Oral, mouth/rinses, povidone-iodine, covid-19, viral load, pcr
Coronavirus disease 19 (COVID-19) pandemic is a public health emergency of international concern [1] that is associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. SARS-CoV-2 initially colonizes the upper respiratory tract of infected individuals [2]. The transmission of virus to humans mainly occurs through a direct transmission of large or aerosolized respiratory droplets [3,4]. Human saliva have a plays crucial role in COVID-19 and the salivary gland is a significant reservoir of the virus [5]. The principal measures for the control of transmission are social distancing and the use of facemasks [6] but the antiviral efficacy of oral rinsing solutions against SARS-CoV-2 has been suggested as a convenient and efficient measure to decrease viral transmission. Povidone-iodine (PVP-I) has a potent anti-viral activity in vitro [7,8]. Although it’s in vivo efficacy is unknown, it has been previously shown to be active against severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses (SARS-CoV and MERSCoV) [9,10]. It has also been recently demonstrated its in vitro virucidal activity against SARS-CoV-2 [11-13]. Those data motivated us to ascertain it’s in vivo efficacy by analyzing the usefulness of mouth rinses and gargling with PVP-I as a mean to decrease the oropharyngeal viral load of SARS-CoV-2 in patients with COVID-19.
This was a “non-interventional trial” approved by the Spanish Agency for Medicines and Health Products (Spanish Health Ministry). We included 62 patients (mean age 43±17 yrs.; range: 19-86 yrs.; 55% male) with a recent diagnosis of SARS-CoV-2 in nasopharyngeal swabs, confirmed by RT-PCR (VIASURE SARS-CoV-2 Real Time PCR Detection Kit, Certest, Zaragoza, Spain).They were volunteers recruited among patients in the Emergency room, medical ward or outpatient clinic between March and June 2020, within 24 hours of a first positive PCR (i.e., CT<35 for either N or R genes). The exclusion criteria were a history of allergy to PVP-I, all forms of thyroid disease, radioactive iodine treatment, lithium therapy, known pregnancy or age < 18 yrs.
Thirty-one patients received mouth rinses/gargling with PVP-I (oral solution 100 mg/ml, minimal duration 30 seconds) every 8 hours for two consecutive days and 31 patients received nothing (control group). Other therapeutic measures were prescribed according to clinical indication. After first PCR, nasopharyngeal swabs and PCR were repeated after 3 (2-4), 11 (9-13) and 17 (14-19) days.
Quantitative reverse transcription PCR (qRT-PCR) was performed using EDX SARS-CoV-2 Standard (Exact Diagnostics, Bio-Rad), an approved commercial kit with synthetic RNA transcripts containing five gene targets (E, N, ORF1a, RdRP and S Genes) of SARS-CoV-2 that are each quantitated at 200,000 cp/ml. Viral load was calculated by plotting Ct values onto the standard curve constructed based on the standard product. The viral load was expressed as Log10 of the average viral load in both genes (N and R) and as the average of the Ct value for gene N and R. Cycle threshold (Ct) values were inversely proportional to viral loads. Serologic studies (IgG) were performed using an automated chemiluminescent immunoassay (Virclia, Monotest, Vircell, S.L., Granada, Spain). The laboratory procedure and interpretation were done blindly of the study group.
The study protocol was approved by the Institutional Review Board and all patients gave informed consent.
The results were expressed as mean and standard deviation (SD) for quantitative variables and percentage for qualitative variables. We used the Kolmogorov-Smirnov test to check for normal distribution. Quantitative variables were analyzed by Student t- test if the variables had a normal distribution, or the nonparametric Mann–Whitney U test were used to compare between-group differences. We used Chi-2 test to compare qualitative variables. All analyses were performed using SPSS 23.0 software (Chicago, IL, USA). A two-sided p < 0.05 was considered statistically significant.
The baseline characteristics of both groups are shown in table 1. There were no differences in age and sex distribution, proportion of symptomatic patients, or the type and duration of symptoms.
