Background: As studies on various cytokines that contribute to hair growth and differentiation have been actively conducted in recent years, a growth factor cocktail (GFC) in combination with microneedling has been used as an effective and safe treatment for patients with androgenetic alopecia (AGA). However, further research on a more efficient injection method and an effective GFC is needed.
Objective: This study aimed to evaluate the therapeutic effect of a new long-acting GFC injected using microneedles in patients with AGA.
Methods: A total of 22 patients (12 men and 10 women) were enrolled in the study. Patients with AGA were treated six times for a total of 12 weeks at 2-week intervals from the first visit. The scalp was divided into two parts on the right and left sides; 2.5 mL of a new long-acting GFC and 2.5 mL of normal saline were injected on the right and left sides, respectively, using a microneedle at a depth of 1.2 mm. Clinical photographs and phototrichogram images were obtained before the initial treatment and at 2 weeks after the sixth treatment to determine the final treatment effect.
Results: The phototrichogram images of the right scalp showed that the hair density significantly increased from 165.9±27.1/cm2 to 178.3±27.8/cm2, and the hair diameter significantly increased from 61.27±10.50 mm to 62.18±10.61 mm (p<0.05). The hair density and diameter after 12 weeks of treatment with normal saline were not significantly different from those at baseline.
Conclusion: The new long-acting GFC injected via microneedling showed its effect in patients with AGA over a period of 12 weeks. However, further studies on the long-term efficacy of this new long-acting GFC injected via microneedling and relief of bleeding and pain caused by this procedure are needed.
androgenetic alopecia, Cellcurex™, growth factor cocktail, microneedle
Androgenetic alopecia (AGA) is the most common hair loss disease and is mediated by dihydrotestosterone (DHT). DHT is a potent form of testosterone that induces miniaturization of hair follicles, causing a decrease in growth and transformation to vellus hair. Without proper treatment, patients experience gradual hair loss [1,2]. To date, oral administration of finasteride and dutasteride and topical application of minoxidil have been commonly used as treatments. Furthermore, surgical methods, such as scalp skin auto-implantation and follicle autografting, have been implemented, and low-level laser light therapy (LLLT) is the only FDA-cleared procedure for the treatment of AGA [1-4].
As studies on various cytokines that contribute to hair growth and differentiation have been actively conducted in recent years, a growth factor cocktail (GFC) in combination with microneedling has been used as an effective and safe treatment for patients with AGA [5,6]. We have been studying a GFC injected via microneedling at a depth ranging from 0.5 to 0.8 mm in patients with AGA since 2016 [7-11]. However, further research on a more efficient injection method and an effective GFC is needed. Unlike previously studied GFCs [11], a GFC consisting of long-acting growth factors, including phloretin, keratinocyte growth factor-1, and Noggin, was evaluated in this study. We also used microneedling to allow greater absorption of the GFC's validity into the scalp. Herein, we investigated the effects of a new long-acting GFC injected using a microneedle at a depth of 1.2 mm.
Patients
We selected 12 men and 10 women as the final subjects of this study among patients who visited the Alopecia Clinic of the Department of Dermatology at Myongji Hospital. The study subjects were adults aged 19 to 49 years, with the men categorized under Hamilton-Norwood types 3, 4, and 5 and women categorized under Ludwig types 1 and 2. One man and three women dropped out owing to protocol violations and pain during the treatment procedures. Patients who had not undergone hair treatment within 12 months from the start of the study; those with severe seborrheic dermatitis, infectious or severe inflammatory skin lesions, keloid disease or collagen and elastic fiber disease, immunodeficiency disease, or psychiatric problems; and those undergoing treatments that can lead to immunodeficiency were excluded.
Materials and treatment regimens
All patients were treated with microneedling (intradermal injection) and did not undergo hair treatment. The scalp was divided into two parts on the right and left sides, and each injection site was 3 cm away from the center point; the right side was treated with a new long-acting GFC and the left side with normal saline using a microneedle injected at a 1.2-mm depth.
