Plasma cell myeloma (PCM) comprises 10-15% of all hematopoietic neoplasms. The disease shows a male predominance with a median age of diagnosis of 70 years. Clinically, patients are either symptomatic or asymptomatic upon presentation. Symptomatic patients present with signs of end-organ damage, such as hypercalcemia, renal insufficiency, anemia, and lytic bone lesions with serum and urine studies showing elevated M-protein levels [1]. PCM demonstrates disseminated bone marrow involvement by a clonal plasma cell infiltrate. IgG PCM represents 70% of cases followed by IgA. While IgD or IgE PCM has been reported, these cases are rarely seen, with less than 50 cases of IgE PCM reported in current literature.
IgE PCM was first described in 1967, with an estimated prevalence of <0.1% of all plasma cell neoplasms [2]. Similar epidemiology and clinical presentation are seen in patients with IgE PCM as compared to patients with other myelomas. Anemia, Bence-Jones proteinuria, and progression to secondary plasma cell leukemia have been seen in higher frequency in IgE myeloma patients [3].
A 78-year-old male was referred to a Nephrologist for renal insufficiency. Routine blood work showed creatinine of 12.2; serum immunofixation showed free monoclonal lambda light chain band and a low kappa/lambda light chain ratio; urine immunofixation also showed a free lambda light chain band. He was admitted to the hospital for worsening uremia and need for arteriovenous access for hemodialysis. His significant past medical history included hypertension, chronic kidney disease, anemia, and prostate cancer (status post radical prostatectomy). Concurrently the patient noted, weight loss, and fatigue. Initial laboratory work up revealed a normocytic anemia (WBC 5.1 k/uL, MCV 96.7 fl, Hgb 10.3 g/dL, platelet 138 k/uL). Creatinine was 14.14 and BUN 84. Calcium was 8.2 with albumin 4.1 and LDH 222. Serum protein electrophoresis showed an abnormal band in the beta region; serum immunofixation showed free lambda M protein. The serum kappa/lambda light chain ratio was 0.12 (reference range 0.26-1.65). Serologic studies revealed abnormal band in the beta fraction on serum protein electrophoresis and free lambda M protein on serum immunofixation. Quantitative serum immunoglobulin levels (IgG, IgA, IgM, and IgE) were obtained (Table 1), confirming the presence of IgE-lambda para-protein. Free lambda light chains (free lambda 4525) in serum and urine with a kappa/lambda ratio of 0.05. Random urine protein was 78 mg/dl and total protein calculated at 404.5 mg/dl. The urine M protein was calculated at 30.34 mg/dl. Urine electrophoresis showed an abnormal band in the gamma regions and urine immunofixation showed monoclonal free lambda light chains. CT scan of the chest revealed numerous lytic lesions within the ribs and vertebral bodies, concerning for a plasma cell neoplasm; skeletal survey showed multiple lytic lesions throughout the bony skeleton.
Table 1. Quantitative serum immunoglobulin levels (IgG, IgA, IgM, and IgE) were obtained, confirming the presence of IgE-lambda para-protein.
| |
Results (July 2017) |
Reference Limits |
IgG |
1210 |
700-1600 mg/dL |
IgA |
164 |
70-400 |
IgM |
27 |
40-230 |
IgE |
1040 |
<214 kU/L |
Lambda |
2227.2 |
5.7-26.3 mg/L |
Kappa |
276.8 |
3.3-19.4 mg/L |
Beta 2 Microglobulin |
33.1 |
0.0-2.6 mg/L |
Immunoglobulin E, Total |
614 |
0-100 IU/mL |
A bone marrow biopsy was performed revealing a plasma cell myeloma with lambda light chain restriction. Therefore, an IgE lambda PCM diagnosis was established. During hospital admission, the patient underwent treatment with cyclophosphamide, bortezomib, and dexamethasone (Table 1).
A bone marrow aspirate and biopsy were performed. The bone marrow aspirate smears contained scattered marrow elements with increased mature appearing plasma cells. The core biopsy revealed a slightly hypocellular (overall 30-40% cellularity) marrow with an interstitial, paratrabecular, and focally diffuse plasma cell infiltrate (Figure 1A). These plasma cells comprised 50% of the total cellularity, showing positivity for CD138 (Figure 1B) and lambda in situ hybridization (Figure 1C). Further immunohistochemistry results revealed these plasma cells to be negative for kappa in situ hybridization, IgG, IgA, IgM, and IgD (Fig. 1D-H).
