The pathogenesis of thrombocytopenia in critically ill patients (TCIP) has not been established yet. Based on “two-activation theory of the endothelium”, TCIP is a manifestation of platelet activation and consumption in association with endotheliopathy. Endotheliopathy occurs in many critical illnesses. An injury to vascular endothelial cells (ECs) from pathogen or insult leads to endothelial dysfunction, which initiates the activation of two distinctly independent molecular pathways (i.e., inflammatory and microthrombotic). The activation of inflammatory pathway occurs due to the release of inflammatory cytokines from injured ECs. Inflammatory cytokines mediate inflammation. The activation of microthrombotic pathway is induced by the activation of platelets and endothelial exocytosis of unusually large von Willebrand factor multimers (ULVWF). Activated platelets are recruited by exocytosed ULVWF, which are anchored to ECs, and together assemble microthrombi consisting of platelet-ULVWF complexes. This microthrombogenesis leads to consumptive thrombocytopenia (i.e., TCIP) and disseminated intravascular microthrombosis (DIT). DIT triggers vascular microthrombotic disease (VMTD), which manifestations include hypoxic multi-organ dysfunction syndrome, and thrombotic microangiopathy (TMA). The combined syndrome due to the activation of both inflammatory pathway and microthrombotiic pathway is called systemic inflammatory response syndrome (SIRS). Also, the true nature of “DIC” is endotheliopathy-associated DIT/VMTD (i.e., TTP-like syndrome).
thrombocytopenia, endotheliopathy, thrombotic thrombocytopenic purpura (TTP), TTP-like syndrome, therapeutic plasma exchange, disseminated intravascular coagulation (DIC), disseminated intravascular microthrombosis, vascular microthrombotic disease (VMTD), microthrombogenesis
Abbreviations:
ADAMTS13: a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13, rADAMTS13: recombinant ADAMTS13, AH/AHNS: acute hepatitis/acute hepatic necrosis syndrome, ARDS: acute respiratory distress syndrome, ARF: acute renal failure, CABG: coronary artery bypass graft, C-APLAS: catastrophic anti-phospholipid antibody syndrome, CNS: central nervous system, CNSD: central nervous system dysfunction, DSS: dengue shock syndrome, DIC: disseminated intravascular coagulation, DIT: disseminated intravascular thrombosis, ECs: endothelial cells, GE: gastroenteritis, HC: hepatic coagulopathy, HCP: hantavirus pulmonary syndrome, HCPS: hantavirus cardio-pulmonary syndrome, HELLPs: hemolysis: elevated liver enzymes: and low platelet count syndrome, HFRS: hemorrhagic fever with renal syndrome, HUS: hemolytic-uremic syndrome, LDH: lactate dehydrogenase, MAHA: microangiopathic hemolytic anemia, aMAHA: atypical microangiopathic hemolytic anemia, MERS-CoV: middle east respiratory syndrome-coronavirus, MODS: multi-organ dysfunction syndrome, MOF: multi-organ failure, MRSA: methicillin-resistant staphylococcus aureus, NOMI: non-occlusive mesenteric ischemia, RMSF: Rocky mountain spotted fever, SARS-CoV: severe acute respiratory syndrome-coronavirus, SIRS: systemic inflammatory response syndrome, SFTS: severe fever with thrombocytopenia syndrome, TAMOF: thrombocytopenia-associated multiple organ failure, TCIP: thrombocytopenia in critically ill patients, TMA: thrombotic microangiopathy, TTP: thrombotic thrombocytopenic purpura, ULVWF: unusually large von Willebrand factor multimers, VMTD: vascular microthrombotic disease
In the critically ill patient, thrombocytopenia is a very common hematological condition that occurs due to several different pathogenic mechanisms, and manifests with a broad clinical spectrum from benign presentation to life-threatening emergency. Mild to moderate thrombocytopenia plays a minor role in short-term clinical course, but the patient outcome related to the thrombocytopenia depends more upon the underlying pathologic disease.
Even after careful exclusion of the known etiology of thrombocytopenia, the cause of thrombocytopenia cannot be clearly determined in more than half of critically ill patients. This etiology-unidentified thrombocytopenia, encountered in critical illnesses (e.g., sepsis/septic shock, severe trauma, and complications of pregnancy, transplant and surgery), has been designated as “thrombocytopenia in critically ill patients” (TCIP) [1]. TCIP is now suspected to be an unfavorable indicator influencing the prognosis of the patient [2-4].
The known mechanisms producing thrombocytopenia in critically ill patients include: 1) decreased production of the platelet due to transient bone marrow suppression or myelodysplasia (e.g., infection-associated), 2) increased destruction due to immune or non-immune response (e.g., drug or transfusion-induced), 3) increased utilization (e.g., disseminated intravascular coagulation - DIC), 4) increased consumption (e.g., heparin-induced thrombocytopenia, and thrombotic thrombocytopenic purpura - TTP), and 5) sequestration secondary to hypersplenism [5,6]. To date, TCIP is the term to use after the exclusion of known mechanisms.
