Introduction: Vitamin D deficiency and type 2 diabetes mellitus (T2DM) remain major health problems. Few data is available on the extent of vitamin D deficiency in Saudi female with T2DM, the aim of this study was to determine vitamin D status in females with T2DM.
Method: A cross-sectional single centre study was conducted in 2908 patients with T2DM attended the Diabetes Centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia between January 2018 and December 2018. The serum concentration of 25-hydroxyvitamin D (25-OHD) and HbA1c were measured.
Results: There were 2908 female patients with T2DM. The mean age was 53.8 ± 16.5 years (20-105 years). The mean and median 25-OHD concentrations were 59.8 ± 31.6 and 55.3 nmol/l respectively. The prevalence of different vitamin D status were; 9.3% severe deficient, 34.7% deficient, 31.5% insufficient and 24.5% sufficient. The mean 25-OHD was the lowest in the fourth decade and highest in the eighth decade. HbA1c was statistically significant lowest in the severe deficient group and highest in the sufficient one. 25-OHD concentration was significantly positively correlated with age (r = 0.212, p < 0.0001) and significantly negatively correlated with HbA1c (r = - 0.135, p < 0.0001).
Conclusions: The prevalence of vitamin D deficiency in females particularly young with T2DM is high.
female patients with type 2 diabetes mellitus, vitamin D deficiency
The global prevalence of vitamin D deficiency is estimated at 30-87% [1,2]. Moreover, in Saudi Arabia, these estimates range between15-87% in the general population [3,4]. Of great importance, the prevalence of type 2 diabetes mellitus (T2DM) in Saudi Arabia is one of the highest reported in the world, reaching up to 30% in a recent study [5]. It has been demonstrated that vitamin D deficiency is associated with T2DM [4,6-10]. It has been reported that insulin secretion is dependent upon vitamin D and there is a positive correlation of vitamin D concentration with insulin sensitivity [11-16].
The prevalence of vitamin D deficiency in patients with T2DM varies from 70 to 90%, depending on the threshold used to define vitamin D deficiency [17-19]. Hollick found that 84% African females in Boston experienced vitamin D deficiency [20]. Meanwhile, Setiati found 35.1% prevalence in the female population [21]. Few published researches have been found that surveyed the prevalence of vitamin D deficiency in patients with T2DM in Saudi Arabia [22].
Updated recommendations on osteoporosis in Saudi Arabia emphasized vitamin D correction but fell short on limiting vitamin D correction on patients suspected and at increased risk of osteoporosis. Since vitamin D deficiency complications extend beyond the bone, vitamin D status should be made a routine clinical test and correction encouraged to the subpopulations most susceptible in harboring vitamin D deficiency (e.g. elderly, women) [23]. In 2017, female population for Saudi Arabia was 14.1 million persons. Female population of Saudi Arabia increased from 2.7 million persons in 1968 to 14.1 million persons in 2017 growing at an average annual rate of 3.5 % [24].
Certain groups such as the elderly, dark-skinned and/or veiled women and their children are at particular risk for vitamin D deficiency [25]. Moreover, since few data is available on the extent of vitamin D deficiency in Saudi female with type 2 DM, the aim of this study was to determine vitamin D status in females with T2DM.
A cross-sectional single centre study was conducted in 2908 female patients with T2DM attended the Diabetes Centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia between January 2018 and December 2018. Eligible patients were 20 years or older. Exclusion criteria were known hepatic or renal disease, metabolic bone disease, pregnancy, malabsorption, hypercortisolism, malignancy, immobility for more than one-week and medications influencing bone metabolism. The serum concentration of 25-OHD was measured by competitive protein binding assay using kits (Immunodiagnostic, Bensheim, Germany). Plasma levels from 75.1 to 250 nmol/l considered sufficient, from 50 to 75 nmol/l insufficient, 25 to 49 deficient and < 25 nmol/l as severe deficient [1]. Glycosylated hemoglobin (HbA1c) was measured by the high-performance liquid chromatography method (Bio-Rad Laboratories, Waters, MA, USA). The total number of cohorts were separated on basis of age values into seven groups: 20-29 years, 30-39 years, 40-49 years, 50-59 years, 60-69 years, 70-79 years and ≥ 80 years. The study was approved by the ethical committee board of King Fahad Armed Forces Hospital.
