Abstract
Unilateral globus pallidus internus (GPi) pallidotomy is one of the therapeutic options for advanced PD patients with medication-refractory motor fluctuation. There are several neurosurgical or neuroradiological treatments for advanced PD and transcranial magnetic resonance imaging-guided focused ultrasound (MRgFUS) is a novel neurosurgical approach. In this article, we review MRgFUS GPi pallidotomy for advanced PD. Previous reports of MRgFUS GPi pallidotomy are limited; however, it could grow a popular treatment for advanced PD because of its minimal invasiveness.
Keywords
Parkinson’s disease, Magnetic resonance imaging-guided focused ultrasound, Globus pallidus internus, Pallidotomy
Introduction
Parkinson disease (PD) is a slowly progressive neurodegenerative disorder manifested by a multiple motor and non-motor symptoms. Current treatments for motor features of PD are based on dopamine replacement therapy, including levodopa, dopamine agonist, monoamine oxidase B inhibitor, and catechol-O-methyltransferase inhibitor [1]; however, many PD patients in advanced stage annoy medication-refractory motor-fluctuation.
Posteroventral globus pallidus internus (GPi) pallidotomy with radiofrequency (RF) thermocoagulation had been recognized as the one of the therapeutic options motor-fluctuation in PD [2]. To date, transcranial magnetic resonance imaging-guided focused ultrasound (MRgFUS), which is a novel technique of intracranial focal ablation characterized by a minimum invasiveness, applied for not only several neurological and psychological disorders [3], but advanced PD [4,5]. In this article, we review MRgFUS GPi pallidotomy for PD.
MRgFUS GPi pallidotomy
Transcranial MRgFUS is a new MR image- and MR thermography-guided neurosurgical approach. The main advantages of MRgFUS are minimum invasiveness, low exposure to radiation, immediate therapeutic effect, and no risk of infection (Table 1) [5]; however, it has also some limitations. Chang and colleagues pointed that skull density ratio (SDR), the mean value for the ratio of Hounsfield units of marrow and cortical bone, and skull volume have relations with the required energy for ablation of the target. SDR is correlated positively with maximal temperature in the target, whereas skull volume is inversely correlated with it [6]. The GPi target is lateral to the ventral intermediate nucleus (Vim) which has been a target for essential tremor [7] and tremor dominant PD [8]. As a result, a higher number of ultrasound rays have high incident angle with the skull on way to the target. This leads to a smaller number of elements that deliver energy effectively in MRgFUS GPi pallidotomy compared with Vim thalamotomy. Therefore, high SDR value is critical for the success of MRgFUS GPi pallidotomy.
Table 1. The features of the conventional neurosurgical and radiological treatments and MRgFUS [5].
? | RF-thermo coagulation | γ-knife | DBS | MRgFUS | |
Hair shaving | partial | none | partial | complete | |
| |
Incision / Burr hole | must | none | must | none | |
| |
Radiation | low (X-ray, CT) | high | low (X-ray, CT) | low (screening CT) | |
General anesthesia | no | no | yes * | no | |
| |
Therapeutic duration | 1hr | 1hr | 2~4hrs | 3~4hrs ** | |
| |
Efficacy onset | immediate | delayed up to | adjustable | immediate | |
1 year | |
Bilateral procedure | impossible | impossible | possible | unknown | |
| |
Post-treatment MRI | possible | possible | limited *** | possible | |
| |
Hemorrhagic complications | possible | almost none | possible | almost none | |
Infection | possible | none | possible | none | |
| |
Long-term data | yes | yes | yes | limited **** | |
| |
*for implantation of pulse generator.
** time spent in MRI room
*** with an MR-conditional cardiac pacemaker system
**** [7]
On the day of the procedure, the patient in the medication-off state is shaved completely and placed in a stereotactic head frame which is coupled to 1,024 elements, phased-array ultrasound transducer. The stereotactic target for GPi is obtained at 3 mm anterior, 1.5 mm inferior, and 20 mm lateral to the midpoint between anterior and posterior commissure. After stereotactic targeting, we increase the total energy of the sonications stepwise by either increasing the intensity or extending the sonication duration with marking ipsilateral optic nerve to monitor its temperature. We have to watch the temporal pain, nausea / vomiting, floating sensation, and visual field deficit during the procedure. We finish the sonications if we confirm the sufficient improvement of parkinsonism.
Previous Reports of MRgFUS GPi pallidotomy for PD
Na and colleagues reported the first PD patient performed unilateral MRgFUS GPi pallidotomy. The patient was a 78-year-old woman with a 12-year history of PD and presented medication-refractory motor fluctuation. Unified Parkinson's Disease Rating Scale (UPDRS) part III score and Unified Dyskinesia Rating Scale (UdysRS) score reduced 60.0% (on medication), 54.8% (off medication), and 70.2%, respectively without any change of levodopa equivalent dose (LED) for 6 months after the procedure. There was no description about adverse events [4,9].