The example of critical illnesses and conditions associated with TICP is listed in Table 1. Thrombocytopenia is typically recognized after admission to the critical care unit for conditions such as sepsis, severe physical injury, acute respiratory distress syndrome (ARDS), and central nervous system dysfunction. In severe infection due to pathogen causing bacterial sepsis, viral pneumonia (e.g., Middle East respiratory distress syndrome due to coronavirus), and viral hemorrhagic fevers (e.g., Ebola, hantavirus, and dengue), TCIP occurs in advancing stage of the illness.
TCIP is mild to moderately severe usually with the platelet count not less than 20,000/mL. Hemorrhagic tendency has been uncommon unless it occurs with severe thrombocytopenia, DIC or hepatic coagulopathy. Thus, to some clinicians TCIP is considered to be not a serious issue in the management of critically ill patients.
The degree of thrombocytopenia has been correlated with the severity of clinical course. Increasing thrombocytopenia was associated with higher mortality and longer length of hospital stay, and the increase of the platelet count was an early sign of clinical improvement [2-4,6-10]. Severe thrombocytopenia commonly has occurred in association with progressive multi-organ dysfunction syndrome (MODS) [4,11,12] and systemic inflammatory response syndrome (SIRS) [13,14]. These observations support TCIP is an important participant in the pathogenesis of the critical illness.
The endothelium is a delicate biological structure that lines the entire circulatory system. It maintains the integrity of the blood supply by protecting the human body from the invasion of pathogen and insult. It also guards the circulatory system against unneeded intravascular coagulation by preventing the intrusion of tissue factor (TF) at the basement membrane of ECs [15]. ECs do not express in vivo TF. In sepsis and other critical illnesses, the membrane barrier of ECs is not disrupted. Thus, TF does not enter into circulation from extravascular compartment, and intravascular coagulation (i.e., DIC) cannot be initiated. However, injured ECs become activated, and endothelial dysfunction leads to endotheliopathy triggering several molecular responses [16-22].
Endotheliopathy is associated with inflammation [23], platelet activation [24] and exocytosis of unusually large von Willebrand factor multimers (ULVWF) [25-27]. It is also associated with thrombocytopenia (i.e., TCIP) and disseminated intravascular microthrombosis (DIT) [28,29]. Other clinical syndromes associated with critical illnesses include SIRS [13,14], ARDS [19,22], MODS [4,6,11,12,14], “DIC” [30-32], thrombotic thrombocytopenic purpura (TTP)-like syndrome [33-39], hepatic coagulopathy, and others [36,40].
Current hypothesis for the pathogenesis of vascular microthrombosis, especially in sepsis, is based on the intricate interaction between inflammation and coagulation system. The release of endothelial cytokines would trigger TF-mediated activation of coagulation leading to disseminated intravascular micro blood clots, inducing to vascular microthrombosis (i.e., “DIC”) [30,41,42].
Contrary to this concept, microthrombogenesis plays a key role in the pathogenesis of TTP and TTP-like syndrome. In endotheliopathy, the platelet is activated and excessive amounts of ULVWF are released from ECs [24-27,40]. The result is the formation of microthrombi made of platelet-ULVWF complexes, which also lead to vascular microthrombosis [26,27,34,36,40].
To annotate inflammation and circulatory disorder in the critical illness, a novel hypothesis of “two-activation theory of the endothelium” is proposed [36,40].
Endotheliopathy initiates two significant molecular events: 1) release of inflammatory cytokines (e.g., interleukin (IL)-1, IL-6, tumor necrosis factor-a, and others) [16-22], and 2) activation of the platelet and exocytosis of ULVWF [24-27]. The former triggers inflammation, which is called “activation of inflammatory pathway”, and the latter initiates microthrombogenesis, which is expressed as “activation of microthrombotic pathway”. These two independent responses are the essence of “two-activation theory of the endothelium” as illustrated in Figure 1. The manifestation of activated inflammatory pathway is inflammation with symptoms such as fever, myalgia, arthralgia, and malaise, and that of activated microthrombotic pathway is consumptive thrombocytopenia, hypoxemia, multi-organ dysfunction and multiple clinical syndromes as presented in Figure 1 and Table 1.
