Abstract
Purpose: To retrospectively analyze the results of our therapeutic recommendations for EMR and surgical operation using thin-probe EUS for patients with early colorectal neoplasms.
Methods: We retrospectively analyzed the therapeutic recommendation for using EUS and the results in 63 lesions of 63 patients. When the depth of the change for the third layer (submucosal layer) was 1 millimeter or more, we recommended surgical operation (EUS operation-recommended group).
Results: Surgical operation was suitable for all of lesions in the EUS-operation-recommended group (23 lesions of 23 patients).
Conclusion: We think that a change of 1 millimeter or more in the third layer (corresponding to the submucosal layer) in EUS for colorectal neoplasms may be a useful finding for surgical decision making.
Key words
colorectal cancer, EUS, endoscopic mucosal resection
Introduction
Surgical operation has a curative role for early colorectal neoplasms for which invasion is limited to within the submucosal layer [1,2]. Recently, high-definition colonoscopy and image-enhanced endoscopy can be used to detect small and shallow lesions [3,4]. Furthermore, the widespread use of endoscopic mucosal resection (EMR) and development of submucosal dissection have made possible local resection for early colorectal neoplasms [5].
Clinicopathological data show that mucosal layer (M) carcinoma or carcinoma with slight submucosal layer (SM) invasion without lymphatic vessel infiltration is mostly safe from metastasis and that EMR is adequate for it. However, it was reported that the lymph node metastasis rate of colorectal carcinoma with an SM invasion depth of 1,000 micrometers (1 mm) or more was 12.5%. Therefore, curative surgical resection is required for such lesions with deep SM invasion. In this situation, pretherapeutic estimation of deep SM invasion by early colorectal neoplasms is needed [6].
Currently we employ thin-probe EUS invasion depth staging for early gastric carcinoma [7]. In the present study, we retrospectively analyzed the results of our therapeutic recommendation for EMR or surgical operation using thin-probe EUS for patients with early colorectal neoplasms.
Materials and methods
Materials
From May 1997 through September 2003 (77 months), 1356 superficial colorectal neoplastic lesions were endoscopically resected and 450 colorectal tumor lesions were surgically resected at the Hofu Institute of Gastroenterology. During this period, we used thin-probe EUS for determining the therapeutic recommendation of EMR or surgical operation for 75 endoscopically examined superficial colorectal neoplastic lesions. From these lesions, 12 lesions of carcinoid tumors and non-epithelial tumors were excluded. Finally, we retrospectively analyzed the therapeutic recommendations and the results for 63 lesions of 63 patients. The patients consisted of 41 males and 22 females, with a mean age of 64.5 years old (range: 32 to 89). There were 43 adenocarcinomas and 20 adenomas. In many cases, the final pathological diagnosis was unknown in the pretherapeutic situation and was made using resected specimens. Therefore, in this study, we analyzed adenocarcinoma and adenoma.
Thin-probe EUS findings
We used a Sonoprobe System (15MHz, FUJI FILM MEDICAL, Tokyo, Japan) for thin-probe EUS. EUS observation was done using the water immersion method. An endoscopist with over 20 years of experience with EUS (HY) performed all EUS procedures.
The tumor invasion depth on the EUS image was determined in the deepest layer with a change in the layered structure visualized at the lesion site. The normal colorectal wall is visualized basically as a five-layered structure [8]. By referring to the view of the five-layered structure from a practical standpoint, the tumor invasion depth was staged as follows: EUS-M-the tumor was limited to the first and second layers (mucosal layer); EUS-M/SM borderline-changes in the third layer of less than 1 millimeter; EUS-SM-depth of the change in the third layer (submucosal layer) of 1 millimeter or more; EUS-AD-the tumor invaded the fourth layer (muscularis propria) or more as an advanced tumor. The fifth layer corresponds to the subserosal layer and serosal layer. When the EUS image was unclear, it was evaluated as EUS-NG [9] (Figures 1-3).
