Parkinson’s Disease (PD), as one neurodegenerative disease, reduce the life quality of our patients extensively. Since the identification and validation of role of alpha-synuclein in PD, several impactful hypotheses pointed out the direction of our research, while the bioinformatic studies initialized to focus on the generation, formation, transportation and aberrant clearance of alpha-synuclein. The pioneer studies of Braak provided a comprehensive classification between alpha-synuclein and the severity of PD. Currently our understanding of bioinformatic on PD are increasing sharply as well from genomic, proteomic and metabolomic. To our best knowledge, here we provide a comprehensive picture of this disease from genomic to metabolomic.
parkinson’s disease, bioinformatics, genomics, proteomisc, metabolomics.
Parkinson’s Disease (PD) is a serious neurodegenerative disease of the central nervous system. The prevalence of population over 65 years old arrived at 1.7% and the global incidence rate is 1.5% [1]. In 2016, there were 6.1 million people suffered from PD, including 2.9 million women and 3.2 million men. It caused 211,296 deaths [2], unfortunately incidence increased with age [3]. According to epidemiological statistics prediction, there will be more than 14 million patients of PD in 2040.
The treatment on PD requires robust financial support. Obviously, it is a huge economic burden not only for patients and his/her families, but also on the society. According to the global epidemiological statistics, the sum of direct and indirect costs of treatment on PD in Europe reached 7.7 billion Euros in 2010. While in United States, the total cost of medical and non-medical treatment for every PD patient has increased by a total of $22,800 [4]. As for China, the annual direct medical expenses for patients with PD per year in Shanghai, amount to 4,305 yuan, and the non-medical expenses are about 3,301 yuan [5].
Date back to 2003, Braak, et al. proposed the PD stage according to the specific pattern of α-syn diffusion, which describing the relationship between α-synuclein and disease severity [6]. These six stages clarified the severity of PD accordingly to deficit from sensory to sport zone in the brain. The key pathological traits of PD is the damage or deficit of dopaminergic neurons in the dense part of the substantia nigra. This can be leading to a decrease in the secretion of dopaminergic neurons [7]. The loss of dopaminergic neurons reaches 50%, and the corresponding clinical manifestations appears [8]. The clinical features are mainly static. Sport symptoms such as tremors, bradykinesia, muscle rigidity, and unstable posture are also associated with non-motor symptoms, including constipation, sensory disturbances, depression, autonomic disorders, and sleep disorders [9] (Figure 1). Non-motor symptoms of PD are earlier with exercise needle symptoms [10].
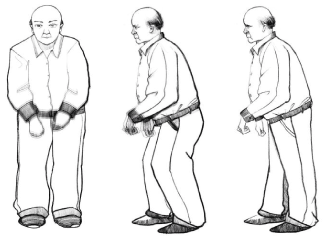
Figure 1. Dominant manifestation Trembling of Parkinson’s Disease
Accumulation of α-synuclein is the main cause of Parkinson's disease [11], but recent studies have shown that dopamine metabolites can increase neuronal deformation, pamine is oxidized to produce reactive sputum substances, hydrogen peroxide and other reactive oxygen species. These metabolites have an accelerating effect on PD [12].
Oxidative stress is a stress injury caused by intracellular oxidation and anti-oxidation imbalance [13]. Reactive oxygen species (ROS) is produced during oxidative stress, leading to mitochondrial disorders and ultimately neuronal death. Under physiological conditions, reactive oxygen is an important redox form that regulates various signaling pathways. It plays an important role in regulating different cellular metabolism, post-transcriptional modification, antioxidant defense mechanisms, and excessive reactive oxygen destroy cellular lipids. Studies disclosed that dopamine neurons in the brain damaged via the path of oxidative stress and mitochondrial dysfunction, due to the reduced dopamine secretion [14].
The accumulation of unfolded or misfolded proteins caused by glucose starvation, hypoxia, calcium homeostasis or oxidative stress is known as endoplasmic reticulum stress [15]. Folding false or unfolded proteins inhibit synaptic function and interfere with signal transduction pathways, these are key factors leading to ubiquitin proteasome system-mediated protein degradation dysfunction, thereby altering the normal physiological morphology of cells, disrupting protein homeostasis, triggering protein accumulation, and causing the neuron degeneration [11].
Neuroinflammatory response is considered to be one of the major pathogenic factors of neurodegenerative disease [16]. Activation of astrocytes and microglia releases harmful reactive oxygen species and pro-inflammatory factors, while over-activated microglia also increase oxidative stress and cause neuronal cell deformation [17]. Intestinal flora and its metabolites increase intestinal permeability and bacterial translocation, stimulate local and systemic inflammation of the intestine, resulting in the production of lewy bodies in the intestine, and the metabolic products (lipopolysaccharides, etc.) [18]. Ring blood-brain barrier, promoting inflammation and the damage of the substantia nigra.
Accompanied with our increased knowledge several interest gene SNCA (PARK1; encoding α-synuclein) [19], LRRK2 (PARK8; encoding dakarin) [20], VPS35 (encoding vacuolar protein sorting 35) [21], PINK1 (PARK6; PTEN-induced kinase 1) [22], DJ-1 (PARK7) [23], Parkin (PARK2) [22], ATP13A2 (PARK9) [24] and FBXO7 [25] lead to autosomal recessive PD and/or Parkinson's disease, have been validated that they associated with development of PD [18].