Table 1. The baseline characteristics of both groups. Mean (SD) or n (percentage). Ct=cycle threshold, RT-PCR=real time reverse transcription polymerase chain reaction.
|
Povidone-iodine
N=31 |
Controls
N=31 |
p-value |
Sex Male n (%) |
17 (55%) |
17 (55%) |
0.25 |
Age yrs. |
40.8 (16.4) |
45.8 (18.0) |
0.25 |
Out-patients % |
27 (87%) |
26 (84%) |
0.50 |
Patients with symptoms n (%) |
22 (72%) |
21 (69%) |
0.53 |
Type of Symptom:
- Fever
- Myalgia
- Anosmia and/or disgeusia
- Cough
- Dyspnea
- Diarrhea |
27 (89%)
18 (60%)
16 (53%)
12 (38%)
8 (27%)
4 (12%) |
26 (86%)
17 (56%)
19 (61%)
13 (43%)
9 (29%)
2 (7%) |
0.26
0.40
0.50
0.80
0.50
0.45 |
Duration of symptoms, days |
4 (2) |
3 (1) |
0.11 |
Ct value N gene
- First PCR
- Second PCR
- Third PCR
- Fourth PCR |
27.7 (6.3)
32.7 (7.0)
39.7 (3.7)
40.3 (3.8) |
26.7 (7.2)
32.9 (7.9)
38.1 (4.9)
40.7 (2.5) |
0.58
0.94
0.16
0.67 |
Ct value R gene
- First PCR
- Second PCR
- Third PCR
- Fourth PCR |
27.0 (7.8)
32.2 (7.6)
39.6 (3.8)
40.5 (3.9) |
26.2 (8.5)
32.8 (8.9)
38.5 (5.3)
41.7 (0.9) |
0.71
0.79
0.37
0.12 |
Also, the mean time elapsed between tests was similar in both groups (between the first and second PCR, 3±1 days in the PVP-I group, 3±1 days in controls; between the first and the third PCR 11±2 vs. 11±3 days; between the first and the fourth PCR, 17±3 vs. 18±3 days; p=0.62).
The percentage of patients with a negative result in the second PCR was 26% in the PVP-I group and 32% in controls (p=0.39); in the third PCR, 58% and 50%, respectively (p=0.47); and in the fourth PCR, 80% in both groups (p=0.62) (Figure 1).
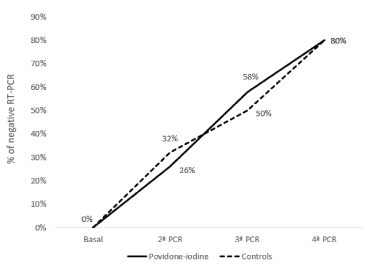
Figure 1. Percentage of patients with negative RT-PCR.
The quantitative estimation of the viral load in the first PCR was also similar in both groups (6.1±2.0 log copies/ml in PVP-I and 6.5±2.3 log copies/ml in controls; p=0.51). After the intervention, the viral load remained similar: 4.0±2.7 vs. 3.9± 3.1 (p=0.84), 1.4±1.8 vs. 1.9±2.3 (p=0.37), and 1.7±2.2 vs.1.2±1.5 copies/ml (p=0.55) at the second, third and fourth PCRs, in the PVP-I and control groups, respectively (Figure 1). Likewise, the Ct values of N and R genes were similar in both groups, both at diagnosis and in the analyses, following the intervention (Table 1). PVP-I rinses did not appear to modify the immune response against the virus. In fact, 87% of patients in the PVP-I group and 95% of controls developed immunoglobulin G (Ig G) in response to SARS-CoV-2 infection, analyzed at an average time of 74±31 and 81± 48 days in the PVP-I and control groups, respectively.
Recent scientific evidence suggests a relevant role of the oral cavity in the transmission and pathogenicity of SARS-CoV-2. Thus, it has been speculated that oral antiseptics could lower the number of infectious aerosolized particles and, consequently, the risk of transmission [14]. PVP-I has a potent antiviral activity, related to the concentration of free iodine, partly due to the disruption of surface proteins essential for the spread of enveloped viruses [15,16]. Recent studies found a clear antiviral action of PVP-I in vitro [17]. A study with four commercial PVP-I-based products (antiseptic solution PVP-I 10%, skin cleanser PVP-I 7.5%, gargle and mouth wash PVP-I 1% and throat spray PVP-I 0.45%) showed 99.99% antiviral activity against SARS-CoV-2 corresponding to ≥4 log10 reduction of virus within 30 seconds of contact [10]. Another study with different commercially available oral rinses showed that PVP-I significantly reduced viral infectivity in vitro up to 3 orders of magnitude to background levels in viral load [11].
Those encouraging results in vitro led several investigators to suggest a possible role PVP-I in patients with COVID-19. However, in a randomized clinical trial in 24 out-patients with COVID-19, the use of PVP-I mouthwash, gargle, and nasal spray had no influence on changes of viral RNA quantification in nasopharyngeal swabs [18]. Our controlled study explores in vivo the utility of mouth rinses and gargling with PVP-I in the reduction of oropharyngeal viral load of SARS-CoV-2. Within this aim, a PVP-I group and a control group, showing similar baseline viral loads, were assayed. However, after intervention the viral load evolved similarly in both groups. In other words, in comparison with the control group, mouth rinses/gargling did not reduce the viral load of SARS-CoV-2 in nasopharyngeal swabs or accelerated the negativization of PCR.