GFC
The GFC used in this study (Cellcurex™; PnP Biopharm, Seoul, Korea) consisted of insulin-like growth factor-1 (IGF-1, 2.5 µg/mL), stem cell factor (SCF, 5 µg/mL), keratinocyte growth factor-1 (KGF-1, 5 µg/mL), fibroblast growth factor 9 (FGF9, 5 µg/mL), Noggin (10 µg/mL), fibroblast growth factor 5-short (FGF5s, 10 µg/mL), stable acidic fibroblast growth factor (aFGF, 5 µg/mL), phloretin (80 µg/mL), and nicotinamide mononucleotide (NMN, 500 µg/mL) (Table 1).
Table 1. Component ratio of the new long-acting growth factor cocktail (Cellcurex™)
No. |
Ingredient |
PPM |
1 |
Insulin-like growth factor-1 |
2.5 |
2 |
Stem cell factor |
5 |
3 |
Keratinocyte growth factor-1 |
5 |
4 |
Fibroblast growth factor 9 |
5 |
5 |
Noggin |
10 |
6 |
Fibroblast growth factor 5-short |
10 |
7 |
Stable acidic fibroblast growth factor |
5 |
8 |
Phloretin |
80 |
9 |
Nicotinamide mononucleotide |
500 |
Microneedle
The microneedle device used in this study (Crystal MESO; Nobamedi Co., Ltd., Yongin, Korea) consisted of five multi-pins, which were injected at a 1.2-mm depth in 32 G, and the needle was mounted on the injector of Crystal MESO (Figure 1).
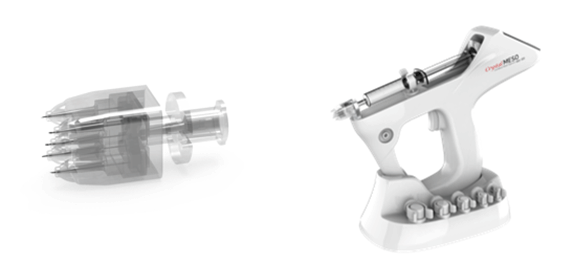
Figure 1. The microneedle device (Crystal MESO™; Nobamedi Co., Ltd., Yongin, Korea) consists of five multi-pins, which are injected at a 1.2-mm depth in 32 G
Treatment
The new long-acting GFC provided as a freeze-dried powder was dissolved in 2.5 mL normal saline before use. Approximately 2.5 mL of the GFC solution was injected on the right side of the scalp and 2.5 mL of normal saline on the left side of the scalp (Figure 2). Both sides were treated with microneedling at a depth of 1.2 mm, and the total area of treatment was 5 cm2 for each site. Each patient received six treatments at 2-week intervals for 12 weeks.
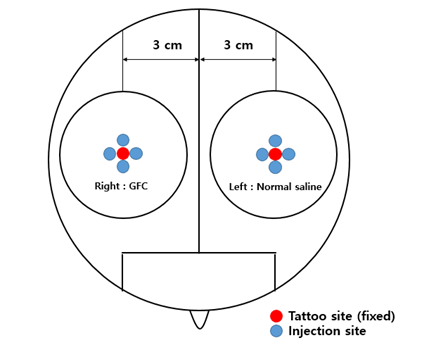
Figure 2. Schematic diagram of the division of the scalp into the right and left sides for the split test. GFC, growth factor cocktail
Parameter measurement
The right and left injection sites were tattooed at locations 3 cm from the designated central point to compare the hair density and diameter. Phototrichogram (Folliscope® 2.8; Lead M, Seoul, Korea) was performed just before treatment and at the 12th week after treatment on the left and right tattooed areas. An unbiased investigator blinded to the study details counted the number of hair and hair diameter using phototrichogram images.
Statistical analysis
All data were expressed as means ± standard deviations. The Wilcoxon signed-rank test was performed using STATA/SE version 15 (Stata Corp LP, College Station, TX, USA) to evaluate the effectiveness of the treatment on the same patients. This method checks whether the difference is positive or negative after treatment by examining the tendency. Statistical significance was calculated at p<0.05.
Patient characteristics
Of the 27 patients, 22 aged 25 to 48 (mean, 34.9±7.5) years completed a total of six treatments every 2 weeks. This study population comprised 12 male patients with a mean age of 38.4±6.7 years and male pattern hair loss type III (n=6), type IV (n=4), and type V (n=2) according to the Hamilton-Norwood classification and 10 female patients with a mean age of 30.7±6.5 years and female pattern hair loss type I (n=5) and type II (n=5) according to the Ludwig scale (Table 2).
