Background: Liver fibrosis is characterized by excessive accumulation of extracellular matrix. In a mouse model of liver fibrosis, systemic injection of Adipose tissue-derived multi-lineage progenitor cells (ADMPCs) was considered to rescue the diseased phenotype.
Objective: The aim of this study was to assess the efficiency of ADMPC-cell-sheets on improving liver fibrosis. Method and results: ADMPCs were isolated from rat inguinal adipose tissues and expanded. Production of fibrinolytic enzymes MMPs from ADMPCs was examined by ELISA and compared to that from BM-MSCs. Secretion of MMP-3, MMP-9 and -13 from ADMPC were higher than those from BM-MSCs, indicating ADMPCs could be fibrinolytic enzyme-delivery vehicles. Eight-week-old male rats were treated with carbon tetra-chloride (CCl4) by intraperitoneal injection twice a week for 5 weeks, followed by a successful transplantation of ADMPC-sheets on to the diseased liver or mock operation. After 5 more weeks of CCl4 injection (10 weeks in all), rats with ADMPC-sheet-transplants exhibited a significant reduction in liver fibrosis, as evidenced by Sirius red staining, compared with rats treated with CCl4 for 10 weeks without ADMPC-sheet-transplants. Moreover, serum glutamic pyruvate transaminase, γ-glutamyl transpeptidase and total bilirubin levels in ADMPC-sheet-treated rats were lower levels than those in mock operated.
Conclusion: These results showed that the mode of action and proof of concept of cell-sheet transplantation of ADMPCs, which is a promising therapeutic intervention for the treatment of patients with liver fibrosis, and that the ADMPC-sheet transplantation should be similar concept to the drug delivery system.
(242 words)
ADMPC-sheets, cell-sheet, liver fibrosis, MMPs, drug delivery system
Liver cirrhosis could be caused by various conditions such as viral hepatitis, nonalcoholic fatty liver disease, autoimmune diseases and bile duct epithelial injury [1,2]. Liver cirrhosis is the end-stage result of fibrosis in liver parenchyma and should be generally irreversible [3]. Liver fibrosis is characterized by excessive accumulation of extracellular matrix, with the formation of scar tissue encapsulating the area of injury [4]. The prognosis of patients with liver cirrhosis, especially non-compensated, is poor, only liver transplantation seems to improve the prognosis [5,6]. However, limited numbers of donor livers could be available for the millions and thousands of waiting patients who need them [7]. Now, we are eager to develop the novel approaches to cure the patients with end staged live cirrhosis.
Recently, cell therapy has been proposed as an attractive tool for treatment of patients with severe liver fibrosis [8-14]. Mesenchymal stem cells, which possess certain characteristics including self-renewal, proliferation, differentiation and/or secretion of bio-active cytokines, are promising tools in cell therapy [14]. The effectiveness of bone marrow or adipose tissue-derived cells in animal models of liver fibrosis and cirrhosis have been demonstrated by several groups including us [14,15-20]. However, others have reported the lack of any changes in the extent of liver fibrosis or liver function tests following the use of BM-MSCs in the animal models of severe chronic liver injury [21]. Thus, the therapeutic efficacy of BM-MSCs transplantation remains controversial at present [21]. Adipose tissue-derived progenitor/ stem cells are an attractive cell source for cell therapy for liver fibrosis, based on several properties of these cells; 1) ample production of fibrinolytic enzymes and cytokines [22], 2) ease of obtaining stem cells compared to other tissue-specific stem cells including BM-MSCs, [23]. The use of adipose tissue-derived multi-lineage progenitor cells (ADMPCs) supports the view that fibrinolytic enzyme-secretion could mediate the therapeutic mode of actions in liver fibrosis.
In the cell therapy, the route of administration is also the critical issue in addition to mode of actions. Major part of previous reports demonstrated their routes of administration were intravenous [14,16-20]. The intravenously administered cells have reported to be accumulated to the lung and then the liver with loss of administrated cell numbers [24], resulted in reduction of efficacy. To prevent the issues, the other routes of administration should be demonstrated, in which we wonder whether the direct transplantation onto the damaged liver with the cell-sheet technology [25].