Environment selectio,,,,,,,,,,, et al. , et al. ,
Di, et al. so contributes as risk factor in the development of PD. In 2019, Anna Sauerbier, et al. reported a correlation existed between diet and Parkinson's disease [30]. Andre Rodrigues Vasconcelos, et al. demonstrated that dietary energy restriction (DER) may induces activation of the transcription factor Nrff2, which activates the expression of phase 2 detoxification enzymes, thereby increasing neuronal resistance against oxidative stress and death, and reducing PD risk [31]. At the same time, X. Gao, et al. disclosed that the intake of some flavonoids may reduce the risk of PD. Oxidative stress has a negative impact on flavonoids such as apoptosis and the formation of α-synuclein fibers. Also it inhibits the use of dopamine neurons [32].
The pathology of PD is caused by the imbalance of alpha-synuclein generation and clearance [33]. In 1998, Polymeropoulos, et al. investigated the beta-synuclein gene was extremely high expressed in brain, this gene showed the inhabitation phospholipase D2 selectively [34]. In 2007, Gasser T, et al. studied the gene function of SNCA, causing the alpha-synuclein gene to develop sporadic PD [35]. Wade-Martins, et al. investigated the function of Leucine gene rich with repeat kinase 2 (LRRK2) in PD. The data disclosed this gene is involved in endosomal-autophagic pathway and the recruitment of specific membrane microdomains under the physiological human gene expression [36]. In 2010, Papapetropoulos S, et al. tested the function of RNA splicing gene SRRM2 (or SRm300), sereine/arginine repetitive matrix 2. It is playing the crucial role in RNA splicing in PD [37]. In 2011, Das F, et al. discovered that oncogene DJ-1 prevents oxidative damage and apoptosis of dopaminergic neurons in animal models of Parkinson's disease [38]. In 2012 Maraganore DM, et al. performed the first genome-wide association study (GWAS), identified a series of candidate gene including, C8orf4 participate in inflammation process. CACNB4 has a calcium channel function. TRPM3 inhibit AKT protein kinase and ITPK 2 encode the ER function [39]. In 2014, Screaton RA, et al. reported upon mitochondrial damage such as PINK1is stabilized on the outer mitochondrial membrane where it phosphorylates ubiquitin, generates a signal for the recruitment and activation of Parkin [40].
Similar research interest, LRRK attracts numerous attentions as well. Saniz J found LRRK2-G2019S participated in Akt signaling, glucose metabolism and immunity in 2014 [41] and cell adhension molecular, complement, coagulation cascade in 2016 [42]; Sealfon SC reported LRRK2-G2019S may increase kinase activity to antagonize specific microRNAs [43]. Moreover, the gene PARKs also draws great research interest. Hoffman-Zacharska D, et al. found it predominantly involved in rearrangement processes in genomic region in 2015 [44]; while Alonso I, et al. validated its similar functions mediate the deletion in homogeneous population in 2016 [45]. More interestingly, Bosch E, et al. discovered correlation between stop codon and PARK in 2017 [46] (Table 1).
Table 1. Genomic understanding of PD
Author |
Year |
Interested Gene |
Function |
Polymeropoulos MH |
1998 |
beta-synuclein gene |
Inhibit phosphatase D2 selectively |
Gasser T |
2007 |
SNCA |
alpha-synuclein |
Wade-Martins R |
2009 |
LRRK2-R1441C |
Abnormal autophagy balance, abnormal MVB formation |
Papapetropoulos S |
2010 |
SRRM2 |
mRNA expression, signal transduction |
Das F |
2011 |
DJ-1 |
Anti-apoptosis |
Maraganore DM |
2012 |
C8orf4 |
Inflammation |
CACNB4 |
Calcium channel complex protein |
TRPM3 |
Inhibition of AKT protein kinase |
ITPK2 |
Calcium channel encoding ER |
Screaton RA |
2014 |
PINK1 |
Phospho-ubiquitin method on the outer membrane of mitochondria, recruitment and activation to generate signals |
Sainz J |
2014 |
LRRK2-G2019S |
Akt signaling, glucose metabolism or immunity |
Sealfon SC |
2015 |
LRRK2-G2019s |
Increases kinase activity to antagonize specific microRNAs |
Hoffman-Zacharska D |
2015 |
PARK2 |
rearrangement processes |
Orr-Urtreger A |
2015 |
GBA |
Peripheral blood leukocyte-associated gene down-regulation |
Lou Z |
2015 |
Parkin |
mitotic defects, genomic instability, tumorigenesis |
Alonso I |
2016 |
PARK2 |
rearrangement processes |
Sainz J |
2016 |
LRRK2-G2019s |
Cell adhesion molecule, complement, coagulation cascade |
Bosch E |
2016 |
PARK2 |
Premature stop codon |
Kim J |
2017 |
GBA-GBAP1 |
Pseudogene rearrangement, gene conversion |
|
|
LK2GS |
gene expression in the intestinal cells. |
Table 2. Proteins Discovered in PD
Author |
Date |
Key protein |
Biological function (Annotation) |
|
Nussbaum RL |
2005 |
Alpha-synuclein |
Preferentially binds to oligomers of the membrane, binds to lipid droplets and affects triglyceride metabolism |
|
Singh MP |
2009 |
albumin precursor, serum albumin chain-A, PRR14 and serum transferrin N-terminal lobe |
Neuronal dysfunction |
|
| |
Fasano M |
2014 |
alpha-synuclein |
reduces nuclear factor kappa B activation |
|
Yang P |
2015 |
apolipoprotein A-I |
lipid metabolism, deposition process of proteins |
|
Burkhard PR |
2016 |
CNDP2 |
oxidative stress, protein aggregation inflammation. |
|
Iseri P |
2016 |
E3-protein ubiquitin ligase |