We reported the second PD patient performed unilateral MRgFUS GPi pallidotomy [5]. This patient was a right-handed 78-year-old woman with a 15-year history of PD and annoyed medication-refractory motor fluctuation. UPDRS part ? score was 19 (on medication) and 33 (off medication), and UdysRS score was 28 (LED: 773.5mg). Left GPi pallidotomy was scheduled; however, her SDR was 0.26 at the enrollment (exclusion criteria: ≤ 0.30). We administered 35mg of alendronate per week, a second-generation bisphosphonate, and it elevated to 0.36 after 6 months. We performed MRgFUS GPi pallidotomy with a 1.5-Tesla MRI (Signa HDx, GE Healthcare, Milwaukee, USA) and a focused ultrasound system (Exablate 4000®, InSightec, Tirat Carmel, Israel). We sonicated 12 sessions and the maximum target temperature reached 51°C (maximum sonication energy: 35740 J). UPDRS part ? score reduced 94.7% (on medication) and 63.6% (off medication) at 6 months after the procedure. Furthermore, dyskinesia disappeared after the procedure according to the reduction of LED to 499.0mg. Left temporal pain during the sonication and swelling of left eyelid after the procedure occurred; however, both were transient (Figure 1).
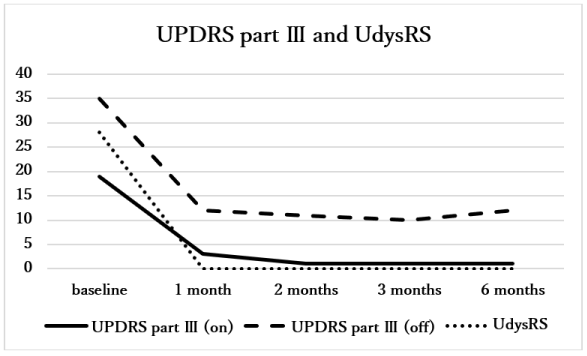
Figure 1. UPDRS part ? scores, UdysRS score, and LED at baseline and up to 6 months (UPDRS: Unified Parkinson’s disease rating scale, UdysRS: Unified dyskinesia rating scale, LED: levodopa equivalent dose [9]).
Conclusion
Although the efficacy and safety of MRgFUS GPi pallidotomy for PD remains to be elucidated, it might become one of therapeutic options for PD patients with intractable motor fluctuation. Further investigations with more patients and longer follow-up periods should be necessary.
Acknowledgments
We thank Dr. Takaomi Taira (Department of Neurosurgery, Tokyo Women’s Medical University), Dr. Toshio Yamaguchi (Research Institute of Diagnostic Imaging, Shin-Yurigaoka General Hospital), and Mr. Itay Rachmilevitch (Application Team, InSightec) for their technical supports to perform MRgFUS GPi pallidotomy.
References
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, et al. (2011) The Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord 26: S2-S41.
- Baron MS, Vitek JL, Bakay RA, Green J, Kaneoke Y, et al. (1996) Treatment of advanced Parkinson's disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol 40: 355-366. [Crossref]
- Weintraub D, Elias WJ (2017) The emerging role of transcranial magnetic resonance imaging–guided focused ultrasound in functional neurosurgery. Mov Disord 32: 20-27.
- Na YC, Chang WS, Jung HH, Kweon EJ, Chang JW (2015) Unilateral magnetic resonance-guided focused ultrasound pallidotomy for Parkinson disease. Neurology 85: 549-551.
- Ito H, Taira T, Fukutake S, Yamamoto K, Baba Y, et al. (2018) Magnetic resonance imaging-guided focused ultrasound unilateral pallidotomy for Parkinson’s disease: a case report. Int J Case Rep 2: 7.
- Chang WS, Jung HH, Zadicario E, Rachmilevitch I, Tlusty T, et al (2016) Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg 124: 411-416.
- Chang JW, Park CK, Lipsman N, Schwartz ML, Ghanouni P, et al. (2018) A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: Results at the 2-year follow-up. Ann Neurol 83: 107-114. [Crossref]
- Ito H, Fukutake S, Yamamoto K, Yamaguchi T, Taira T, et al. (2018) Magnetic resonance imaging-guided focused ultrasound thalamotomy for Parkinson’s disease: a case report. Inter Med 57: 1027-1031.
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, et al. (2010) Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25: 2649-2653. [Crossref]