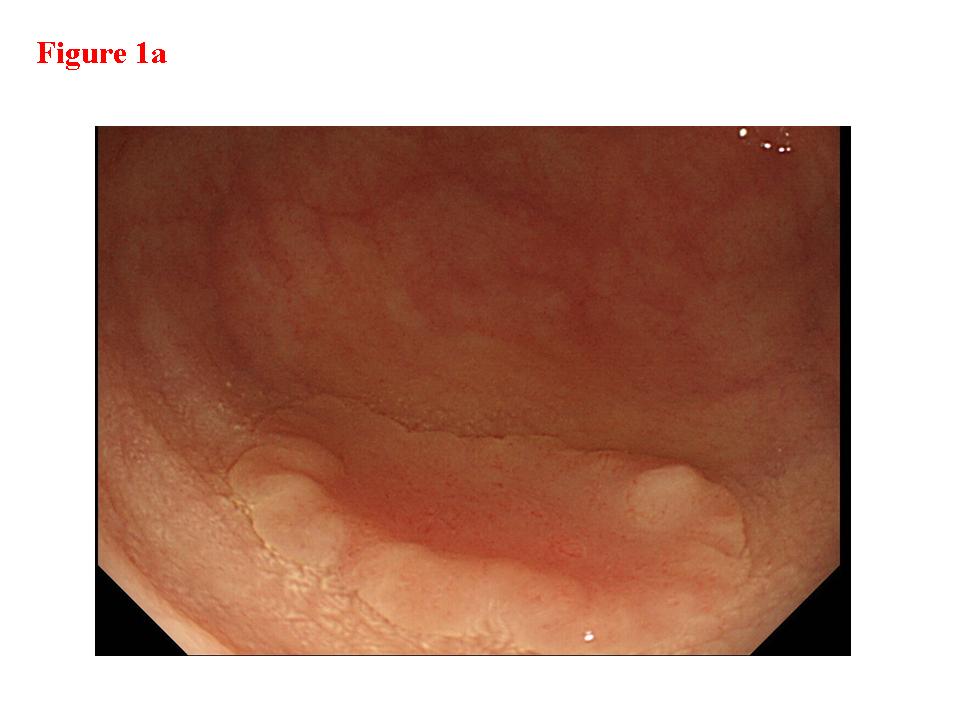
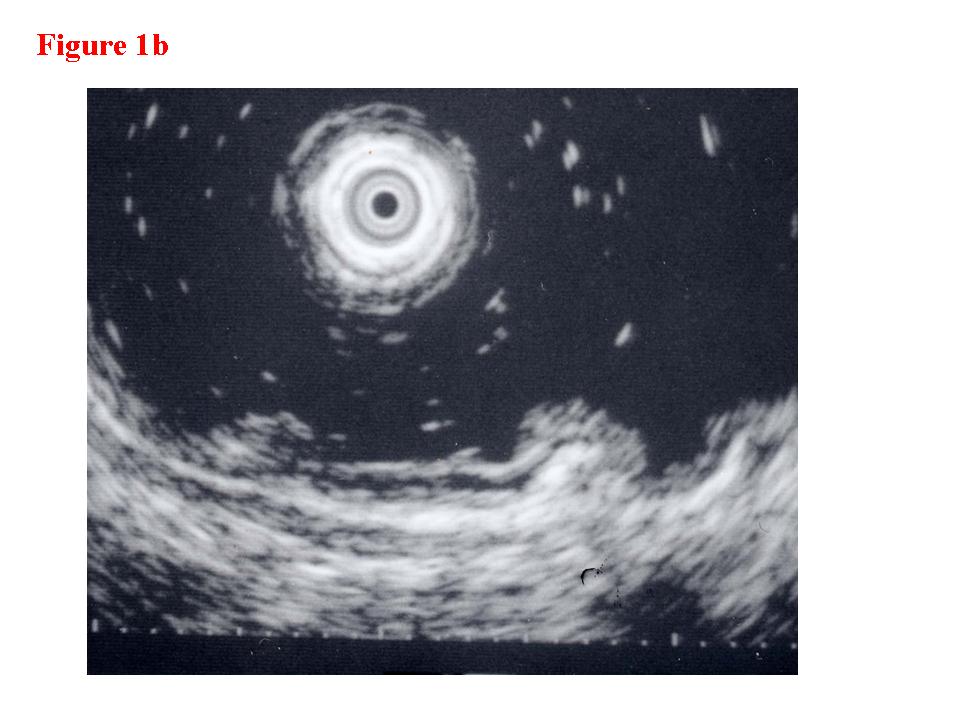
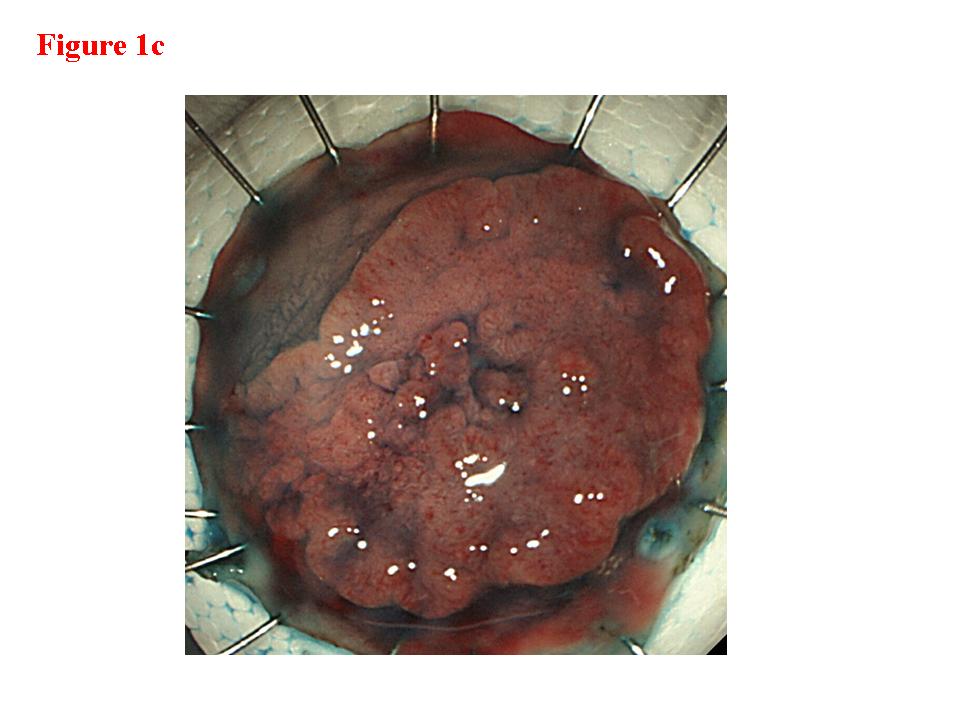
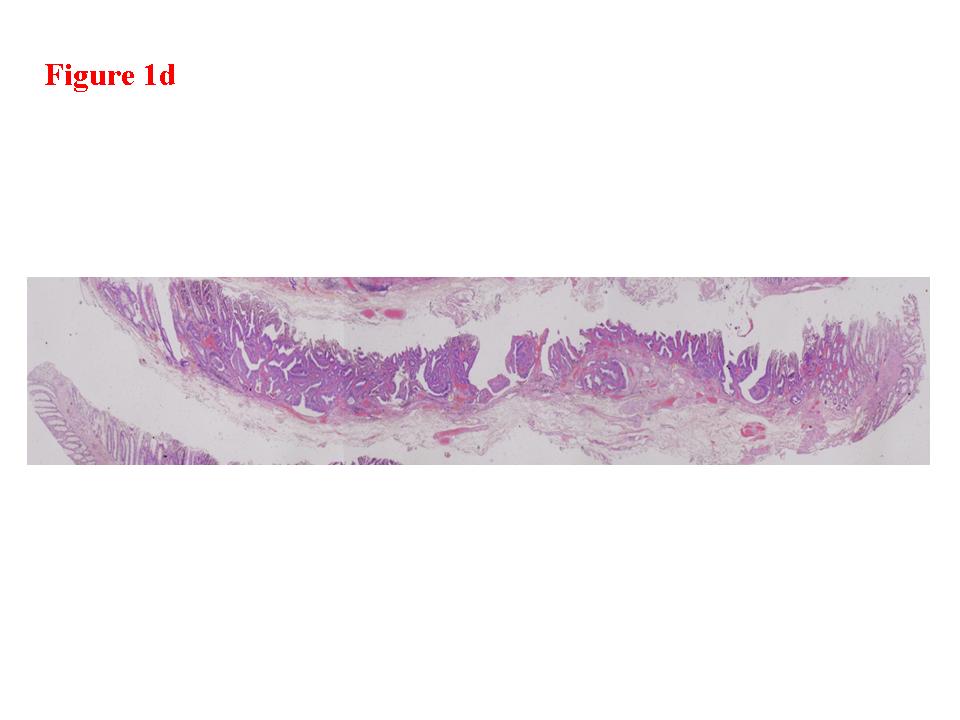
Figure 1. Early rectal cancer lesion (EUS-M). a) Endoscopic features of superficial depressed type early rectal cancer lesion. b) The tumor was