cell metabolism |
|
Robinson PA |
2017 |
Parkin |
regulating mitochondrial activity within cells |
|
Mandell JW |
2018 |
caspases |
synapse loss |
|
Ketterman AJ |
2018 |
Human glutathione transferase omega 1 |
modulating stress response |
|
Arenas E |
2018 |
Leucine-rich repeat kinase 2 |
Maturation of substantia nigra dopaminergic neurons |
|
Yen SH |
2018 |
alpha-synuclein |
compromise cell viability. |
|
Maguire-Zeiss KA |
2018 |
Alpha-synuclein |
Oxidative stress, inflammation |
|
Rubinsztein DC |
2018 |
lrrk2 |
impairs the activity of the ubiquitin-proteasome pathway, |
|
Lindquist S |
2018 |
Alpha-synuclein |
Inhibition of vesicle transport |
|
Cookson MR |
2018 |
DJ-1 |
Protected neurons against death |
|
Harvey K |
2019 |
lrrk2 |
protein translation and trafficking |
|
Zhang Y |
2019 |
(MTHSP75), (PHGDH), (LBP), (14-3-3epsilon) and YWHAZ protein(14-3-3zeta) |
mitochondrial dysfunction, serine synthesis, amyloidclearance, apoptosis process and neuroprotection |
|
Mann M |
2019 |
lrrk2 |
Vesicle transport |
|
To our best knowledge, the imbalance between generation and clearance of alpha-synuclein is playing dominant role in PD [47]. The CSF is produced by the brain and participates in the internal circulation of the brain. Therefore, it is the best indicator to reflecting the structural characteristics of the pathophysiology of the brain [48]. The pathological marker of PD is the formation of Lewy bodies, while α-synuclein (α-syn) is the main component. alpha-synuclein may resist damage through aggregation. So when the damage occurs, alpha-synuclein will be aggregate continuously, resulting in mitochondrial damage caused by oxidative stress, beyond the ability of the cell to withstand, and cannot clear abnormal proteins, thereby producing toxic effects on cells and accelerating cell deaths [49].
Studies also uncovered that the level of alpha-synuclein in CSF of patients with PD gradually decreases with the progression of the disease. Decrease in the total α-syn entering the systemic circulation after α-syn fibrosis in the brain tissue may result in total alpha CSF [50]. With respects to the function of alpha-synuclein, Nussbaum RL, et al. found that the alpha-synuclein may bind to the membrane of oligomers, lipid droplet and affected triglyceride metabolism [51]. In 2018, Yen SH demonstrated that it may participate in cell viability [52]. Maguire-Zeiss KA, et al. found that it was involve in oxidative stress and inflammation [53], while Lindquist S, et al. demonstrated that it inhibit the vesical transport [54].
Regarding to other researches interest proteins, Singh MP, et al. reported that the albumin precursor, serum albumin chain-A, PRR14 and serum transferrin N-terminal lobe can be reduce the neuronal dysfunction in 2009 [55]. Yang P, et al. summarized that apolipoprotein A-I is involve in lipid metabolism and deposition process of proteins in 2015 [56]. Burkhard PR, et al. disclosed that cytosolic non-specific dipeptidase 2 (CNDP2) participate in oxidative stress, protein aggregation and inflammation [57]. Robinson PA, et al. validated that protein Parkin participate in the regulating mitochondrial activity [58].
In 2018, Ketterman AJ validated that the human glutathione transferase omega 1 modulating stress response [59]. Arenas E, et al. reported that leucine-rich repeat kinase 2 accelerate the maturation of substantia nigra dopaminergic neurons [60]. While, Rubinsztein DC, et al. revealed that Leucine-rich repeat kinase 2, LRRK2, may impair the activity of ubiquitin proteasome pathway [61]. Harvey, et al. demonstrated that LRRK2 participate in protein translation and trafficking [62]. Mann M, et al. figured out that LRRK2 involve in vesicle transport [63].
Moreover, Cookson MR, et al. disclosed that the DJ-1 may protect the neurons against death [64]. Zhang Y, et al. reported that the mitochondrial heat shock protein 75 (MTHSP75), phosphoglycerate dehydrogenase (PHGDH), laminin binding protein (LBP), tyrosine,,,
Kaddurah-Daouk R, et al. disclosed that uric acid may reduce the antioxidative damage in 2009 [66]; Jones DP, et al. found that the polyamine regulate cell growth and its size value in 2013 [67]; Pamplona R, et al. reported that glutathione participate in buffering oxidative stress [68]; Kong L, et al. found that creatine may protect neurons from oxidative stress in 2014 [69]; Rango M, et al. reported in 2015 that HEP involved in oxidative phosphorylation, mitochondrial damage and lactic acid may reduce mitochondrial damage [70]; Powers R, et al. reported that sorbitol sugar can cause the mitochondrial dysfunction, oxidative stress. Pyruvate is involved in Tricarboxylic acid cycle. This is known since 2015 [71]; Liu H, et al. reported that serotonin participate in metabolic pathways including antioxidant activities and citrate cycle [72]. Cai H, et al. found that guanosine participate in neurodegeneration to prevent 1-methyl-4-phenylpyridinium (MPP+)-induced PC12 cell, and carnosine protect brains mainly through antioxidant, and antiglycative properties in 2015 [73]. Gao H, et al. found that Glu, Gln, GABA inhibit neurotransmitter GABA and lactate may increase the lactate level in the striatum of 6-OHDA-induced PD ratsin 2015 [74].