In the present study, we investigated the therapeutic efficacy using ADMPC-sheets in rats with CCl4-induced chronic liver dysfunction and the mechanism of their action in improvement of liver fibrosis.
isolation and expansion of ADMPCs
Adipose tissues were procured from inguinal of 8-week-old male rats (F344) (body weight of 120-150 g purchased from CLEA, Tokyo) in accordance with the guidelines for animal experiments of the National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan. Rat ADMPCs were prepared as described previously [8-10,14]. Briefly, the rat inguinal adipose tissue was minced and then digested at 37°C for 1 h in Hank’s balanced salt solution (HBSS, GIBCO Invitrogen, Grand Island, NY) with Liberase (Roche Diagnostics, Germany) as indicated by the manufacturer. Digests were filtered through a cell strainer (BD Bioscience, San Jose, CA) and centrifuged at 800 x g for 10 min. Red blood cells were excluded using density gradient centrifugation with Lymphoprep (d=1.077; Nycomed, Oslo, Norway), and the remaining cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO Invitrogen) with 10% defined fetal bovine serum (FBS, Biological Industries., Israel.) for 24 h at 37°C. Following incubation, the adherent cells were washed extensively and then treated with 0.2 g/l ethylenediaminetetraacetate (EDTA) solution (Nacalai Tesque, Kyoto, Japan). The resulting suspended cells were replated on fibronectin-coated dishes (AGC, Tokyo, Japan) in SteMedia (Nipro, Osaka, Japan), 1 x insulin-transferrin selenium (GIBCO Invitrogen), 1 nM dexamethasone (MSD, Tokyo, Japan), 100 μM ascorbic acid 2-phosphate (Sawai Pharmaceuticals Co., Osaka), 10 ng/ml epidermal growth factor (EGF, PeproTec, Rocky Hill, NJ), and 5% FBS (FBS, Biological Industries, Israel). The culture medium was changed twice a week and then the cells were applied for the experiments after 5 to 6 passages.
Flowcytometrical analysis of rat ADMPCs
Rat ADMPCs were characterized by flowcytometry. Cells were detached and stained with fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibodies to rat CD34, Cd44, CD45, CD73, CD90 and CD105 (BD PharMingen). Isotype-identical antibodies served as controls. After washing with Dulbecco's phosphate-buffered saline (PBS, Nacalai Tesque), cells were incubated with PE-labeled goat anti-mouse Ig antibody (BD PharMingen) for 30 min at 4°C. After three washes, cells were resuspended in PBS and analyzed by flow cytometry using a guava easy Cyte flow cytometry systems (Merck Millipore. Darmstadt, Germany).
Measurement of MMP-3, -9 and -13 secretions by ADMPCs
One million cells of rat ADMPCs and rat BM-MSCs (DS Pharma Biomedical, Osaka) were seeded onto 6 well plates and then cultured for 24 h. The supernatants were harvested, centrifuged, and frozen at -80°C until analysis. MMP-3, MMP-9 and MMP-13 were measured by enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) using the instructions supplied by the manufacturer.
Preparation of monolayered cell sheets
The passaged rat ADMPCs were trypsinized and then replated onto 35-mm temperature-responsive dishes (Cell Seed, Tokyo, Japan) in 2mL of expansion medium at 1x106 cells per dish. After culture at 37°C for 2 days, the cells were incubated again at 20°C. Within 20min, the rat ADMPC-sheets detached spontaneously and floated up into the medium for use as monolayered cell grafts [26].
Animal model of liver fibrosis and cell-sheet-transplantation
Chronic liver fibrosis was induced in rats using the procedure described previously [14, 27, 28] with some modification. Briefly, 8-week-old male rats (F344) (body weight of 120-150 g purchased from CLEA, Tokyo) were treated with a mixture of CCl4 (Wako Pure Chemicals, Osaka) (0.3 mL/kg) and olive oil (Wako Pure Chemicals) (1:1 vol/vol) by intra-peritoneal injection twice a week for 5 weeks, and this was followed by a transplantation of 3 pieces of ADMPC-sheets on the liver (n=4) or mock operation (n=5), and followed by 5 more weeks of CCl4 treatment. All animal experiments were conducted in accordance with the guidelines for animal experiments of the National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan.
Liver function tests and histological analysis
Blood specimens were collected by cardiac puncture at the end of the experiment. Measurement of serum albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (γ-GTP) and total-bilirubin levels by routine laboratory methods was outsourced to Oriental Yeast Co. (Shiga, Japan).
Sirius Red (SR) staining were performed to determine the extent of liver fibrosis. The stained slides were viewed on a BioZero laser scanning microscope (Keyence, Osaka, Japan.). The area of liver fibrosis was quantified with SR staining. Briefly, the fibrotic area (red staining) was assessed at 40× magnification using computer-assisted image analysis with All-in-One analysis software (Keyence, Osaka). Sixteen fields were randomly selected for each animal. The examiner was blinded to the experimental procedure.