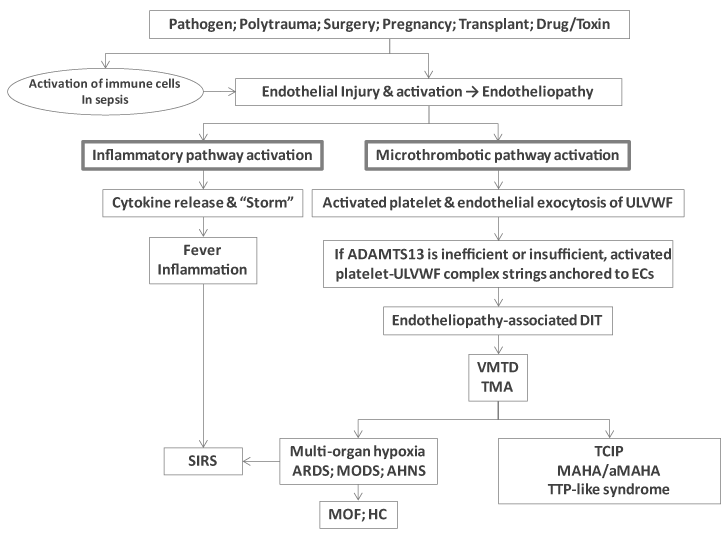
Figure 1. Pathogenesis of TCIP and related syndromes in critically ill patients.
Table 1. Examples of thrombocytopenia (TCIP)-associated conditions seen in critical care.
|
Causes |
Involved organs |
Associated syndromes |
Infectious agent Virus
|
Ebola
H1N1 influenza
MERS-CoV
SARS-CoV
Hantavirus
Dengue
SFTS virus
|
Lungs; liver; multi-organs
Brain; lungs; multi-organs
Lungs; multi-organs
Lungs; multi-organs
Heart; lung; kidneys
Adrenals; multi-organs
Multi-organs
|
ARDS; hepatic necrosis; MODS
Encephalopathy; ARDS; MODS
ARDS; MODS
ARDS; MODS
HCPS; HPS; HFRS
DSS; MODS
SFTS; MODS
|
Bacteria
|
Neisseria meningitides
E.Coli O157:H7
MRSA
Klebsiella pneumonia
Various bacterial sepsis
|
Adrenals
Bowels; kidneys
Multi-organs
Lungs; multi-organs
Lungs; multi-organs
|
Waterhouse-Friderichsen syndrome
Hemolytic-uremic syndromes; GE
MODS; SIRS
ARDS; MODS; SIRS
ARDS; MODS; SIRS; TAMOF; C-APLAS |
Rickettsia
|
Rickettsia rickettsii
|
Skin; multi-organs
|
RMSF; MODS |
Fungus
|
Candida albicans
|
Multi-organs
|
MODS; SIRS |
Parasite
|
Plasmodium falciparum
Plasmodium vivax
|
Brain; multi-organs
Lungs; multi-organs
|
Cerebral malaria; MODS; ARDS; SIRS
ARDS |
Trauma
Lungs/chest trauma
CNS trauma
|
Motorcycle accident
Head injury
|
Lungs; multi-organs
Brain; lungs; multi-organs
|
ARDS; MODS; SIRS
Encephalopathy; ARDS; MODS; SIRS
|
Surgery
Cardiac surgery
Vascular surgery
Bowel surgery
|
CABG; open heart surgery
Aortic aneurysm surgery
Mesenteric inflammation |
Lungs; heart; multi-organs
Lungs; multi-organs
Mesentery; multi-organs
|
ARDS; myocardial ischemia; MODS
ARDS; MODS
NOMI; MODS
|
Pregnancy
Preeclampsia
|
Toxin (?); infection (?)
|
Lungs; uterus; multi-organs
|
ARDS; HELLPs; Abruptio placenta; MODS |
Transplant
Liver transplant
Kidney transplant |
Infection (?)
Infection (?) |
Lungs; multi-organs
Lungs; multi-organs |
ARDS; MODS
ARDS: MODS |
The activation of inflammatory pathway occurs due to release of cytokines in both sepsis and non-septic critical illnesses. Unlike in non-septic illnesses, sepsis also promotes inflammation through another loop of activated circulating immune cell pathway (e.g., macrophages, monocytes, neutrophils, and lymphocytes). This pathway also interacts with activated ECs as shown in Figure 1 [43,44]. This additional cytokine expression accentuates the inflammatory pathway that could result in “cytokine storm”. This mechanism explains why severer inflammation occurs in sepsis, which might lead to SIRS [11,13,14,45].
On the other hand, the activation of microthrombotic pathway is initiated by activated platelets and excessively exocytosed ULVWF that are anchored to ECs as long elongated strings [46,47]. If protease ADAMTS13, which cleaves ULVWF to smaller molecular weight VWF, is under expressed [36,48], activated platelets under shear stress of blood flow are recruited to the uncleaved ULVWF strings. This microthrombogenesis generates intravascular microthrombi consisting of platelet-ULVWF complexes at ECs [46,47]. This process sets off DIT and could lead to multiple clinical syndromes.
DIT is the underlying pathology provoking vascular microthrombotic disease (VMTD) [36,40], which triggers hypoxic multi-organ dysfunction and thrombotic microangiopathy (TMA). Three kinds of disseminated VMTD are known to exist: 1) antibody-associated VMTD (i.e., acquired TTP), 2) gene mutation-associated VMTD (i.e., hereditary TTP), and 3) endotheliopathy-associated VMTD (TTP-like syndrome). Endotheliopathy-associated DIT/VMTD is the underlying pathologic condition producing TTP-like syndrome. It is characterized by TCIP, microangiopathic hemolytic anemia (MAHA)/atypical MAHA (if fewer schistocytes are present) with/without MODS.