Madine J, et al. found that taurine may stabilise cell membranes because assisting in ion transport to antioxidant and increase cognitive function; Dimethylamine may produce NO which can induce oxidative/nitrative stress conditions and in turn damage mitochondrial complex I, complex II and mitochondrial aconitase [75]; Cai Z, et al. established that Tryptophan influent CNS inflammation and amino acid play a notable role in signal exchange between neurons [76] (Table 3.1).
Table 3.1. Metabolism of PD understanding from 2009-2017
Author |
Date |
Metabolites |
Pathway |
Kaddurah-Daouk R |
2009 |
uric acid |
Antioxidative damage |
Jones DP |
2013 |
Polyamine |
Regulate cell growth and increase value |
Pamplona R |
2014 |
Glutathione |
Buffering oxidative stress |
Kong L |
2014 |
Creatine |
Protect neurons from oxidative stress |
Rango M |
2015 |
HEP |
Oxidative phosphorylation, mitochondrial damage |
| |
|
Lactic acid |
Mitochondrial damage |
Powers R |
2015 |
Sorbitol sugar |
Mitochondrial dysfunction, oxidative stress |
| |
|
Pyruvate |
Tricarboxylic acid cycle |
Liu H |
2015 |
Serotonin |
metabolic pathways, antioxidant activities, citrate cycle |
Cai H |
2015 |
Guanosine |
neurodegeneration, prevent 1-methyl-4-phenylpyridinium (MPP+)-induced PC12 cell |
|
|
α-synuclein |
the first genetic causal factor linked to PD |
| |
|
Carnosine |
protect brains mainly through antioxidant, metal chelating, and antiglycative properties |
Gao H |
2015 |
Glu, Gln,GABA |
inhibitory neurotransmitter GABA |
| |
|
lactate |
Increased the lactate level in the striatum of 6-OHDA-induced PD rats relative to normal rats |
Jolicoeur M |
2017 |
ATP |
Energy metabolism, oxidative stress |
Madine J
|
2017
|
Taurine |
stabilise cell, membranes assisting in ion transport and is suggested to have antioxidant properties and to increase cognitive function |
Creatinine |
anti-oxidant |
Dimethylamine |
produce NO which can induce oxidative/nitrative stress conditions and in turn damage to mitochondrial complex I, complex II, and mitochondrial aconitase |
Cai Z |
2017 |
Tryptophan |
influence CNS inflammation |
fatty acids |
They play roles in metabolic and inflammatory disorders |
Tauroursodeoxycholic acid |
neuro protective agent |
Amino acid neurotransmitters |
play a notable role in signal exchange between neurons |
Roy R, et al. found that branched-chain amino acids regulate the mitochondrial respiration and participate in the synthesis of neurotransmitters, while histidine helps in scavenging ROS [77]; Lamberts JT, et al. found in 2019 that urate involved in antioxidant and reactive oxygen species (ROS) scavenger, meanwhile, adenosine play anti-inflammatory effects [78]; Eggers C, et al. found that mannose regulate the immune signal and mediate inflammatory response [79]; Le W, et al. found that cerebrospinal fluid metabolome involved in antioxidative stress responses and metabolic pathways of sphingolipid, glycerophospholipid and amino acid. Consistently, urate participate in against oxidative stress in 2019 [80]. Hattori N, et al. discovered that benzoate-related metabolites may altered in gut microbiota and caffeine and its metabolites affect the malabsorption [81]. Xu F, et al. reported that adenosine may attenuating oxidative stress, excitotoxicity and neuroinflammation, promoting sleep, improving cognitive function and exerting anti-depressive effects in 2019 [82] (Table 3.2).
Table 3.2. Metabolism of PD understanding from 2018-2019
Author |
Date |
Metabolites |
Pathway |
Roy R |
2018 |
branched-chain amino acids |
mitochondrial respiration, participate in the synthesis of neurotransmitters |
histidine |
an antioxidant, helps in scavenging ROS |
Lamberts JT |
2019 |
Urate |
antioxidant and reactive oxygen species (ROS) scavenger |
Adenosine |
anti-inflammatory effects |
Eggers C |
2019 |
fatty acids |
fatty acid oxidation |
mannose |
immune signal, mediate inflammatory response |
Le W |
2019 |
Cerebrospinal fluid metabolome |
involved in antioxidative stress responses, and metabolic pathways of sphingolipid, glycerophospholipid and amino acid |
Urate |
against oxidative stress |
Hattori N |
2019 |
fatty acid |
lipid metabolism |
benzoate-related metabolites |
alteration in gut microbiota. |
caffeine and its metabolites |
malabsorption |
Xu F |
2019 |
Adenosine |
attenuating oxidative stress, excitotoxicity and neuroinflammation,promoting sleep, improving cognitive function and exerting anti-depressive effects |
Philosophy on computational
Thanks to the knowledge of central dogma, it disclosed the basic life rules from replication, transcription, reverse transcription and translation of DNA. It maintains the general life process and responses the modification from inner and outer of body [83]. Several databases, such as GEO, GenBank etc., which collected the data from all over the world. This is not only an opportunity, but also a risk. Due to ununified samples and different design principles, the data we see may only one tip of iceberg.