Statistical analysis
MMP-3, -9 and -13 levels, fibrotic area and liver panel area are presented as mean ± SE. Differences between groups were assessed for statistical significance by the Student’s t-test, with P<0.05 considered statistically significant.
Characterization of rat ADMPCs
To characterize the rat ADMPCs, first, the representative positive or negative surface markers were examined by flowcytometric analysis (Figure 1A). The cells were negative for markers of hematopoietic lineage (CD34 and CD45) and positive for markers of mesenchymal stromal cells, CD44, CD73 and CD105.
Next, to show the fibrinolytic enzyme secretory potentials, we measured the amount of MMP-3 and MMP-9 and MMP-13, as the representatives of them, secreted from rat ADMPCs by ELISA and compared to those of rat BM-MSC (Figure 1B). After 1-day culture, the supernatants were applied for measurement. MMP-3 and MMP-9 production levels were significantly higher in ADMPCs than BM-MSCs (2.40±0.24 vs 1.03±0.34 ng/mL, p<0.05, 0.048±0.006 vs 0.016±0.003 ng/mL, p<0.05, from 1.0 x 104 cells cultured for 1 days, respectively). There was no significant difference in MMP-13 levels, but the level of rat ADMPCs was higher than that of rat BM-MSCs (0.18±0.02 vs 0.12±0.02 ng/mL).
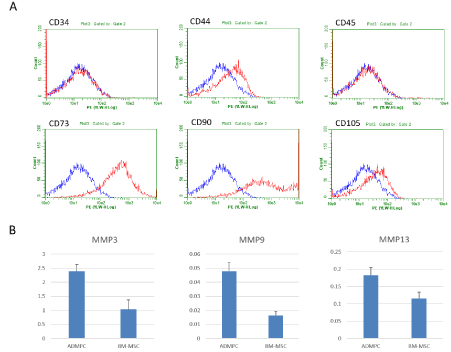
Figure 1: Characterization of rat ADMPCs.
A. Flow cytometric characterization of rat ADMPCs. An isotype-matched negative control indicated as blue curve. Rat ADMPCs were positive for CD44, CD73 and CD105 and negative for CD34 nor CD45.
B. Quantitative analysis of MMP-3, MMP-9 and MMP-13 level produced by rat ADMPCs and BM-MSCs after 1 days of culture. Secretion of MMP-3, -9 and -13 were higher than that of rat BM-MSCs. Data are mean ± SE. Statistical analysis were performed by Student's t-test.
Effects of rat ADMPC-sheet transplantation on CCl4-induced chronic liver dysfunction in rats
As shown in Figure 2A,2B, 9 male rats were injected intraperitoneally with CCl4 twice weekly for 5 weeks, and then divided into two groups, 4 animals received rat ADMPC-sheet transplantation onto their liver surface and the other 5 control received mock operation. All animals were followed for 5 weeks after the last injection.
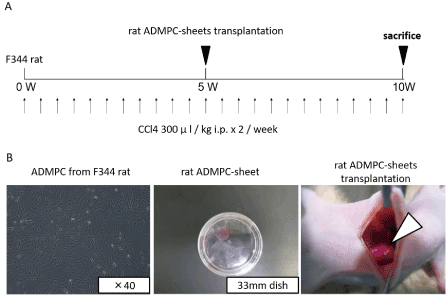
Figure 2: Diagram of the treatment protocol.
A. CCl4 injection and rat ADMPC-sheets transplantation schedule. Rats were treated with CCl4 by intra-peritoneal injection twice a week for 5 weeks, and this was followed by a transplantation of 3 pieces of ADMPC-sheets on the liver or mock operation, and followed by 5 more weeks of CCl4 treatment.
B. Cell-sheet-formation of rat ADMPCs and their transplantation procedures. After culture at 37°C for 2 days, the cells were incubated again at 20°C. Within 20min, the rat ADMPC-sheets detached spontaneously and floated up into the medium for transplantation as monolayered cell grafts.
Sirius Red (SR) staining of sections from ADMPC-sheet-transplanted rats showed mild liver fibrosis, while that of sections from control group rats showed moderate fibrosis (Figure 3A). Quantitative image analysis of the fibrotic area in SR-stained sections confirmed the efficacy of ADMPC-sheet-transplantation on liver fibrosis (Figure 3B). The mean fibrotic area was significant lower in ADMPC-sheet-transplanted CCl4-injured rats (10.8±1.3% of fibrotic areas) than control rats (22.2±3.1% of fibrotic areas) (p<0.05), indicating that ADMPC-sheet-transplantation ameliorated liver fibrosis, and resulted in the recovery of the hepatic parenchymal area.