limited to be the first and second layers (EUS-M). c) Macroscopic feature of the tumor specimen. d) The cancer invasion was limited within mucosa and muscularis mucosae.
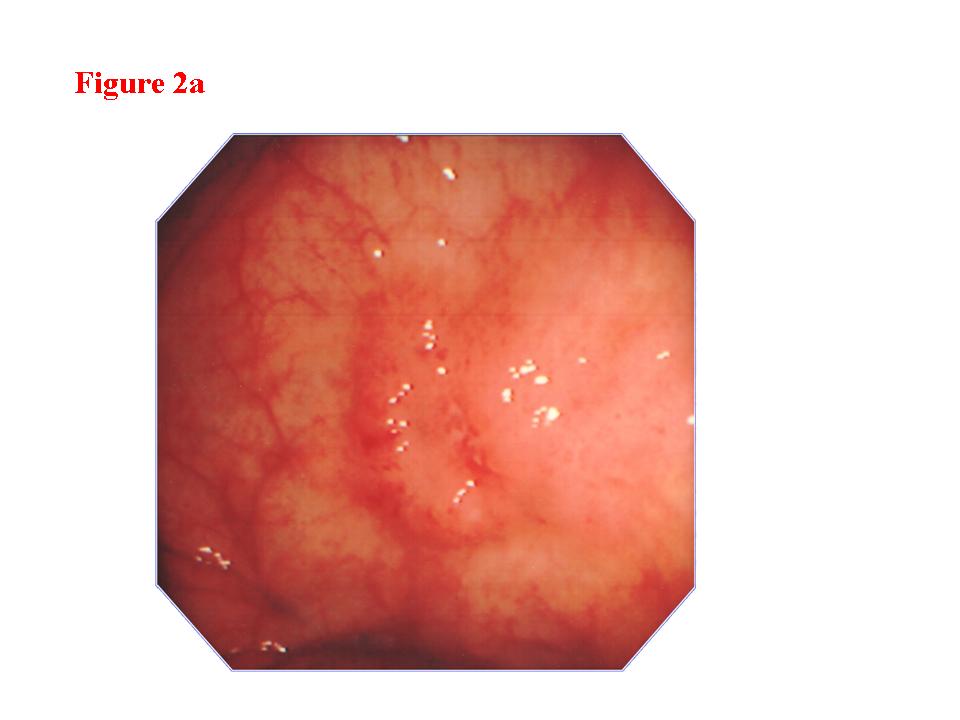
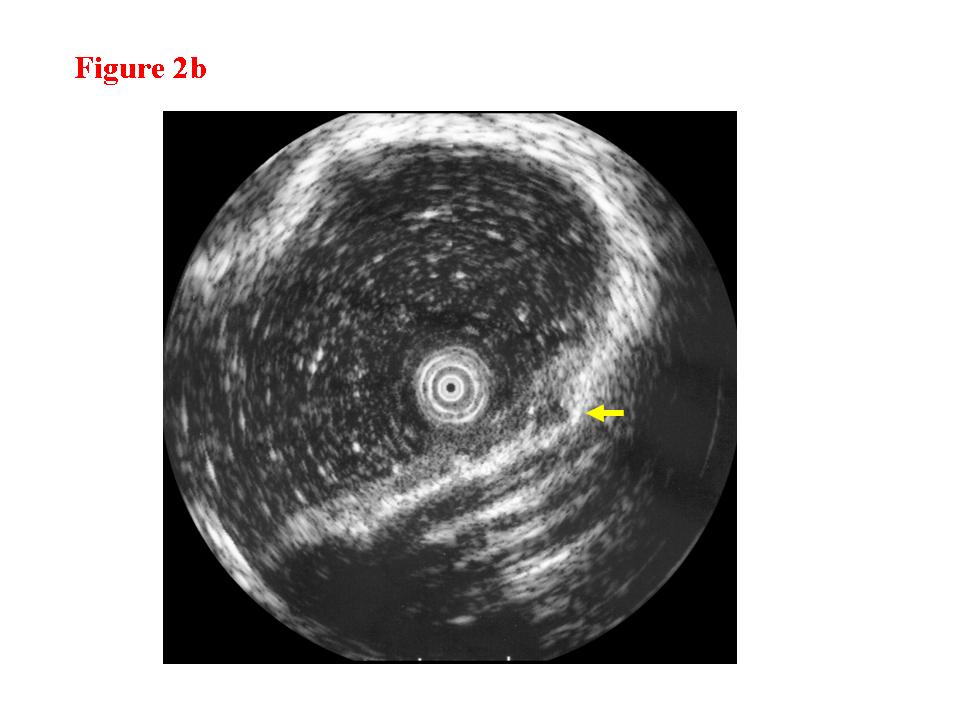
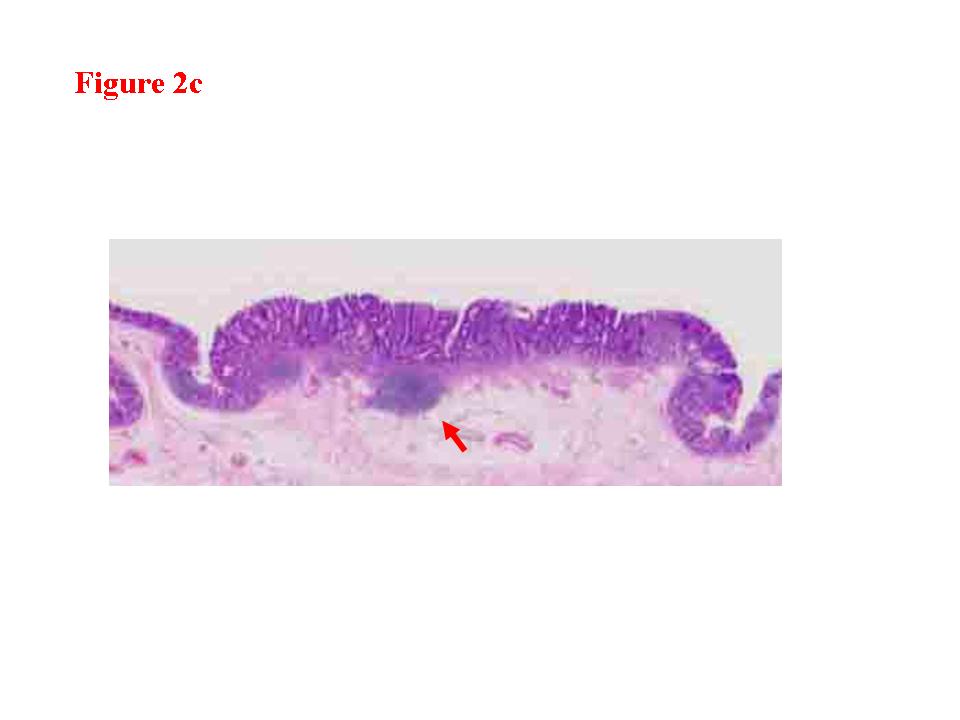
Figure 2. Early colon cancer lesion (EUS-M/SM border line). a) Endoscopic features of superficial depressed type early colon cancer lesion. b) The change in the third layer was less than 1 millimeter (EUS-M/SM borderline). c) The cancer invasion was limited within the mucosa. There was lymphoid follicle in the submucosal layer.