Robust algorithm and powerful online platform
Due to numerous customized packaged developed and test in R, including sequencing [84], mapping [85], comparing [86], identifying [87], finding [25] and predicting [88]. More particular, the package named as “Bioconductor”, so far there are 1714 packages. They can be classified into assay domain, biological question, annotation data, experiment data and workflow, which almost cover full aspects of bioinformatics. While many online platform developed to disclose the cellular pathway [89], including over-representation analysis tool, geneset enrichment analysis, network module-based pathway analysis. Furthermore, aim to get a comprehensive picture of changes, molecular network alteration became another emerging hot topic. For example, the network perturbation analysis, causal reasoning analysis, were developed as valuable complementary tools to conventional pathway analyses.
It is worth noting that no matter the robust code or powerful online platform, it is only handling the unique dataset. Because the experiment or trial on Parkinson’s patient was customized, including the inclusive and exclusive criteria, specific intervention, biological sampling, samples processing method. Any kind of different middle term may introduce the variation. It furtherly strength the difficult in data merge. Therefor the current data only reflect one perspective, not comprehensive and globe view of the object. The reason behind may explain as the below cartoon, every investigator may report one fact based on his/her dataset. But another challenge remains, that is how to merge the data orderly
Big data need big idea
An obvious tendency accompanied with the big data is that the mainstream data are overload with much noise, and the discovery of the truths that underly big data poses various challenges. Here are some challenges remaining, such as the sample uniformity in different bioinformatic databases including DNA, RNA and metabolism. The biggest challenge is heterogeneity, the bioinformatic data were generated by different methods from different biological samples at different periods. Therefore, it is obvious that the principle of current data merge is not suitable or reliable. Even though comprehensive data were being introduced more and more under the surroundings of big data era, however, the error fact or noise were also being introduced.
Big data needs big idea. Traditional data analysis explain the modifications under a certain period with specific purpose. As for the Parkinson’s Disease, the pathological changes occurred in time sequence. For example, with unknown etiologic reason, several toxins from environment such as rotenone, MPTP, paraquat, pesticide and heavy metal may induced the damage on structure of DNA, consequently, the balance between clearance and generation of alpha-synuclein was broken. The abnormal deposition of alpha-synuclein among neurons may reduce the depletion of dopamine. Consequently, the movement disorder may be presented from the early stage of PD, (Figure 2). Therefore, the concept of transomic is emerged. The integral data need to be drawn from different databases with unified samples, while the data flow needs to be merged or combined according to the time-series
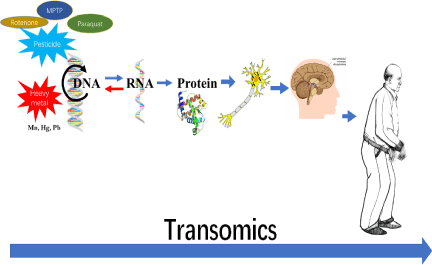
Figure 2. The concept of data flow of reasoning in PD
Thanks to huge development of “-omic” studies, our understanding of PD grows rapidly as well. From the genomic perspective, to identifying and validating the key driver gene become a critical issue. Because more and more genes may be triggered and to modified the pattern of translation and transcription due to increased entropy, while it may be reported due to a single research. However, the general picture may missing. That is of importance to discover the key driver gene. While the treatment effect and adverse event of deep brain stimulation on PD receive great satisfactory from both patients and doctors. It has been serviced in front clinic for two decades. Numerous evidence validated the modification of electric field on micro-environment among neurons can be rearrange the dopamine distribution. Therefore, to rearrange and generate the neuron transmitters may become the new strategy to against the Parkinson’s Disease. We place great expectations on these technologies to personalize treatment for patients with Parkinson’s Disease.
We thank the Dr Huang Kun from Chengdu TME Co.,Ltd for providing a robust and valuable big data analysis. This work was supported by the Chengdu Medical College Natural Science Foundation (CYZ18-08, CYZ18-20, CYZ18-33), the Sichuan Provincial Education Department (17ZA0134), the Sichuan Medical Association (S18023), and Sichuan undergraduate innovation and startup program funding support (S201913705080, S201913705130, S201913705059)
The authors declare no potential conflict of interest with respect to research, authorship and/or publication of this manuscript.
To the best of our knowledge, the materials included in this chapter do not violate copyright laws. All original sources have been appropriately acknowledged and/or referenced. Where relevant, appropriate permissions have been obtained from the original copyright holder(s).
- Benito-Leon J (2018) Epidemiology of Parkinson's disease in Spain and its contextualisation in the world. Rev Neurol 66: 125-134.
- Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, et al. (2018) Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17: 939-953.
- Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson's disease: A systematic review and meta‐analysis. Mov Disord 29: 1583-1590.