Perhaps the dissimilar clinical features (e.g., central nervous system dysfunction in TTP and ARDS in TTP-like syndrome) are related to microthrombogenesis occurring at different sites, resulting in different clinical syndromes due to divergent localization of intravascular microthrombi, even among the TTP-like syndromes (Table 2). TTP seems to be the result of microvascular microthrombosis, but TTP-like syndrome is the result of vascular microthrombosis. In the former, microthrombogenesis occurs in the circulation and formed microthrombi become lodged in microvasculatures [49], predominantly in the brain and kidneys. But in the latter, it occurs at ECs-anchored long elongated ULVWF strings [46,47] in smaller and larger vasculatures, commonly involving the lungs (i.e., ARDS), kidneys (i.e., acute renal failure, hemolytic-uremic syndrome), liver (i.e., acute hepatic necrosis syndrome), intestines (i.e., gastroenteritis), pancreas (i.e., acute pancreatitis), muscles (i.e., rhabdomyolysis), heart (i.e., acute myocardial ischemia), skin (purpura fulminans), and others.
Table 2. Genesis and characteristics of DIT/VMTD in TTP and TTP-like syndrome.
|
ADAMTS13 gene mutation-associated VMTD (Hereditary TTP) ADAMTS13 antibody-associated VMTD (Acquired TTP) |
Endotheliopathy-associated VMTD (TTP-like syndrome) |
Primary event
Secondary event
Tertiary event
Final event
|
Hereditary ADAMTS13 gene mutation
Acquired ADAMTS13 antibody formation
↓
Excessive circulating ULVWF & platelet aggregation
↓
Microthrombogenesis leading to platelet-ULVWF complexes
↓
Microthrombi lodged in arteriolar capillary lumens
↓
VMTD
↓
TMA (microthrombotic microangiopathy)
↓
TTP |
Sepsis/septic shock due to pathogens (e.g., viruses; bacteria; fungi; rickettsia; parasites)
Polytrauma (e.g., chest/lungs; bones; skull/brain injury)
Pregnancy complications (e.g., preeclampsia; abruptio placenta; amniotic fluid embolism)
Cancer (e.g., stomach; breast; lung)
Transplant (e.g., liver; kidney; bone marrow)
Drug and chemical (e.g., cyclosporine; mytomycin C; Shiga toxin; ricin)
↓
Endothelial injury & platelet activation® ECs activation & endotheliopathy
↓
Cytokine release and cytokine storm ® Inflammation ® SIRS
Endothelial exocytosis of ULVWF & anchored to ECs as a long elongated strings® DIT
↓
Vascular microthrombogenesis leading to platelet-ULVWF complexes anchored to ECs
↓
VMTD
↓
TMA (microthrombotic angiopathy)
↓
TTP-like syndrome |
Hematologic features
Platelet
Red blood cell
Clinical syndromes
Inflammation/fever
Cytokine storm
SIRS
CNSD
ARDS
GE
AH/AHNS
ARF/HUS
Hepatic coagulopathy
DIC*(see text) |
Consumptive thrombocytopenia
MAHA
Fever may be present (?)
Absent
Absent
Very common
Absent
Uncommon
Uncommon
Very common
Not reported
Doesn’t occur |
Consumptive thrombocytopenia
MAHA/aMAHA
Very common
Often present in sepsis/septic shock
Often present in sepsis/septic shock
Common
Very common
Common
Common
Common
Common
Doesn’t occur |
Laboratory features
ADAMTS13 activity
ADAMTS13 antibody
LDH
Haptoglobin
Schistocytosis |
Markedly decreased (<5% of normal)
Positive in acquired TTP
Increased
Markedly decreased
++ to ++++ |
Mild to moderately decreased (20-70% of normal)
Negative
Increased
Markedly decreased
None to +++ |
Therapeutic response to
TPE
Platelet transfusion
rADAMTS13 |
Very good response
Contraindicated
Unknown at this time; expected to be effective in hereditary TTP |
Excellent and fast response if treated in early stage
Contraindicated
Unknown at this time; expected to be very effective |
According to the “two-activation theory”, DIT induced by microthrombogenesis is completely different from true DIC occurring as a result of activated TF coagulation pathway. DIT is a microthrombotic disorder, but true DIC is a coagulation disorder. Additionally, the current concept of pathologic coagulation (i.e., “DIC”) through TF pathway in the critical illness cannot be correct because in vivo sufficient TF is not available in the ECs. The characteristic difference between DIT and true DIC is shown in Table 3.