This study has some limitations. First, it was not randomized nor blinded. However, both groups were well balanced at baseline, and investigators performing viral analyses were unaware of the patient’s group. Second, since we did not perform viral cultures, we cannot exclude the possibility of some between-group differences in the load of viable viruses. Nevertheless, given the remarkable similarity in the PCR-assessed viral abundance, both at the short-term and the long-term, we consider that a remarkable difference in viable virus is also unlikely.
Given the limited activity against SARS-COV2 of currently available systemic drugs [19], there is considerable interest in local therapies and other measures aimed to decrease the infectivity of patients with COVID-19. In that sense, topical antiviral agents, such as PVP-I, with potent virucidal activity in vitro, are attractive candidates. However, our results do not support the clinical usefulness of PVP-I mouth washes in patients with COVID-19.
Funded by grants from the Superior Council of Scientific Investigations (CSIC) and Javier Xercavins. Ministerio de ciencia e innovación. Spain.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733. [Crossref]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465-469. [Crossref]
- Meselson M (2020) Droplets and aerosols in the transmission of SARS-CoV-2. N Engl J Med 382: 2063.
- Liu J, Liao X, Qian S, Yuan J, Wang F, et al. (2020) Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis 26: 1320-1323. [Crossref]
- Fini MB (2020) Oral saliva and COVID-19. Oral Oncol 108: 104821. [Crossref]
- Coronavirus Disease 2019 (COVID-19) Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings.
- Ito H, Hikida M, Yashiro J, Kida H, Ito T (2009) Virucidal efficacy of povidone-iodine products against swine influenza viruses. J Chemother 57: 508-510.
- Kawana R, Kitamura T, Nakagomi O, Matsumoto I, Arita M, et al. (1997) Inactivation of human viruses by povidoneiodine in comparison with other antiseptics. Dermatology 2: 29-35. [Crossref]
- Eggers M, Eickmann M, Zorn J (2015) Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect Dis Ther 4: 491-501. [Crossref]
- Kariwa H, Fujii N, Takashima I (2004) Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Dermatology 212: 119-123. [Crossref]
- Anderson DE, Sivalingam V, Zheng Kang AE, Ananthanarayanan A, Arumugam H, et al. (2020) Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect Dis Ther 9: 669-675. [Crossref]
- Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, et al. (2020) Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2. J of Infect Dis 222: 1289-1292.
- Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, et al. (2020) Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J Prosthodont 29: 599-603. [Crossref]
- Herrera D, Serrano J, Roldán S, Sanz M (2020) Is the oral cavity relevant in SARS-CoV-2 pandemic?. Clin Oral Investig 24: 2925-2930. [Crossref]
- Shirai J, Kanno T, Tsuchiya Y, Mitsubayashi S, Seki R (2000) Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses. J Vet Med Sci 62: 85-92. [Crossref]
- Sriwilaijaroen N, Wilairat P, Hiramatsu H, Takahashi T, Suzuki T, et al. (2009) Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol J 6: 124. [Crossref]
- Meyers C, Robison R, Milici J, Alam S, Quillen D, et al. (2020) Lowering the transmission and spread of human coronavirus. J Med Virol 93: 1605-1612. [Crossref]
- Guenezan J, Garcia M, Strasters D, Jousselin C, Lévêque N, et al. (2021) Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 147: 400-401. [Crossref]
- Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J (2020) Early Dynamics of Transmission and Control of COVID-19: A Mathematical Modelling Study. Lancet Infect Dis 20:553-558. [Crossref]
Editorial Information
Editor-in-Chief
Tomoko Hasunuma
Kitasato University School of Medicine
Article Type
Research Article
Publication history
Received: April 23, 2021
Accepted: May 05, 2021
Published: May 17, 2021
Copyright
©2021 Pablo-Marcos D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Pablo-Marcos D, Abascal B, Lloret L, Cuadra MG, Velasco N, et al. (2021) Utility of mouth rinses and gargling with povidone-iodine in patients with covid-19. J Clin Invest Stud. 4. DOI: 10.15761/JCIS.1000128
Corresponding author
Carmen Valero Diaz de Lamadrid
Department of Internal Medicine, Hospital Marqu�s de Valdecilla Avenida de Valdecilla s/n. 39008 Santander, Spain
E-mail : bhuvaneswari.bibleraaj@uhsm.nhs.uk