- Afentou N, Jarl J, Gerdtham UG, Saha S (2019) Economic Evaluation of Interventions in Parkinson's Disease: A Systematic Literature Review. Mov Disord Clin Pract 6: 282-290.
- Wang G, Cheng Q, Zheng R, Tan YY, Sun XK, et al. (2006) Economic burden of Parkinson's disease in a developing country: a retrospective cost analysis in Shanghai, China. Mov Disord : Movement Disorder Society 21: 1439-1443.
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, et al. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197-211.
- Forno LS (1996) Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 55: 259-272.
- Gaenslen A, Swid I, Liepelt-Scarfone I, Godau J, Berg D (2011) The patients' perception of prodromal symptoms before the initial diagnosis of Parkinson's disease. Mov Disord: Movement Disorder Society 26: 653-658.
- Bridi JC, Hirth F (2018) Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson's Disease. Front Neurosci 12: 80.
- Lee HM, Koh SB (2015) Many Faces of Parkinson's Disease: Non-Motor Symptoms of Parkinson's Disease. J Mov Disord 8: 92-97.
- Cabral-Miranda F, Hetz C (2018) ER Stress and Neurodegenerative Disease: A Cause or Effect Relationship? Curr Top Microbiol Immunol 414: 131-157.
- Li SQ, Li WB, Zhang M, Wu YZ, Hu YY (2013) The role of neuroglobin in the neuroprotection of limb ischemic preconditioning in rats. Mol Neurobiol 47: 197-208.
- Hemmati-Dinarvand M, Saedi S, Valilo M, Kalantary-Charvadeh A, Alizadeh Sani M, et al. (2019) Oxidative stress and Parkinson's disease: conflict of oxidant-antioxidant systems. Neurosci Lett 709: 134296.
- Crotty GF, Ascherio A, Schwarzschild MA (2017) Targeting urate to reduce oxidative stress in Parkinson disease. Exp Neurol 298: 210-224.
- Segura-Aguilar J (2019) On the Role of Aminochrome in Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Parkinson's Disease. Front Neurosci 13: 271.
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140: 918-934.
- Kalia LV, Lang AE (2015) Parkinson's disease. Lancet (London, England) 386: 896-912.
- Hernandez DG, Reed X, Singleton AB (2016) Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem 139: 59-74.
- Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK (2016) Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat Disord 22: S1-S6.
- Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, et al. (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol 7: 583-590.
- Rahman AA, Morrison BE (2019) Contributions of VPS35 Mutations to Parkinson's Disease. Neuroscience 401: 1-10.
- Bonello F, Hassoun SM, Mouton-Liger F, Shin YS, Muscat A, et al. (2019) LRRK2 impairs PINK1/Parkin-dependent mitophagy via its kinase activity: pathologic insights into Parkinson's disease. Human molecular genetics 28: 1645-1660.
- Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, et al. (2003) DJ-1( PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci 24: 159-160.
- Bento CF, Ashkenazi A, Jimenez-Sanchez M, Rubinsztein DC (2016) The Parkinson's disease-associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat Commun 7: 11803.
- Chen R, Peng Y, Choi B, Xu J, Hu H (2014) A private DNA motif finding algorithm. J biomed infor 50: 122-132.
- James KA, Hall DA (2015) Groundwater pesticide levels and the association with Parkinson disease. Int J Toxicol 34: 266-273.
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, et al. (2012) The outdoor air pollution and brain health workshop. Neurotoxicology 33: 972-984.
- Sarfo FS, Ovbiagele B (2017) Mobile health for stroke: a promising concept for research and practice. m Health 3: 4.
- Chen CY, Hung HJ, Chang KH, Hsu CY, Muo CH, et al. (2017) Long-term exposure to air pollution and the incidence of Parkinson's disease: A nested case-control study. PloS one 12: e0182834.
- Sauerbier A, Schrag A, Martinez-Martin P, Hall LJ, Parry M, et al. (2018) Dietary Variations in a Multiethnic Parkinson's Disease Cohort and Possible Influences on Nonmotor Aspects: A Cross-Sectional Multicentre Study. Parkinson's disease 2018: 7274085.
- Vasconcelos AR, Dos Santos NB, Scavone C, Munhoz CD (2019) Nrf2/ARE Pathway Modulation by Dietary Energy Regulation in Neurological Disorders. Front Pharmacol 10: 33.
- Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A (2012) Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 78: 1138-1145.
- Jan A, Jansonius B, Delaidelli A, Bhanshali F, An YA, et al. (2018) Activity of translation regulator eukaryotic elongation factor-2 kinase is increased in Parkinson disease brain and its inhibition reduces alpha synuclein toxicity. Acta Neuropathol Commun 6: 54.
- Lavedan C, Leroy E, Torres R, Dehejia A, Dutra A, et al. (1998) Genomic organization and expression of the human beta-synuclein gene (SNCB). Genomics 54: 173-175.
- Fuchs J, Tichopad A, Golub Y, Munz M, Schweitzer KJ, et al. (2008) Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB journal 22: 1327-1334.
- Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, et al. (2009) LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Human molecular genetics 18: 4022-4034.
- Shehadeh LA, Yu K, Wang L, Guevara A, Singer C, et al. (2010) SRRM2, a potential blood biomarker revealing high alternative splicing in Parkinson's disease. PloS one 5: e9104.