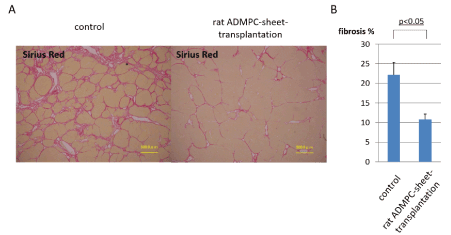
Figure 3: Assessment of liver fibrosis in rat ADMPC-sheet-transplanted rats and controls.
A. Photomicrographs of representative liver sections with hematoxylin/eosin (HE) staining. The peri-lobular regions were the major areas affected by hepatotoxicity while the centrilobular regions seemed to be less affected.
B. Extracellular deposition of collagen fibers on the liver sections stained with Sirius Red. Rats with ADMPC-sheets transplants exhibited a significant reduction in liver fibrosis, as evidenced by Sirius red staining in comparison with those treated with CCl4 for 10 weeks without cell-sheet-transplants. C. Quantification of Sirius red positive fibrotic area by image analysis. The mean fibrotic area was significantly recovered in ADMPC-sheet-transplanted rats than controls. Data are mean ± SE. Statistical analysis were performed by Student's t-test.
Functional recovery of liver damage following transplantation of ADMPC-sheet
We next evaluated the effects of cell-sheet-transplantation on the extent of liver injury and liver panel (Figure 4). Serum transaminase levels (AST, ALT and γ-GTP) were significantly higher in rats with liver damage (mock control), but the increase was attenuated by ADMPC-sheet-transplantation. These results confirmed the efficacy of ADMPC-sheets in the treatment of liver damage associated with fibrosis. Considered together, the results suggest the beneficial effects of ADMPC-sheets in attenuating liver damage and recovery of liver function.
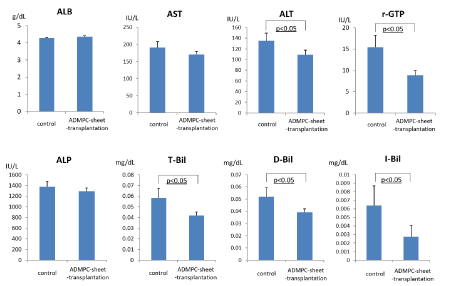
Figure 4: Examination of serum parameters, albumin, AST, ALT, γ-GTP, Alkaline phosphatase, T-Bil, D-Bil and I-Bil. ALT, γ-GTP, T-Bil, D-Bil and I-Bil were significantly reduced by rat ADMPC-sheet-transplantation. Data are mean ± SE. Statistical analysis were performed by Student's t-test.
The major finding of this study was improvement of CCl4-induced rat-liver fibrosis models after ADMPC-sheet-transplantation onto their damaged liver directly, and that these effects were mediated through the production of fibrinolytic MMPs from ADMPCs at least in part, suggesting that these cells could be particularly effective in resolving liver fibrosis.
Liver transplantation 2021 Copyright OAT. All rights reserv end staged liver cirrhosis, although the limited number of donation prevent the amounts of patients who could be treated from treatment [5]. Cellular therapies have been proposed as the attractive and alternative tools for treating patients with many kinds of liver disease [8-14]. There are two strategies in the cellular therapy, first is the replacement therapy and the second the bystander effect-therapy. Among the cellular therapeutic strategies examined so far, replacement therapy had been first examined. Isolated hepatocytes or hepatic progenitor cells from human liver [11,13], regenerated hepatocyte-like or -progenitor cells from pluripotent stem cells [29,30], and in situ reprogrammable cells [9,10, 31] have been tried for their efficacy in animal models. The replacement strategy has also been successful in clinical trials involving patients with certain inherited diseases [32]. Although huge amounts of hepatocytes or hepatocyte-like cells are necessary for meaningful treatment and there should be no room for the replaceable cells in fibrotic hepatic parenchyma to engraft, however, do not seem to be clinically fruitful for liver fibrosis. Thus, we chose the bystander effect strategy in which fibrinolytic enzymes produced by ADMPCs could lyse and diminish the fibrosis of liver parenchyma, and therefore shifted the treatment strategy to improvement of liver fibrosis with cell-based fibrinolytic enzymes delivery. ADMPCs delivered-MMPs should lysis the excess extracellular matrices and make room for the patient's own proliferative hepatocytes. This strategy is similar to those of drug delivery system (DDS), in which the delivered drugs are fibrinolytic enzymes and the delivery vehicles cells.