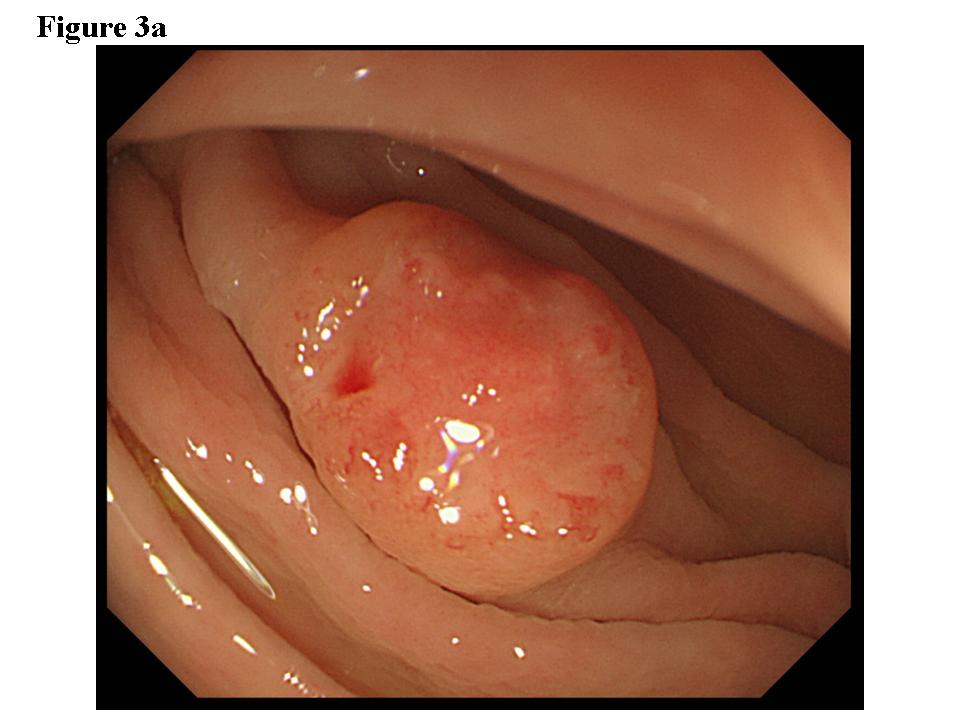
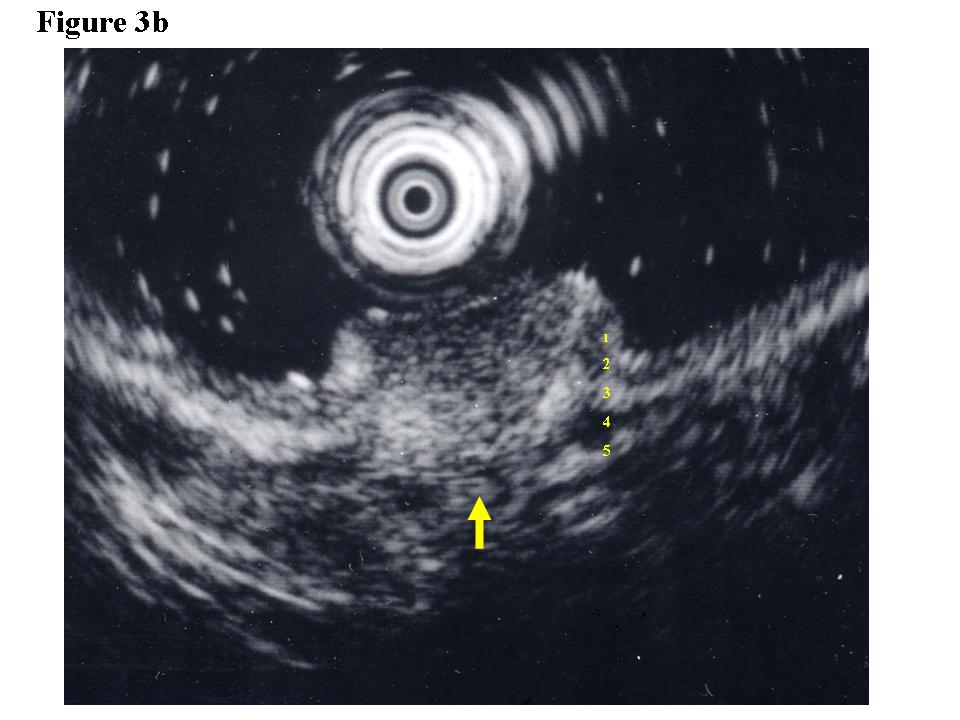
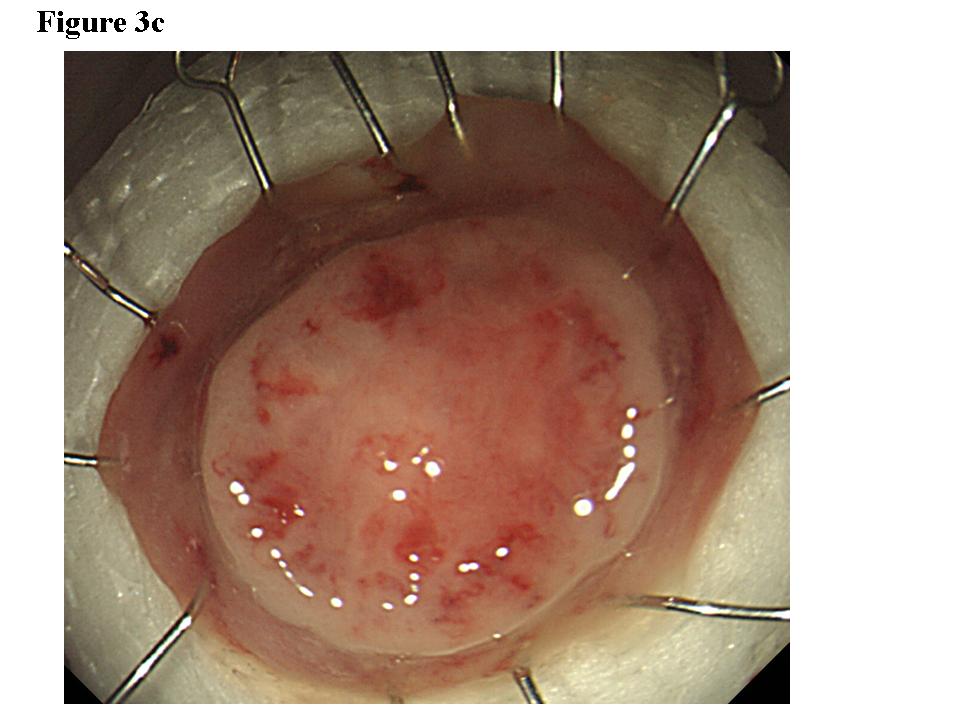
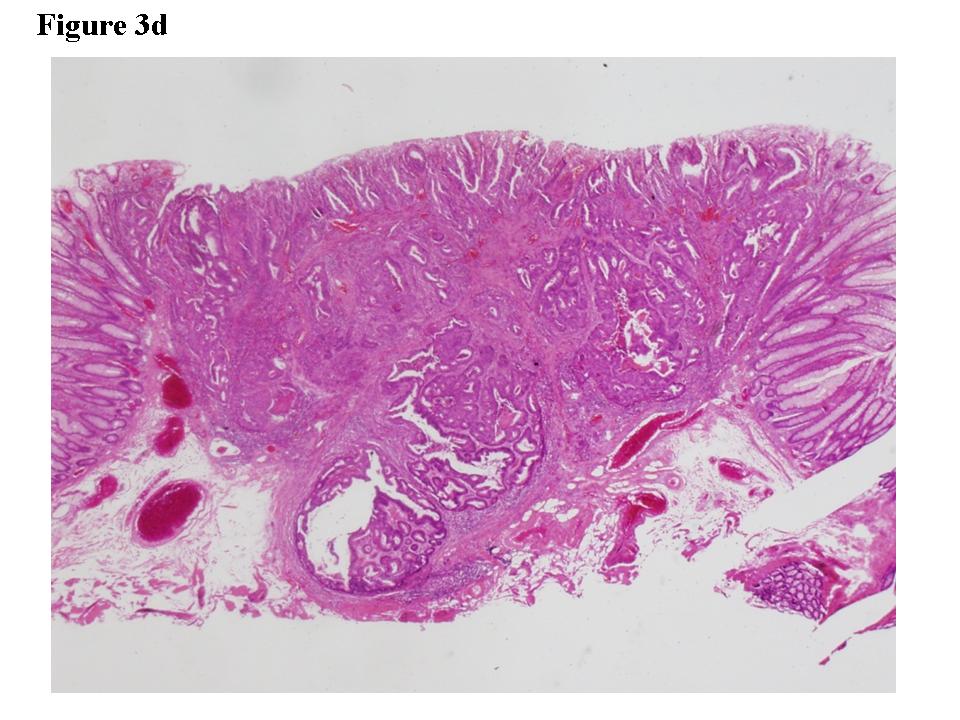
Figure 3. Early colon cancer lesion (EUS-SM). a) Endoscopic features of superficial depressed type early colon cancer lesion. b) The depth of the change for the third layer (submucosal layer) is more than 1 millimeter. EUS finding is EUS-SM. Surgical operation was recommended. c) The patient once refused surgical operation and chose palliative EMR. d) Pathological result of EMR specimen is SM deep invasion over 1 millimeter. With this information, the patient’s mind changed and an additional surgical operation was performed.