- Das F, Dey N, Venkatesan B, Kasinath BS, Ghosh-Choudhury N, et al. (2011) High glucose upregulation of early-onset Parkinson's disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal 23: 1311-1319.
- Chung SJ, Armasu SM, Biernacka JM, Anderson KJ, Lesnick TG, et al. (2012) Genomic determinants of motor and cognitive outcomes in Parkinson's disease. Parkinsonism Relat Disord 18: 881-886.
- Ng AC, Baird SD, Screaton RA (2014) High-content functional genomic screening to identify novel regulators of the PINK1-Parkin pathway. Methods Enzymol 547: 1-20.
- Infante J, Prieto C, Sierra M, Sanchez-Juan P, Gonzalez-Aramburu I, et al. (2015) Identification of candidate genes for Parkinson's disease through blood transcriptome analysis in LRRK2-G2019S carriers, idiopathic cases, and controls. Neurobiol Aging 36: 1105-1109.
- Infante J, Prieto C, Sierra M, Sanchez-Juan P, Gonzalez-Aramburu I, et al. (2016) Comparative blood transcriptome analysis in idiopathic and LRRK2 G2019S-associated Parkinson's disease. Neurobiol Aging 38: 214.e1-.e5.
- Chikina MD, Gerald CP, Li X, Ge Y, Pincas H, et al. (2015) Low-variance RNAs identify Parkinson's disease molecular signature in blood. Mov Disord : Movement Disorder Society 30: 813-821.
- Ambroziak W, Koziorowski D, Duszyc K, Gorka-Skoczylas P, Potulska-Chromik A, et al. (2015) Genomic instability in the PARK2 locus is associated with Parkinson's disease. J Appl Genet 56: 451-461.
- Morais S, Bastos-Ferreira R, Sequeiros J, Alonso I (2016) Genomic mechanisms underlying PARK2 large deletions identified in a cohort of patients with PD. Neurology Genetics 2: e73.
- Spataro N, Roca-Umbert A, Cervera-Carles L, Valles M, Anglada R, et al. (2017) Detection of genomic rearrangements from targeted resequencing data in Parkinson's disease patients. Mov Disord: Movement Disorder Society 32: 165-169.
- Sevlever D, Jiang P, Yen SH (2008) Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry 47: 9678-9687.
- Marques TM, van Rumund A, Bruinsma IB, Wessels H, Gloerich J, et al. (2018) Cerebrospinal Fluid Galectin-1 Levels Discriminate Patients with Parkinsonism from Controls. Mol Neurobiol.
- Poschmann G, Seyfarth K, Besong Agbo D, Klafki HW, Rozman J, et al. (2014) High-fat diet induced isoform changes of the Parkinson's disease protein DJ-1. J Proteome Res 13: 2339-2351.
- Majbour NK, Vaikath NN, van Dijk KD, Ardah MT, Varghese S, et al. (2016) Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson's disease. Mol Neurodegener 11: 7.
- Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, (2002) Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem 277: 6344-6352.
- Kulathingal J, Ko LW, Cusack B, Yen SH (2009) Proteomic profiling of phosphoproteins and glycoproteins responsive to wild-type alpha-synuclein accumulation and aggregation. Biochim Biophys Acta 1794: 211-224.
- Beraud D, Hathaway HA, Trecki J, Chasovskikh S, Johnson DA, et al. (2013) Microglial activation and antioxidant responses induced by the Parkinson's disease protein alpha-synuclein. J Neuroimmune Pharmacol 8: 94-117.
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, et al. (2008) The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA 105: 145-150.
- Sinha A, Srivastava N, Singh S, Singh AK, Bhushan S, et al. (2009) Identification of differentially displayed proteins in cerebrospinal fluid of Parkinson's disease patients: a proteomic approach. Clin Chim Acta 400: 14-20.
- Zhang X, Yin X, Yu H, Liu X, Yang F, et al. (2012) Quantitative proteomic analysis of serum proteins in patients with Parkinson's disease using an isobaric tag for relative and absolute quantification labeling, two-dimensional liquid chromatography, and tandem mass spectrometry. The Analyst 137: 490-495.
- Licker V, Cote M, Lobrinus JA, Rodrigo N, Kovari E, et al. (2012) Proteomic profiling of the substantia nigra demonstrates CNDP2 overexpression in Parkinson's disease. J Proteomics 75: 4656-4667.
- Davison EJ, Pennington K, Hung CC, Peng J, Rafiq R, et al. (2009) Proteomic analysis of increased Parkin expression and its interactants provides evidence for a role in modulation of mitochondrial function. Proteomics 9: 4284-4297.
- Wongtrakul J, Saisawang C, Kumrapich B, Wipasa J, Roytrakul S, et al., (2018) Proteomic analysis of human glutathione transferase omega (hGSTO1) stable transfection in a 6-hydroxydopamine-induced neuronal cells. Gen Physiol Biophys 37: 141-152.
- Salasova A, Yokota C, Potesil D, Zdrahal Z, Bryja V, et al. (2017) A proteomic analysis of LRRK2 binding partners reveals interactions with multiple signaling components of the WNT/PCP pathway. Mol Neurodegener 12: 54.
- Lichtenberg M, Mansilla A, Zecchini VR, Fleming A, Rubinsztein DC (2011) The Parkinson's disease protein LRRK2 impairs proteasome substrate clearance without affecting proteasome catalytic activity. Cell death & disease 2: e196.
