: 957-959. [Crossref]
- Osterman AL (1988) The double crush syndrome. Orthop Clin North Am 19: 147-155. [Crossref]
- Simpson RL, Fern SA (1996) Multiple compression neuropathies and the double-crush syndrome. Orthop Clin North Am 27: 381-388. [Crossref]
- Upton AR, McComas AJ (1973) The double crush in nerve entrapment syndromes. Lancet 2: 359-362. [Crossref]
- Nakano KK (1978) The entrapment neuropathies. Muscle Nerve 1: 264-279. [Crossref]
- Hurst LC, Weissberg D, Carroll RE. The relationship of the double crush to carpal tunnel syndrome: an analysis of 1,000 cases of carpal tunnel syndrome. J Hand Surg Br 10: 202-204. [Crossref]