Table 3.Hematological and Clinical Characteristics of endotheliopathy-associated DIT and true DIC.
|
Endotheliopathy-associated DIT (including “DIC” of McKay) |
True DIC |
Examples
|
TTP-like syndrome
|
DIC associated with APL
|
Nature of the disorder
|
Microthrombosis made of platelet-ULVWF complexes
|
Coagulation activated by TF-FVIIa complexes
|
Mechanism of the genesis
|
Intravascular microthrombogenesis
|
Intravascular coagulation
|
Inciting events
|
Sepsis, complications of surgery, pregnancy, cancer,
and transplant, and drugs/toxins leading to endotheliopathy
|
APL and drugs (?) leading to TF expression
|
Hematological manifestations
|
TTP-like syndrome
|
Hemorrhagic disorder of APL
|
Pathogenesis
Mechanism
Site of activation
Pathology
Result of pathogenesis
|
Activation of microthrombotic pathway
Intravascular membrane of the endothelium
Endothelial activation/dysfunction ® endotheliopathy
Formation of platelet-ULVWF microthrombi
|
Activation of TF-FVIIa complex pathway
In circulation of the Intravascular space
TF expression ® coagulation and factor consumption Depletion of fibrinogen, FVIII, FV
|
Essence of pathology
|
Arteriolar and capillary luminal hyaline microthrombi
|
Incoagulable blood/unstable blood clots
|
Effect on the involved organs
|
Vascular microthrombosis leading to organ hypoxia
|
Hemorrhage leading to organ damage
|
Coagulation tests
Fibrinogen
PT; aPTT; TT
FDP
FVIII activity
Thrombocytopenia
|
Normal
Prolonged
Normal
Normal or markedly increased
Moderately severe
|
Decreased
Prolonged
Increased
Markedly decreased
Mild to very severe
|
Associated clinical syndromes
|
TTP-like syndrome
TMA
MODS
SIRS
|
Hemorrhagic disorder
|
Associated hematologic features
Schistocytes
MAHA/aMAHA
Consumptive thrombocytopenia
Hepatic coagulopathy
|
0 - +++
Often present
Always present
May occur
|
0 - + (?)
Absent
Present (?)
Unusual
|
Incidence in clinical practice
|
Very common
|
Extremely rare
|
Therapy
Platelet transfusion
Treatment |
Contraindicated
TPE; rADAMTS13 (expected to be very effective) |
May be needed for APL
Treat underlying pathology (e.g., ATRA in APL) |
Donald McKay in early1950s coined the term “DIC” [50] for a coagulation disorder that is caused by abnormally activated intravascular thrombotic state. He and his associates believed intravascular microthrombi in the luminal arterioles and capillaries in the pathologic tissue examination were micro blood clots made of platelets, coagulation factors and fibrins. His followers also supported the diagnosis of “DIC” with the laboratory result of prolonged prothrombin time and activated partial thromboplastin time, hypofibrinogenemia, and increased fibrin degradation products. The most of the coagulopathy associated with thrombocytopenia in the critical illnesses has been ascribed to “DIC” [51-53].
It should be emphasized that since no single laboratory test or set of tests is sensitive or specific enough to allow a definite diagnosis of “DIC” [54]. In most cases the diagnosis is based on the combination of results of non-specific abnormal coagulation profile in the patient with clinical conditions known to be associated with “DIC” [55].
In clinical medicine, “DIC” mainly has been diagnosed on clinical pretense and is accepted based on the scoring system of the International Society on Thrombosis and Haemostasis (ISTH). Because of the misconception of “DIC”, DIT in the critically ill patient has been diagnosed as “DIC”. “DIC” diagnosis has not been based on more reliable coagulation factor assay of FVIII and FV, which are typically depleted in true DIC [40,56-59] as seen in acute promyelocytic leukemia. In many patients with “DIC”, the coagulation profile is perfectly normal and hemorrhagic tendency does not occur. Puzzled but conveniently, the concept of “chronic/compensated/ covert” was introduced. This description, however, cannot explain inexplicably extensive microthrombi in the absence of depleted coagulation factors.
“DIC” and endotheliopathy-associated DIT/VMTD (i.e., TTP-like syndrome) are exactly the same in their underlying risk factors and presentation. Both almost always occur in critical illnesses (e.g. sepsis/septic shock, trauma, immunologic and collagen-vascular diseases, and complications of surgery, pregnancy and transplant) [38,60,61]. Pathologically both are characterized by arteriolar and capillary hyaline microthrombi with variable fibroblastic proliferation [49,62]. Hematologically they also present with TCIP and MAHA/aMAHA. Therefore, “DIC” and DIT are exactly the same disorder.
Considering the different pathogenic mechanisms between DIC and DIT, “DIC” must have been started with a incorrect concept. Hence, “DIC” is a misnomer. For more than 60 years, this unfortunate misconception on “DIC” has created confusion in medical science and practice, including diagnostic dilemma [54,55] and treatment failures to date [63].