Data are presented as means ± standard deviation (SD) or numbers (%). Quantitative variables were compared between two groups by using the Student’s test. Differences in categorical variables were analyzed using the chi-square test. The relationship between continuous variables was assessed using coefficients of correlation. P value < 0.05 indicates significance. The statistical analysis was conducted with SPSS version 23.0 for Windows.
There were 2908 female patients with T2DM (Table 1). The mean age was 53.8 ±16.5 years (20-105 years). The mean and median 25-OHD concentrations were 59.8 ± 31.6 and 55.3 nmol/l respectively. The prevalence of different vitamin D status were; 9.3% severe deficient, 34.7% deficient, 31.5% insufficient and 24.5% sufficient.
The mean 25-OHD was the lowest in the fourth decade and highest in the eighth decade (Figure 1).
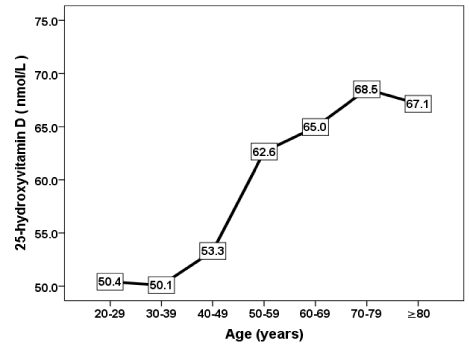
Figure 1. The mean of vitamin D concentration in correlation to age groups
HbA1c was statistically significant lowest in the severe deficient group and highest in the sufficient one (Figure 2). 25-OHD concentration was significantly positively correlated with age (r = 0.212, p < 0.0001) and significantly negatively correlated with HbA1c (r = - 0.135, p <0.0001) (Figure 3A and 3B).
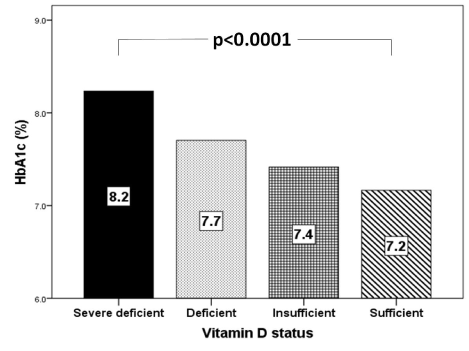
Figure 2. Mean HbA1c (%) in correlation to vitamin D status categories
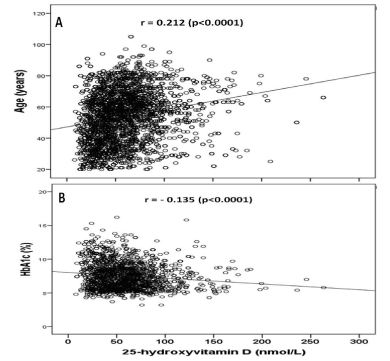
Figure 3. Correlation of 25-hydroxyvitamin D concentration and age (A) and HbA1c (B) in the study population
The high prevalence of T2DM worldwide and in Saudi Arabia, in addition, the accumulated evidence on the status of vitamin D under different conditions make it extremely important to determine the relationship between vitamin D and T2DM [5,26]. This cross-sectional study is the first study that observed an association between 25(OH)D levels in a sample of female subjects with T2DM, as far as we know. Garg et al found that the prevalence of vitamin D level < 50 nmol/l in the Indian women was 64.06% and the prevalence of vitamin D level < 75 nmol/l was 98.75% [27]. This is in accordance to the study conducted by Kritiar et al. in which vitamin D deficiency was 93% and out of which 34% subjects were vitamin D insufficient and 59% had frank deficiency and also in accordance to the study in which the prevalence of Vitamin D deficiency (levels < 50 nmol/l) was found in 90.8% [28]. Three studies from Saudi Arabia reported that 30%, 71% and 87% of women were vitamin D deficient respectively [29-31] The lower vitamin deficiency in our study compared to others might be due to the different subjects characteristics, which were not limited only to the patients with T2DM as the previous studies. Our study involved female subjects of general population and not only on limited population such as subjects who lived in nursing home. It is of importance to state that the sample size is representative for a number of subjects suffering from T2DM in the area and study population of one institution does not represent the entire city of Jeddah, in addition the study sample confined to patients with T2DM but without comparable groups.