To establish the cell-based fibrinolytic enzyme delivery system (DDS) first line next to liver transplantation, some challenging points should be discussed with; 1) the cells act as the vehicles for the delivery of fibrinolytic enzymes, 2) cell sheets could be technically deliverable in practice and 3) the “DDS” should improve liver fibrosis and liver panel.
First an important issue “the cells act as the vehicles for the delivery of fibrinolytic enzymes” is whether the cells secrete sufficient amount of fibrinolytic enzymes or not. One study reported that matrix metalloproteinase gene delivery could decrease collagen fibers and reduce liver fibrosis [33]. The mode of action was considered to be the strong expression MMPs on the transplanted cells, indicating that MMPs-producing cells other than BM-MSCs [34] are suitable for use for the cell-based enzyme delivery. The present study showed that ADMPCs expressed MMP-3, -9 and -13 (Figure 1B). The production of MMP-3, MMP-9 and MMP-13 from ADMPCs was superior to that from BM-MSCs. MMP-3 and MMP-9 are known to lyse and destruct collagen types III and I, which are major compartment of liver fibrotic lesion [33]. These data spotlight the potential efficacy of ADMPC-cell-sheet transplantation onto the fibrotic liver directly.
The second issue is whether the cell-sheet technology could be applicable to the disease liver in clinical practice. As shown in Figure 2B, the monolayered ADMPC sheet could easily de-touch from temperature-sensitive culture dish without fragmentation nor torn, and could be pasted easily. Surgeons could transplant them easily in not only open surgery but also endoscopic surgery. New endoscopic cell-sheet transfer devices are now developed [35,36], these devices shall make our ADMPC-cell-sheet applicable for the patient with the end stage liver cirrhosis in near future.
Finally, we need to show that ADMPC-sheet transplantation results in improvement of liver fibrosis and liver panel, as proof-of-concept of our therapy. As shown in Figure 2, ADMPC significantly improved liver fibrosis in CCl4-treated rats as a model of chronic liver cirrhosis. The treatment also resulted in improvement of liver panel, serum transaminase ALT levels. In this model, massive fibrosis was mainly noted in the peri-hepatic lobular region but not in the centrilobular regions surrounding the central veins [14]. Albumin is known to be mainly produced by hepatocytes in the centrilobular region. This is the most likely reason for the lack of difference in serum albumin levels between ADMPC-sheet-transplanted animals and controls. These results indicate that ADMPC-cell-sheet transplantation showed the proof-of-concept to liver dysfunction associated with fibrosis.
The present study demonstrated that the ADMPC-sheet-transplantation onto damaged liver surface significantly attenuated liver fibrosis and improved liver function. These proof of concept and mode of action prompted us to choose ADMPC-sheets for liver cirrhosis next line to liver transplantation.
The authors declare no conflict of interest.
This study was supported by JSPS KAKENHI Grant-in-Aid for Young Scientists (A) 15H05679 for H.O.
- Kasahara A, Tanaka H, Okanoue TI (2004) Interferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver-related death. J Viral Hepat 11: 148-156.
- Saito T, Misawa K, Kawata S (2007) Fatty liver and non-alcoholic steatohepatitis. Intern Med 46: 101-103.
- Manne V, Akhtar E, Saab S (2014) Cirrhosis regression in patients with viral hepatitis B and C: a systematic review. J Clin Gastroenterol 48: e76-84.
- Mallat A, Lotersztajn S (2013) Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am J Physiol Cell Physiol 305: C789-C799.
- Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K (2003) Living-donor liver transplantation: 12 years of experience in Asia. Transplantation 75: S6-S11.
- Takada Y, Ueda M, Ito T (2006) Living donor liver transplantation as a second-line therapeutic strategy for patients with hepatocellular carcinoma. Liver Transpl 12: 912-919.
- Rai R (2013) Liver transplantation- an overview. Indian J Surg 75: 185-191.
- Okura H, Komoda H, Saga A (2010) Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods 16: 761-770.
- Okura H, Saga A, Fumimoto Y (2011) Transplantation of human adipose tissue-derived multilineage progenitor cells reduces serum cholesterol in hyperlipidemic Watanabe rabbits. Tissue Eng Part C Methods 17: 145-154.