If one accepts the fact that “DIC” is a misnomer and its euonym must be endotheliopathy-associated DIT, “DIC” can be explained perfectly well by the concept of DIT. The only remaining question is how “DIC” sometimes is associated with hemorrhagic disorder. Another word, “What is the correct diagnosis for acute “DIC” that is associated with abnormal coagulation profile?” The hemorrhagic disorder in “DIC” can be explained by hepatic vascular microthrombosis. Endotheliopathy-associated DIT/VMTD can trigger acute hepatic necrosis syndrome leading to hepatic coagulopathy [40]. Indeed, hepatic coagulopathy shows exactly the same coagulation profile as seen in “acute DIC”.
True DIC is very rare but perhaps occurs in acute promyelocytic leukemia, presumably due to TF expression from leukemic cells [64]. The predominant feature of true DIC is hemorrhagic disorder without MAHA/aMAHA, hypoxic organ dysfunction and MODS [56-58]. In differentiating true DIC from hepatic coagulopathy, the appropriate test is the assay of coagulation factors, especially FVIII and FV, which are depleted in true DIC. More importantly, in hepatic coagulopathy, FVIII is normal or increased although it is markedly decreased in true DIC [40,58,59]. Also, a markedly decreased liver dependent FVII occurs in hepatic coagulopathy. A suggested guideline for laboratory tests is presented in Table 4 to aid the differential diagnosis among complicated thrombopathies and coagulopathies [36].
Table 4. Differential characteristic hematologic features among thrombopathies and coagulopathies (Adapted and modified from Chang JC (36) with permission).
|
TTP & TTP-like syndrome (DIT) |
TTP-like syndrome (DIT) associated with HC (e.g., Ebola) = acute “DIC” |
DIC (e.g., acute promyelocytic leukemia) |
PF (e.g., amyloidosis) |
Thrombocytopenia |
Always present |
Always present |
Always present |
Not present |
MAHA/aMAHA |
Almost always present |
Usually present |
Very unlikely to be present |
Not present |
Fibrinogen |
Normal |
Decreased |
Always decreased |
Always decreased |
Factor VIII |
Normal |
Normal or increased |
Markedly decreased |
Decreased |
Factor V |
Normal |
Decreased |
Decreased |
Decreased (?) |
Factor X |
Normal |
Decreased |
Usually normal |
Normal |
Factor VII |
Normal |
Markedly decreased |
Normal |
Normal |
Factor IX |
Normal |
Decreased |
Normal |
Normal |
FDP |
Normal |
Positive |
Positive |
Strongly positive |
Thrombin time |
Normal |
Prolonged |
Prolonged |
Prolonged |
Thrombosis form |
Microthrombi |
Microthrombi |
Friable macrothrombi (?) or not formed |
Absent |
Bleeding: Character |
Rare, mild petechiae |
May cause serious bleeding |
Common, serious bleeding |
Slow & persistent bleeding |
Treatment |
Usually no need of treatment |
Controllable with FFP |
Abrogated with ATRA & chemotherapy |
Treatable with AFA |
Platelet transfusion |
Contraindicated |
Contraindicated |
May be used with ATRA |
Not needed |
In the critically ill patient, TCIP is the earliest sign suggestive of microthrombogenesis in progress. In addition to inflammation, endotheliopathy-associated DIT/VMTD may lead to MODS, TMA, TTP-like syndrome and SIRS. “DIC” presents with the same clinical, pathologic and hematologic features as TTP-like syndrome. “DIC” should be correctly renamed as TTP-like syndrome.
The author Jae C. Chang, M.D. has neither actual nor potential conflicts of interest in regard to this article. The author has no connection to or personal interest in any company.