Some factors estimated as affecting factors on the development of vitamin D deficiency were analyzed and we found evidence that age and HbA1c were statistically significant. Female subjects often used sun protectors, which consequently reduced the direct sun light exposure. We found serum 25-OHDconcentration was strongly correlated with age, moreover, was highest in the eighth decade and lowest in the fourth decade, that is similar to the findings of Hashemipour et al, in a cohort of 1210 Iranians adult [32]. The strong correlation of 25(OH)D to age is also in agreement with a study carried out in the US, where vitamin D deficiency was found to be more common among the young, and less common among the elderly [33].
We found vitamin D deficient patients have statistically significant higher HbA1c than the insufficient and sufficient groups. Moreover, 25-OHD concentration was inversely correlated with HbA1c. These findings are supported by a number of international studies. In contrast some studies show no association of a low vitamin D with HbA1c levels [34]. But inverse correlation between the level of vitamin D and glucose level is well known [35-37]. In many studies vitamin D levels were low in subjects having higher HbA1c values in patients with T2DM indicating that they are inversely related [38]. Vitamin D has various effects on glucose homeostasis. Besides its role in insulin secretion, it also has an influence on insulin resistance directly or via Ca indirectly [39]. Changes in Ca in primary insulin target tissues may contribute to peripheral insulin resistance via impaired insulin signal transduction, leading to decreased glucose transporter-4 activity [40]. The results from the trials on the effect of vitamin D and/or Ca supplementation on insulin resistance have showed improvement on insulin action [41].
We had several limitations. Our study was a cross-sectional study; therefore, it could not evaluate the causal association directly among the studied variables as observed in longitudinal or interventional study. In addition, the study was done at one centre and was done at one point of time. The study sample confined to patients with T2DM but without comparable groups. Another limitation in our study includes the limited sample size. The high prevalence of vitamin D deficiency in our study consequently will call for greater sample size. Parathyroid level should also be studied as an indicator of vitamin D deficiency.
In conclusion, the prevalence of vitamin D deficiency in females particularly young with T2DM is high. We recommend larger scale studies for detecting vitamin D deficiency in our female population with T2DM and suggest planning strategies to supplement our female population with vitamin D.
There is no financial support or relationships that may pose conflict of interest.
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, et al. (2013) A systematic review of vitamin D status in populations worldwide. Br J Nutr 9: 1-23. [Crossref]
- Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81: 353-373. [Crossref]
- Sedrani SH, Elidrissy AW, El Arabi KM (1983) Sunlight and vitamin D status in normal Saudi subjects. Am J Clin Nutr 38:129-132. [Crossref]
- Al-Turki HA, Sadat-Ali M, Al-Elq AH, Al-Mulhim FA, Al-Ali AK (2008) 25-Hydroxyvitamin D levels among healthy Saudi Arabian women. Saudi Med J 29: 1765-1768. [Crossref]
- Alqurashi KA, Aljabri KS, Bokhari SA (2011) Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med 31: 19-23. [Crossref]
- Matilla C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA et al. (2007) Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care 30: 2569-2570. [Crossref]
- Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, et al. (2006) Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29: 650-656. [Crossref]
- Thorand B, Zierer A, Huth C, Linseisen J, Meisinger C, et al. (2011) Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care 34: 2320-2322. [Crossref]
- Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, et al. (2006). Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 29: 722-724. [Crossref]
- Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, et al. (1995) Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract 27: 181-188. [Crossref]
- Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, et al. (2003). Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB 17: 509-511. [Crossref]
- Clark SA, Stumpf WE, Sar M (1981) Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes 30: 382-386. [Crossref]
- Johnson JA, Grande JP, Roche PC, Kumar R (1994) Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 267: 356-360. [Crossref]
- Maestro B, Campion J, Davila N, Calle C (2000) Stimulation by 1, 25 dihydroxy vitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 47: 383-391. [Crossref]
- Williams PF, Caterson ID, Cooney GJ, Zilkens RR, Turtle JR (1990) High affinity insulin binding and insulin receptor effector coupling: modulation by Ca 2+. Cell Calcium 11: 547-556. [Crossref]
- Zemel MB (1998) Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem 188: 129-136. [Crossref]
- Mori H, Okada Y, Tanaka Y (2015) Incidence of vitamin D deficiency and its relevance to bone metabolism in Japanese postmenopausal women with type 2 diabetes mellitus. Intern Med 54: 1599-1504. [Crossref]
- Tahrani AA, Ball A, Shepherd L, Rahim A, Jones AF, et al. (2010) The prevalence of vitamin D abnormalities in South Asians with type 2 diabetes mellitus in the UK. Int J Clin Pract 64: 351-355. [Crossref]
- Miñambres I, Sánchez-Quesada JL, Vinagre I, Sánchez- Hernández J, Urgell E, et al. (2014) Hypovitaminosis D in type 2 diabetes: relation with features of the metabolic syndrome and glycemic control. Endocr Res 40: 160-165. [Crossref]
- Hollick MF (2004) Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. AmJ Clin Nutr 79: 362-371. [Crossref]
- Setiati S, Oemardi M, Sutrisna B (2007) The role of ultraviolet B from sun exposure on vitamin D and parathyroid hormone level in elderly women in Indonesia. Asian J Gerontol Geriatr 2: 126-132.