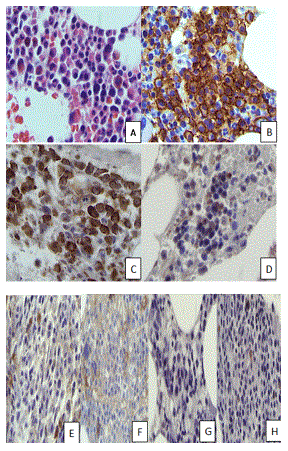
Figure 1. Histopathology of bone marrow. (A) Sheets of mature plasma cells with clumped chromatin, abundant cytoplasm, and low nuclear-cytoplasmic ratio were seen on bone marrow biopsy. The plasma cells were positive for CD138 (B), lambda in situ (C), and negative for kappa in situ (D). The plasma cells were also negative for IgG (E), IgA (F), IgM (G), and IgD (H). (A, H&E, original magnification x100, B, C, D, E, F, G, H, original magnification x 100 respectively).
Four-color flow cytometry performed on the bone marrow aspirate revealed no evidence of increased blasts or lymphoproliferative disorder.
FISH was performed on bone marrow cells and 11% of cells (above laboratory normal reference range of 4%) showed CCND-1-IGH [t(11;14)] fusion POSITIVE hybridization pattern (Figure 1).
IgE myeloma is a rare type of myeloma, reported to comprise about 0.01% of all myelomas. The first case was reported in 1967 by Johansson and Bennich and since then only individual case reports have appeared in the literature [4]. Although the clinical presentation is similar to other myelomas, hepatosplenomegaly is reported to be more common [5] as is amyloidosis [6] and plasma cell leukemia [7]. IgE myeloma is thought to have a worse clinical course than other types, Renal insufficiency is seen in IgE myelomas as in other types of myeloma and also is an indicator of poor prognosis. Average survival time was reported by Kairemo et al in 1999, to be shorter than other forms of myeloma (one year versus 30 months) and to be 33 months after autologous transplantation by Morris et al in 2010. Whether current advances in therapy which include proteasome inhibitors, immunomodulators and novel agents would result in significant improvements is to be determined.
All multiple myeloma patients with an apparently free light chain without an IgG or IgA M-protein must be screened for the presence of IgD and IgE. The amount of IgD and IgE immunoglobulin in the serum may be very low and can escape detection with electrophoresis. Patients are sometimes given a false diagnosis of nonsecretory or light chain myeloma,
The first case was reported in 1967, and fewer than 50 cases have been described to date [8]. In one reported case, a patient with IgE monoclonal gammopathy of undetermined significance was followed for 12 years before developing symptomatic MM [9]. Given the rarity of IgE MM, knowledge about this condition is gathered from isolated case reports and a few small case series. A review of 29 published cases by Macro et al reported a mean age at diagnosis of 62 years, with a slight preponderance of male patients. The clinical features of IgE MM are similar to those of IgG MM, IgA MM, and light chain MM, as well as IgD MM [10]. Bone pain, anemia, renal failure, hypercalcemia, BJP, amyloidosis, and an increased incidence of PCL are frequently noted. The median survival of the 29 patients reported by Macro et al was 16 months. Although survival time is generally short, a patient diagnosed with IgE MM at the age of 56 survived for more than 20 years and died of chronic comorbidities at age of 77 years [10].
The presence of t(11;14)(q13;q32) was reported in 83% of patients with IgM MM, IgE MM, and non-secretory MM. This was five-fold greater than the rate reported in patients with IgD MM. Thus, this translocation is a hallmark of IgE MM [11]. It is characterized by the translocation of CCND1 gene and the immunoglobulin heavy chain enhancer resulting in overexpression of cyclin D1. Distinct morphologic and immunophenotypic characteristics have been associated with t(11;14) PCM. In up to 50% of reported cases, small lymphocyte-like or lymphoplasmacytoid features have been seen, often presenting as a diagnostic challenge as these entities can mimic B-cell lymphoma. In addition to these morphologic features, t(11;14) PCM have been reported to occasionally express both mature-B cell markers, CD19, CD20, PAX5, and plasmacytic markers, CD138 with surface light chain restriction. Therefore, clinical correlation in combination of cyclin D1 expression demonstrated by immunohistochemistry with detection of the IGH/CCND1 fusion by FISH studies are essential to formulating a correct diagnosis [12].