EUS findings and therapeutic recommendations
When the EUS finding was EUS-M or EUS-M/SM borderline, we recommended EMR for total biopsy and therapy for the patient (EUS-EMR-recommended group). For EUS-SM or EUS-AD patients, we recommended surgical operation (EUS operation-recommended group) (Table 1).
Table 1. EUS findings and therapeutic recommendations.
EUS-EMR- recommended group |
EUS-M |
Tumor limited to the first and second layers (mucosal layers) |
EUS-M/SM |
Changes in the third layer of less than 1 millimeter |
EUS Operation-recommended group |
EUS-SM |
Depth of change in the third layer (submucosal layer) of 1 millimeter or more |
EUS-AD |
Tumor invasion of the fourth layer (muscularis propria) or more as an advanced tumor |
Therapeutic decision made based on ordinary endoscopic findings |
EUS-NG |
EUS image was unclear |
Even for EUS-SM lesions, palliative EMR was done when the patient rejected surgery. After palliative EMR, the pathological results and risk of metastasis were explained for the patients’ second decision. For EUS-NG lesions, the therapeutic decision was made based on ordinary endoscopic findings.
Pathological findings
Pathological diagnosis was performed by an experienced pathologist according to the standard procedure using H&E stain [10]. The histological SM invasion depth was directly measured using a micrometer on the pathological specimen slide.
Statistical analysis
We used the Mann-Whitney U test for statistical analysis and p<0.05 was considered significant.
Results
Results of EUS findings
We staged 21 of the 63 neoplastic colorectal lesions as EUS-M, 14 as EUS-M/SM borderline, 10 as EUS-SM, 13 as EUS-AD, and 5 as EUS-NG (Table 2). The accuracy rates for EUS results were 81.0% (17 of 21) for EUS-M and 80% (8 of 10) for EUS-SM. The EUS-M/SM borderline lesions (14) consisted of 9 M lesions, 4 SM lesions and 1 lesion invading the muscularis propria. The EUS-AD (13 lesions) consisted of 9 advanced lesions and 4 SM deeply invaded lesions. EUS-NG (5 lesions) consisted of 3 M and 2 SM lesions.
Table 2. Results of EUS findings for superficial colorectal neoplasms (63 lesions).
Histological invasion depth |
M |
SM |
MP over |
EUS-EMR- recommended group |
EUS-M (21) |
17(81.0%) |
4 |
0 |
EUS-M/SM borderline (14) |
9 |
4 |
1 |
EUS Operation-recommended group |
EUS-SM (10) |
0 |
8(80.0%) |
2 |
EUS-AD (13) |
0 |
4 |
9 |
EUS-NG (5) |
3 |
2 |
|
The main error factors in EUS staging were large lesion size, US attenuation, high protrusion, and lesions located on the fold.
EUS findings and histological SM invasion depths
The mean histological SM invasion depth of EUS-M and EUS-M/SM borderline lesions was 0.4 mm. In contrast, that of EUS-SM lesions was 4.3 mm. The histological invasion depth of EUS-SM lesions was over 1 mm and significantly deeper than EUS-M and EUS-M/SM borderline lesions (p<0.001) (Table 3).
Table 3. EUS findings and histological SM invasion depth.
The mean histological SM invasion depth |
EUS-M (21) and EUS-M/SM borderline (14) |
0.4 mm |
|
EUS-SM (10) |
4.3 mm |
P<0.001 |
EUS-AD (13) |
Not measured |
|
EUS findings and clinical courses
We recommended EMR for 35 lesions of 35 patients and surgical operation for 23 lesions of 23 patients (Table 4). All 35 lesions of the EUS-EMR-recommended group were initially treated using EMR. Eighty percent (28/35) of them were curatively treated by EMR only. The other 7 lesions (20%) with deep SM invasion or lymphatic vessel infiltration in the EMR specimens were additionally treated by surgical operation.
Table 4. EUS findings and clinical courses.
|
EMR |
EMR+Operation |
Operation |
EUS-EMR- recommended group (80% curatively treated by EMR only) |
EUS-M (21) |
20 |
1 |
0 |
EUS-M/SM borderline (14) |
8 |
5 |
1 |
EUS Operation-recommended group (100% suitable for surgical operation) p<0.001 |
EUS-SM (10) |
1 (operation rejected) |
6 |
3 |
EUS-AD (13) |
0 |
0 |
13 |
EUS-NG (5) |
4 |
1 |
|
Of the 23 patients in the EUS operation-recommended group, 16 underwent surgery. The other 7 patients of chose palliative EMR; however, all of the lesions were accompanied by deep SM invasion or lymphatic vessel invasion in EMR specimens. Therefore, surgical operation was suitable for all of members of the EUS operation-recommended group. Of the palliative EMR patients, 6 eventually underwent an additional operation and one refused it.