- Saga A, Okura H, Soeda M (2011) HMG-CoA reductase inhibitor augments the serum total cholesterol-lowering effect of human adipose tissue-derived multilineage progenitor cells in hyperlipidemic homozygous Watanabe rabbits. Biochem Biophys Res Commun 412: 50-54.
- Semeraro R, Cardinale V, Carpino G (2013) The fetal livers as cell source for the regenerative medicine of liver and pancreas. Ann Transl Med 1: 13.
- Sun K, Xie X, Xi J (2014) Cell-based therapy for acute and chronic liver failures: Distinct diseases, different choices. Sci Rep 4: 6494.
- Jorns C, Ellis EC, Nowak G (2012) Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med 272: 201-2123.
- Okura H, Soeda M, Morita M (2015) Therapeutic potential of human adipose tissue-derived multi-lineage progenitor cells in liver fibrosis. Biochem Biophys Res Commun 456: 860-865.
- Pittenger MF, Mackay AM, Beck SC (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147.
- Saito T, Okumoto K, Haga H (2011) Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev 20: 1503-1510.
- Terai S, Sakaida I (2008) Current status of autologous bone marrow cell infusion therapy for liver cirrhosis patients. Hepatol Res 38: S72-s75.
- Abdel Aziz MT, Atta HM, Mahfouz S (2007) Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem 40: 893-899.
- Hardjo M, Miyazaki M, Sakaguchi M (2009) Suppression of carbon tetrachloride-induced liver fibrosis by transplantation of a clonal mesenchymal stem cell line derived from rat bone marrow. Cell Transplant 18: 89-99.
- Haraguchi T, Tani K, Takagishi R (2012) Therapeutic potential of canine bone marrow stromal cells (BMSCs) in the carbon tetrachloride (CCl4) induced chronic liver dysfunction mouse model. J Vet Med Sci 74: 607-611.
- Carvalho AB, Quintanilha LF, Dias JV (2008) Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury, Stem Cells 26: 1307-1314.
- Ding Y, Xu D, Feng G (2009) Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58: 1797-1806.
- Gimble JM, Bunnell BA, Frazier T (2013) Adipose-derived stromal/stem cells: a primer. Organogenesis 9: 3-10.
- Zheng B, von See MP, Yu E (2016) Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells In Vivo. Theranostics 6: 291-301.
- Owaki T, Shimizu T, Yamato M, Okano T (2014) Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol J 9: 904-914.
- Shudo Y, Miyagawa S, Ohkura H (2017) Adipose tissue-derived multi-lineage progenitor cells improve left ventricular dysfunction in porcine ischemic cardiomyopathy model. J Heart Lung Transplant 36: 237-9.
- Takami T, Terai S, Sakaida I (2012) Advanced therapies using autologous bone marrow cells for chronic liver disease. Discov Med 14: 7-12.
- Rengasamy M, Singh G, Fakharuzi NA (2017) Transplantation of human bone marrow mesenchymal stromal cells reduces liver fibrosis more effectively than Wharton's jelly mesenchymal stromal cells. Stem Cell Res Ther 8: 143.
- Chistiakov DA, Chistiakov PA (2012) Strategies to produce hepatocytes and hepatocyte-like cells from pluripotent stem cells Hepatol Res 42: 111-119.
- Imamura M, Aoi T, Tokumasu A (2010) Induction of primordial germ cells from mouse induced pluripotent stem cells derived from adult hepatocytes. Mol Reprod Dev 77: 802-811.
- Okura H, Morita M, Fujita M (2016) Spermine Treated-Adipose Tissue-Derived Multi-Lineage Progenitor Cells Improve Left Ventricular Dysfunction in a Swine Model of Chronic Myocardial Infarction. J Stem Cell Res Ther 6: 2.
- Meyburg J, Hoffmann GF (2010) Liver, liver cell and stem cell transplantation for the treatment of urea cycle defects. Mol Genet Metab 100: S77-S83.
- Iimuro Y, Brenner DA (2008) Matrix metalloproteinase gene delivery for liver fibrosis. Pharm Res 25: 249-258.
- Usunier B, Benderitter M, Tamarat R, Chapel A (2014) Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int 340: 257.
- Kobayashi S, Kanai N, Tanaka N (2016) Transplantation of epidermal cell sheets by endoscopic balloon dilatation to avoid esophageal re-strictures: initial experience in a porcine model. Endosc Int Open 4: E1116-E1123.
- Maeda M, Kanai N, Kobayashi (2015) Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest Endosc 82: 147-152.