- Thachil J, Warkentin TE (2017) How do we approach thrombocytopenia in critically ill patients? Br J Haematol 177: 27-38. [Crossref]
- Tsirigotis P, Chondropoulos S, Frantzeskaki F, Stamouli M, Gkirkas K, et al. (2016) Thrombocytopenia in critically ill patients with severe sepsis/septic shock: Prognostic value and association with a distinct serum cytokine profile. J Crit Care 32: 9-15. [Crossref]
- Zhang S, Cui YL, Diao MY, Chen DC, Lin ZF1 (2015) Use of platelet indices for determining illness severity and predicting prognosis in critically Ill patients. Chin Med J (Engl) 128: 2012-2018. [Crossref]
- Nguyen TC, Carcillo JA (2006) Bench-to-bedside review: thrombocytopenia-associated multiple organ failure--a newly appreciated syndrome in the critically ill. Crit Care 10: 235. [Crossref]
- Greinacher A, Selleng S (2016) How I evaluate and treat thrombocytopenia in the intensive care unit patient. Blood 128: 3032-3042. [Crossref]
- Katz JN, Kolappa KP, Becker RC (2011) Beyond thrombosis: the versatile platelet in critical illness. Chest 139: 658-668. [Crossref]
- Moreau D, Timsit JF, Vesin A, Garrouste-Orgeas M, de Lassence A, et al. (2007) Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest 131: 1735-1741. [Crossref]
- Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, et al. (2002) Time course of platelet counts in critically ill patients. Crit Care Med 30: 753-756. [Crossref]
- Krishnan J, Morrison W, Simone S, Ackerman A (2008) Implications of thrombocytopenia and platelet course on pediatric intensive care unit outcomes. Pediatr Crit Care Med 9: 502-505. [Crossref]
- Thiele T, Selleng K, Selleng S, Greinacher A, Bakchoul T (2013) Thrombocytopenia in the intensive care unit-diagnostic approach and management. Semin Hematol 50: 239-250. [Crossref]
- Stravitz RT, Ellerbe C, Durkalski V, Reuben A, Lisman T, Lee WM (2016) Acute liver failure study group. Thromobcytopenia is associated with multi-organ system failure in patients with acute liver failure. Clin Gastroenterol Hepatol 14: 613-620. [Crossref]
- Nydam TL, Kashuk JL, Moore EE, Johnson JL, Burlew CC, et al. (2011) Refractory postinjury thrombocytopenia is associated with multiple organ failure and adverse outcomes. J Trauma 70: 401-406. [Crossref]
- Tang DM, Zhang HJ, Jing BW, Chen DC (2003) Analysis of platelets in the monitoring of systemic inflammatory response syndrome of critically ill patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 15: 35-37. [Crossref]
- Ogura H, Gando S, Iba T, Eguchi Y, Ohtomo Y, et al. (2007) SIRS-associated coagulopathy and organ dysfunction in critically ill patients with thrombocytopenia. Shock 28: 411-417. [Crossref]
- Mackman N (2009) The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 108: 1447-1452. [Crossref]
- Aird WC (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101: 3765-3777. [Crossref]
- Srikiatkhachorn A, Spiropoulou CF (2014) Vascular events in viral hemorrhagic fevers: a comparative study of dengue and hantaviruses. Cell Tissue Res 355: 621-633. [Crossref]
- Xing K, Murthy S, Liles WC, Singh JM (2012) Clinical utility of biomarkers of endothelial activation in sepsis--a systematic review. Crit Care 16: R7. [Crossref]
- Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, et al. (2007) Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med 35: 2243-2250. [Crossref]
- Dobson GP (2015) Addressing the global burden of trauma in major surgery. Front Surg 2: 43. [Crossref]
- Powe CE, Levine RJ, Karumanchi SA (2011) Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123: 2856-2869. [Crossref]
- Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE (2008) Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 49: 119-133. [Crossref]
- Zhang C (2008) The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol 103: 398-406. [Crossref]
- Janicek MJ, Van den Abbeele AD, Hollenberg NK, Kassis AI, Holman BL, Tumeh SS (1990) Platelet activation and aggregation after endothelial injury. Assessment with indium-111-labeled platelets and angiography. Invest Radiol 25: 988-993. [Crossref]
- van Hinsbergh VW (2012) Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol 34: 93-106. [Crossref]
- Bockmeyer CL, Claus RA, Budde U, Kentouche K, Schneppenheim R, et al. (2008) Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica 93: 137-140. [Crossref]
- Valentijn KM, van Driel LF, Mourik MJ, Hendriks GJ, Arends TJ, et al. (2010) Multigranular exocytosis of Weibel-Palade bodies in vascular endothelial cells. Blood 116: 1807-1816. [Crossref]
- Turner NA, Moake J (2013) Assembly and activation of alternative complement components on endothelial cell-anchored ultra-large von Willebrand factor links complement and hemostasis-thrombosis. PLoS One 8: e59372. [Crossref]
- Moake JL (2004) von Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura. Semin Hematol 41: 4-14. [Crossref]
- Levi M, Schultz M, van der Poll T (2013) Sepsis and thrombosis. Semin Thromb Hemost 39: 559-566. [Crossref]
2021 Copyright OAT. All rights reserv
- Erez O, Mastrolia SA, Thachil J (2015) Disseminated intravascular coagulation in pregnancy: insights in pathophysiology, diagnosis and management. Am J Obstet Gynecol 213: 452-463. [Crossref]
- Lippi G, Cervellin G (2010) Disseminated intravascular coagulation in trauma injuries. Semin Thromb Hemost 36: 378-387. [Crossref]
- Chang JC, Shipstone A, Llenado-Lee MA (1996) Postoperative thrombotic thrombocytopenic purpura following cardiovascular surgeries. Am J Hematol 53: 11-17. [Crossref]
- Chang JC (2004) The understanding of thrombotic thrombocytopenic purpura: Dyadic, triadic, pentadic, and other manifestations. J Clin Apher 19: 2-4. [Crossref]
- Chang JC, Aly ES (2001) Acute respiratory distress syndrome as a major clinical manifestation of thrombotic thrombocytopenic purpura. Am J Med Sci 321: 124-128. [Crossref]
- Chang JC (2016) A thought on possible pathogenesis of ebola viral hemorrhagic disease and potential treatments: Could it be thrombotic thrombocytopenic purpura-like syndrome? J Ther Aph Dialysis 20: 93-98. [Crossref]
- Goldberg RJ, Nakagawa T, Johnson RJ, Thurman JM (2010) The role of endothelial cell injury in thrombotic microangiopathy. Am J Kidney Dis 56: 1168-1174. [Crossref]
- Booth KK, Terrell DR, Vesely SK, George JN (2011) Systemic infections mimicking thrombotic thrombocytopenic purpura. Am J Hematol 86: 743-751. [Crossref]
- Chang JC, Newman RS (2004) Redefining the syndromes of thrombotic microangiopathy. Ther Apher Dial 8: 73-74. [Crossref]
- Chang JC (2017) Viral hemorrhagic fevers due to endotheliopathy-associated disseminated inrtravascular microthrombosis and hepatic coagulopathy: pathogenesis based on “two-activation theory of the endothelium”. Clin Micro Infec Dis.