There were significantly more lesions with a surgical operation indication in the EUS operation-recommended group than in the EUS-EMR recommended group (p<0.001). All 4 EUS-NG lesions were initially treated using EMR and one of them required an additional surgical operation.
Discussion
Meining et al. [11] reported that lymphatic vessel infiltration, poor grading of the tumor stage, and incomplete endoscopic resection were risk factors for unfavorable outcomes after endoscopic removal of submucosal invasive colorectal tumors. Among these factors, it might be difficult to predict lymphatic vessel infiltration and incomplete endoscopic resection. Therefore, better pretherapeutic tumor staging is needed. Recently, considering the risk of lymph node metastasis, additional treatment after endoscopic resection for cancer of the colon and rectum is recommended for lesions with deep submucosal invasion of over one millimeter in Japan [12].
EUS has been considered useful for tumor staging of colorectal cancers; however, its real clinical impact for deciding on the indication for surgical operation is still not clear [13-15]. In the present EUS study, when the change for the third layer (submucosal layer) was 1 millimeter or more the histological SM invasion depth was 4.3 mm. Furthermore, using the cutoff value of a 1 mm change of the third layer, surgical operation was suitable for all of the lesions in the EUS operation-recommended group based on the pathological results. Therefore, we think that a change of 1 mm or more in the third layer in EUS for colorectal neoplasms may be a useful finding for surgical decision making.
2021 Copyright OAT. All rights reserv
Kobayashi et al. [16] reported the clinical usefulness of pit patterns for detecting colonic lesions requiring surgical treatment, and the combination of EUS and magnifying endoscopy is expected to be useful to detect such patterns.
In the present study, EUS images were unclear for 7% of all lesions. For the colon and rectum, there are some negative conditions that affect EUS performance such as the existence of large folds and difficulty of maintaining water immersion. In the future, mechanical or technical advances will be necessary to ensure stable EUS scanning of the colon and rectum.
Conclusion
We propose that a change of 1 millimeter or more in the third layer (corresponding to the submucosal layer) in EUS for colorectal neoplasms is a useful finding for surgical decision making.
Ethical statement
This study was approved by the institutional review board.
All EUS and EMR were ordinary clinical procedure, and were in accordance with the ethical standard of the responsible committee on human clinical study and with the Helsinki Declaration of 1964 and later versions.
Informed consent was obtained from all patients for being included in the study.
References
- Weitz J, Koch M, Debus J, Höhler T, Galle PR, et al. (2005) Colorectal cancer. Lancet 365: 153-165. [Crossref]
- Kashida H, Kudo SE (2006) Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol 11: 1-8. [Crossref]
- Dekker E, East JE (2010) Does advanced endoscopic imaging increase the efficacy of surveillance colonoscopy? Endoscopy 42: 866-869.
- Gupta N, Bansal A, Rao D, Early DS, Jonnalagadda S, et al. (2012) Accuracy of in vivo optical diagnosis of colon polyp histology by narrow-band imaging in predicting colonoscopy surveillance intervals. Gastrointest Endosc 75: 494-502. [Crossref]
- Tanaka S, Oka S, Chayama K (2008) Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 43: 641-651. [Crossref]
- Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, et al. (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39: 534-543. [Crossref]
- Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, et al. (1999) A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut 44: 361-365. [Crossref]
- Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, et al. (1989) Histologic correlates of gastrointestinal ultrasound images. Gastroenterology 96: 433-441. [Crossref]
- Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, et al. (1999) A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut 44: 361-365. [Crossref]
- Japanese Society for Cancer of the Colon and Rectum (2009) Japanese classification of colorectal carcinoma. (2nd English edn). Tokyo: Kanehara & Co., Ltd.
- Meining A, von Delius S, Eames TM, Popp B, Seib HJ, et al. (2011) Risk factors for unfavorable outcomes after endoscopic removal of submucosal invasive colorectal tumors. Clin Gastroenterol Hepatol 9: 590-594. [Crossref]
- Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, et al. (2012) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 17: 1-29. [Crossref]
- Saitoh Y, Obara T, Einami K, Nomura M, Taruishi M, et al. (1996) Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc 44: 34-39. [Crossref]
- Fu KI, Kato S, Sano Y, Onuma EK, Saito Y, et al. (2008) Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 53: 1886-1892.
- Urban O, Kliment M, Fojtik P, Falt P, Örhalmi J, et al. (2011) High-frequency ultrasound probe sonography staging for colorectal neoplasia with superficial morphology: its utility and impact on patient management. Surg Endosc 25: 3393-3399.
- Kobayashi Y, Kudo SE, Miyachi H, Hosoya T, Ikehara N, et al. (2011) Clinical usefulness of pit patterns for detecting colonic lesions requiring surgical treatment. Int J Colorectal Dis 26: 1531-1540. [Crossref]