The process of evaluation and management of IgE MM is similar to that of the other isotypes [13]. Monitoring of disease response in IgE MM may be difficult, because of excess antigen levels [14]. Hua et al. reported an increase in serum Krebs von den Lungen-6 (KL-6) levels in IgE MM and suggested that KL-6 be used for disease monitoring.
Morris et al. reporting on a series of 13 patients with IgE MM, noted CR rates of 60% following ASCT, compared with 28% CR overall for patients with IgG MM, IgA MM, and light chain MM. The median PFS was the same in both groups. The median OS was 33 months in the 13 patients with IgE MM, compared with a median OS of 62 months for the common myeloma types.
IgD MM and IgE MM are uncommon variants of myeloma. Their clinical features are similar to those of the other isotypes, but there appears to be an increased incidence of amyloidosis and EMD in IgD MM, and an increased incidence of PCL in IgE MM. When there is a suspicion of the diagnosis of myeloma and only monoclonal light chain is detected in the serum or urine, the patient must be screened for the presence of IgD and IgE monoclonal protein. The unique morphologic and immunophenotypic characteristics of IgD and IgE t(11;14) PCMs may require additional FISH studies and be highly dependent on the clinical presentation in order establish an accurate diagnosis for these patients. Although the response to chemotherapy and ASCT is satisfactory, the OS has been shorter. However, most of the reported data on IgD MM and IgE MM were reported before availability of the novel agents that are now used in this setting (thalidomide, bortezomib, and lenalidomide). The response to treatment in patients with IgD MM is similar to that of patients with other myeloma isotypes; however, survival time is generally shorter than in patients with the common myelomas. In the current era of novel therapy and autologous transplantation, reported survival was improved for patients with IgD MM who underwent ASCT, compared with those who did not. More studies are needed to help us better understand the biology of rare myelomas and to further improve outcomes for patients.
To our knowledge, our patient is the first patient with IgE myeloma with ESRD on hemodialysis. Though the patient’s IgE and beta 2 microglobulin level fell with combination chemotherapy his renal function did not improve. Whether this was because of another etiology or simply irreversibility of myeloma renal failure could not be determined.
- Ke Li, Grace K, Min Y, Elizabeth B, Michael K, et al. (2012) A rare and unique case of aggressive IgE-k plasma cell myeloma in a 28-year-old woman presented initially as an orbital mass. Human Pathology 43: 2376-2384.
- Jaffe ES, Harris NL, Stein H, Vardiman JW (2001) World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press, Lyon, pp: 142-148.
- Chiu W, Pullon H, Woon ST, Oei P, The R, et al. (2010) IgE-type multiple myeloma with the late development of IgA2 kappa and plasma cell leukaemia. Pathology 42: 82-84. [crossref]
- Macro M, André I, Comby E, Chèze S, Chapon F, et al. (1999) IgE multiple myeloma. Leuk Lymphoma 32: 597-603. [crossref]
- Gamundí GE, Morandeira RF, Clap&eacut2021 Copyright OAT. All rights reserv new case report. Clinical Chemistry and Laboratory Medicine (CCLM) 55: e37-e40.
- Alexander RL Jr, Roodman ST, Petruska PJ, Tsai CC, Janney CG (1992) A new case of IgE myeloma. Clin Chem 38: 2328-2332. [crossref]
- Lorsbach, Robert B, Eric DH, Ahmet D, Falko F (2011) Plasma cell myeloma and related neoplasms. Am J Clin Pathol 136: 168-182.
- Pandey, Shivlal, Kyle, Robert A (2013) Unusual Myelomas: A review of IgD and IgD variants. Oncology (Williston Park) 27: 798-803.
- Avet-Loiseau H, Garand R, Lode L (2003) Translocation t(11;14)(q13; q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood 101: 1570-1571.
- Macro M (1999) IgE Multiple Myeloma. Leukemia and Lymphoma 32: 597-603.
- Jako JM, Gesztesi T, Kaszas I (1997) IgE lambda monoclonal gammopathy and amyloidosis. Int Arch Allergy Immunol 112: 415-421. [crossref]
- Johansson SG, Bennich H (1967) Immunological studies an an atypical (myeloma) immunoglobulin. Immunology 4: 381-394.
- Richardson, Paul G (2013) IgD and IgD Variants of Myeloma: Valuable insights and therapeutic opportunities. Oncology (Williston Park) 27: 803-804.
- Curly M, Mary D, Jane A, Simona I, Anjavan B, et al. (2010) Efficacy and outcome of autologous transplantation in rare myelomas. Haematologica 95: 2126-2133.