- Al-Zaharani M (2013) The prevalence of Vitamin D deficiency in Type 2 Diabetic patients. Majmaah J Health Sciences 1: 18-22.
- Al-Saleh Y, Sulimani R, Sabico S, Raef H, Fouda M, et al. (2015) 2015 guidelines for osteoporosis in Saudi Arabia: recommendations from the saudi osteoporosis society. Ann Saudi Med 35: 1-12. [Crossref]
- Maalouf G, Gannagé-Yared MH, Ezzedine J, Larijani B, Badawi S, et al. (2007) Middle east and north africa consensus on osteoporosis. J Muskuloskelet Neuronal Interact 7: 131-143. [Crossref]
- Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med 1364: 248-254. [Crossref]
- Hashemipour S, Larijani B, Adibi H, Javadi E, Sedaghat M, et al. (2004) Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health 4: 38. [Crossref]
- Ruchika G, Vishy A, Prabhat A, Saroj S, Neharika M (2018) Prevalence of vitamin D deficiency in Indian women. Int J Reprod Contracept Obstet Gynecol 7: 2222-2225.
- Shukla K, Sharma S, Gupta A, Raizada A, Vinayak K (2016) Current Scenario of Prevalence of Vitamin D deficiency Ostensibly Healthy Indian Population.A Hospital based Retrospective Study. Indian J Clin Biochem 31: 452-457. [Crossref]
- Elsammak MY, Al-Wossaibi AA, Al-Howeish A, Al Saeed J (2011) High prevalence of vitamin D deficiency in the sunny eastern region of Saudi Arabia: a hospital-based study. East Mediterr Health J 17: 317-322. [Crossref]
- Burti, CA, Ashwood ER (1999) Tietz Textbook of clinical chemistry. Philadelphia: WB Saunders 39-56. [Crossref]
- Al-Elq AH (2012) The status of Vitamin D in medical students in the pre-clerkship years of a Saudi medical school. J Family Community Med 19: 100-104. [Crossref]
- Hashemipour S, Larijani B, Adibi H, Javadi E, Sedaghat M, et al. (2004) Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health 4: 38. [Crossref]
- Plotnikoff GA, Quigley JM (2003) Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc 78: 1463-1470. [Crossref]
- Husemoen LLN, Thuesen BH, Fenger M, Jorgensen T, Glumer C, et al. (2012) Serum 25 (OH)D and Type 2 Diabetes Association in a General Population: A prospective study. Diabetes Care 35: 1695-1700. [Crossref]
- Palomer X, Gonzalez-Clemente J, Blanco-Vaca F, Mauricio D (2008) Role ofvitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes ObesMetab 10: 185-197. [Crossref]
- Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ (1995) Glucose intoleranceand impairment of insulin secretion in relation to vitamin D deficiency in eastLondonAsians. Diabetologia 38: 1239-1245. [Crossref]
- Hutchinson MS, Figenshau Y, NjølstadI, Schirmer H, Jorde R (2011) Serum25-hydroxyvitamin D levels are inversely associated with glycatedhaemoglobin (HbA(1c)). The TromsøStudy. 2011. Scand J Clin Lab Invest 71: 399-406. [Crossref]
- Kositsawat J, Freeman VL, Gerber BS, Geraci S (2010) Association of A1Clevels with vitamin D status in U.S. adults: data from the National Health and Nutrition Examination Survey. Diabetes Care 33: 1236-1238. [Crossref]
- Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R (2003) The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 57: 258-261. [Crossref]
- Zemel MB (1998) Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem 188: 129-136. [Crossref]
- Nazarian S, St Peter JV, Boston RC, Jones SA, Mariash CN (2011) Vitamin D3 supplementation improves insulin sensitisity in subjects with impaired fasting glucose. Transl Res 158: 276-281. [Crossref]