- Esmon CT (2005) The interactions between inflammation and coagulation. Br J Haematol 131: 417-430. [Crossref]
- Levi M, van der Poll T (2010) Inflammation and coagulation. Crit Care Med 38: S26-34. [Crossref]
- Langer HF, Chavakis T (2009) Leukocyte-endothelial interactions in inflammation. J Cell Mol Med 13: 1211-1220. [Crossref]
- Barreiro O, Sánchez-Madrid F (2009) Molecular basis of leukocyte-endothelium interactions during the inflammatory response. Rev Esp Cardiol 62: 552-562. [Crossref]
- Balk RA (2014) Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence 5: 20-26. [Crossref]
- De Ceunynck K, De Meyer SF, Vanhoorelbeke K (2013) Unwinding the von Willebrand factor strings puzzle. Blood 121: 270-277. [Crossref]
- Padilla A, Moake JL, Bernardo A, Ball C, Wang Y, et al. (2004) P-selectin anchors newly released ultralarge von Willebrand factor multimers to the endothelial cell surface. Blood 103: 2150-2156. [Crossref]
- Zheng XL (2015) ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med 66: 211-225. [Crossref]
- Tsai HM (2010) Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol 91: 1-19. [Crossref]
- McKay DG, Margaretten W (1967) Disseminated intravascular coagulation in virus diseases. Arch Intern Med 120: 129-152. [Crossref]
- Sundberg E, Hultdin J, Nilsson S, Ahlm C (2011) Evidence of disseminated intravascular coagulation in a hemorrhagic fever with renal syndrome-scoring models and severe illness. PLoS One 6: e21134. [Crossref]
- Hunt BJ (2014) Bleeding and coagulopathies in critical care. N Engl J Med 370: 847-859. [Crossref]
- Levi M, Opal SM (2006) Coagulation abnormalities in critically ill patients. Crit Care 10: 222. [Crossref]
- Slofstra SH, Spek CA, ten Cate H (2003) Disseminated intravascular coagulation. Hematol J 4: 295-302. [Crossref]
- Franchini M, Lippi G, Manzato F (2006) Recent acquisitions in the pathophysiology, diagnosis and treatment of disseminated intravascular coagulation. Thromb J 4: 4. [Crossref]
- Cooperberg AA (1967) Acute promyelocytic leukemia. Can Med Assoc J 97: 57-63. [Crossref]
- Chang JC, Gross HM, Jang NS (1990) Disseminated intravascular coagulation due to intravenous administration of hetastarch. Am J Med Sci 300: 301-303. [Crossref]
- Senzolo M, Burra P, Cholongitas E, Burroughs AK (2006) New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol 12: 7725-7736. [Crossref]
- Castellone D. http://www.aniara.com/Blog/Coagulation-Corner/archives/2010/11/LIVER-DISEASE-AND-COAGULATION-OUTCOMES.aspx
- Franchini M1, Lippi G, Manzato F (2006) Recent acquisitions in the pathophysiology, diagnosis and treatment of disseminated intravascular coagulation. Thromb J 4: 4. [Crossref]
- Nguyen TC, Kiss JE, Goldman JR, Carcillo JA (2012) The role of plasmapheresis in critical illness. Crit Care Clin 28: 453-468. [Crossref]
- Sueishi K, Takeuchi M (1993) Pathology of disseminated intravascular coagulation. Nihon Rinsho 51: 30-36.
- Marshall JC (2014) Why have clinical trials in sepsis failed? Trends Mol Med 20: 195-203. [Crossref]
- Tallman MS, Hakimian D, Kwaan HC, Rickles FR (1993) New insights into the pathogenesis of coagulation dysfunction in acute promyelocytic leukemia. Leuk Lymphoma 11: 27-36. [Crossref]