- Pellegrini L, Hauser DN, Li Y, Mamais A, Beilina A, et al. (2018) Proteomic analysis reveals co-ordinated alterations in protein synthesis and degradation pathways in LRRK2 knockout mice. Human molecular genetics 27: 3257-3271.
- Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, et al. (2017) Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife 6.
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, et al. (2004) The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 101: 9103-9108.
- Jiang H, Yu Y, Liu S, Zhu M, Dong X, et al. (2019) Proteomic Study of a Parkinson's Disease Model of Undifferentiated SH-SY5Y Cells Induced by a Proteasome Inhibitor. Int J Med Sci 16: 84-92.
- Quinones MP, Kaddurah-Daouk R (2009) Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol Dis 35: 165-176.
- Roede JR, Uppal K, Park Y, Lee K, Tran V, et al. (2013) Serum metabolomics of slow vs. rapid motor progression Parkinson's disease: a pilot study. PloS one 8: e77629.
- Jove M, Portero-Otin M, Naudi A, Ferrer I, Pamplona R (2014) Metabolomics of human brain aging and age-related neurodegenerative diseases. J Neuropathol Exp Neurol 73: 640-657.
- Lu Z, Wang J, Li M, Liu Q, Wei D, et al. (2014) (1)H NMR-based metabolomics study on a goldfish model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Chem Biol Interact 223: 18-26.
- Rango M (2015) Parkinson's disease: in vivo brain metabolomics by MRS. Int Rev Neurobiol 122: 81-94.
- Lei S, Powers R (2013) NMR Metabolomics Analysis of Parkinson's Disease. Current Metabolomics 1: 191-209.
- Weng R, Shen S, Tian Y, Burton C, Xu X, et al. (2015) Metabolomics Approach Reveals Integrated Metabolic Network Associated with Serotonin Deficiency. Sci Rep 5: 11864.
- Chen X, Xie C, Sun L, Ding J, Cai H (2015) Longitudinal Metabolomics Profiling of Parkinson's Disease-Related alpha-Synuclein A53T Transgenic Mice. PloS one 10: e0136612.
- Zheng H, Zhao L, Xia H, Xu C, Wang D, et al. (2016) NMR-Based Metabolomics Reveal a Recovery from Metabolic Changes in the Striatum of 6-OHDA-Induced Rats Treated with Basic Fibroblast Growth Factor. Mol Neurobiol 53: 6690-6697.
- Phelan MM, Caamano-Gutierrez E, Gant MS, Grosman RX, Madine J (2017) Using an NMR metabolomics approach to investigate the pathogenicity of amyloid-beta and alpha-synuclein. Metabolomics 13: 151.
- Luan H, Wang X, Cai Z (2019) Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom Rev 38: 22-33.
- Nagesh Babu G, Gupta M, Paliwal VK, Singh S, Chatterji T, et al. (2018) Serum metabolomics study in a group of Parkinson's disease patients from northern India. Clin Chim Acta 480: 214-219.
- Nybo SE, Lamberts JT (2019) Integrated use of LC/MS/MS and LC/Q-TOF/MS targeted metabolomics with automated label-free microscopy for quantification of purine metabolites in cultured mammalian cells. Purinergic Signal 15: 17-25.
- Glaab E, Trezzi JP, Greuel A, Jager C, Hodak Z, et al. (2019) Integrative analysis of blood metabolomics and PET brain neuroimaging data for Parkinson's disease. Neurobiol Dis 124: 555-562.
- Shao Y, Le W (2019) Recent advances and perspectives of metabolomics-based investigations in Parkinson's disease. Mol Neurodegener 14: 3.
- Okuzumi A, Hatano T, Ueno SI, Ogawa T, Saiki S, et al. (2019) Metabolomics-based identification of metabolic alterations in PARK2. Ann Clin Transl Neurol 6: 525-536.
- Huang W, Xu Y, Zhang Y, Zhang P, Zhang Q, et al. (2019) Metabolomics-driven identification of adenosine deaminase as therapeutic target in a mouse model of Parkinson's disease. J Neurochem.
- Liu CC, Jewett MC, Chin JW, Voigt CA (2018) Toward an orthogonal central dogma. Nat Chem Biol 14: 103-106.
- Krall A, Brunn J, Kankanala S, Peters MH (2014) A simple contact mapping algorithm for identifying potential peptide mimetics in protein-protein interaction partners. Proteins 82: 2253-2262.
- Flower R, Roulis E, Hyland C (2018) Whole-genome sequencing algorithm for blood-group typing. Lancet Haematol 5: e233-e234.
- Swerdel JN, Hripcsak G, Ryan PB (2019) FPheValuator: Development and Evaluation of a Phenotype Algorithm Evaluator. J Biomed Inform 2019: 103258.
- Naor Z, Yeshurun Y, Myslobodsky M (1996) Bilateral comparison of occipital lobe sulci: a sulcus identifying algorithm. Neuropsychol Rev 6: 95-105.
- Wang L, Sha L, Lakin JR, Bynum J, Bates DW, et al. (2019) Development and Validation of a Deep Learning Algorithm for Mortality Prediction in Selecting Patients with Dementia for Earlier Palliative Care Interventions. JAMA Netw Open 2: e196972.
- Glaab E (2018) Computational systems biology approaches for Parkinson's disease. Cell Tissue Res 373: 91-109.