Table 2. Baseline characteristics of the study patients.
|
|
Total |
Men |
Women |
Patients |
|
22 |
12 |
10 |
Mean age (year) |
|
34.9±7.5 |
38.4±6.7 |
30.7±6.5 |
Pattern hair loss |
|
|
|
|
Hamilton-Norwood types |
MPHL III |
|
6 |
|
MPHL IV |
|
4 |
|
MPHL V |
|
2 |
|
Ludwig types |
FPHL I |
|
|
5 |
FPHL II |
|
|
5 |
MPHL, male pattern hair loss.
Treatment efficacy and adverse effects
Clinical photographs and phototrichogram images were obtained before the initial treatment and at 2 weeks after the sixth treatment to compare the hair density and thickness (Figure 3). The change of hair density and diameter between the right and left sides after treatment are shown in Table 3. The phototrichogram images of the right scalp showed that the hair density significantly increased from 165.9±27.1/cm2 to 178.7±27.8/cm2 (p=0.0024), and the hair diameter significantly increased from 61.27±10.50 mm to 62.18±10.61 mm (p=0.0485). Meanwhile, the phototrichogram images of the left scalp showed that the hair density decreased from 176.7±30.6/cm2 to 176.3±31.3/cm2 (p=0.9352), and the hair diameter decreased from 62.18±9.67 mm to 59.55±10.00 mm (p=0.1156). Both hair density and diameter after 12 weeks of treatment with normal saline were not significantly different from those at baseline (Figure 4). However, microneedling at a 1.2-mm depth resulted in pain at the injection site and minimal bleeding in some patients. The patients had an average numeral pain rating scale (NRS) score of 4.04 points; some patients had an NRS score of 9 points, causing the experiment to cease. No other adverse effects were identified.
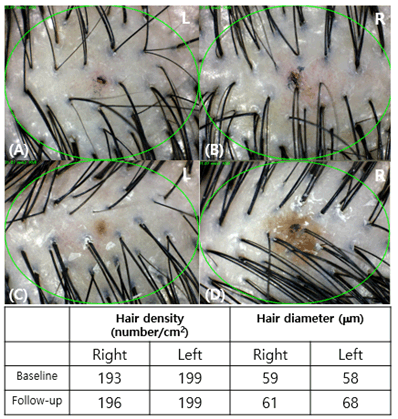
Figure 3. Evaluation of the phototrichogram images after treatment for 12 weeks in patient no. 8, who was categorized under male pattern hair loss type III. (A: baseline, right side of the scalp; B: baseline, left side of the scalp; C: after 12 weeks, right side of the scalp; D: after 12 weeks, left side of the scalp)
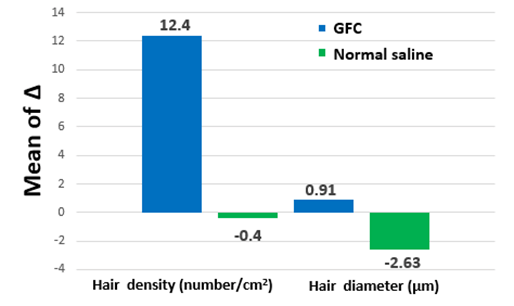
Figure 4. Change from baseline to 12 weeks after treatment. The graph shows that the increase in the right-side parameters (GFC) is greater than that in the left-side parameters (normal saline). GFC, growth factor cocktail
Table 3. Statistical analysis of the changes in the hair density and diameter between baseline and 2 weeks after treatment
| |
|
Baseline |
12 weeks |
| |
|
Mean ± SD |
Mean ± SD |
Δ |
p-value |
Hair density |
GFC |
165.9±27.1 |
178.3±27.8 |
12.4 |
0.0024 |
(number/cm2) |
Normal saline |
176.7±30.6 |
176.3±31.3 |
-0.4 |
0.9352 |
Hair diameter |
GFC |
61.27±10.50 |
62.18±10.61 |
0.91 |
0.0485 |
(mm) |
Normal saline |
62.18±9.67 |
59.55±10.00 |
2.63 |
0.1156 |
GFC, growth factor cocktail; SD, standard deviation.
AGA is the most common hair loss disease and is mediated by DHT. DHT is a potent form of testosterone that induces miniaturization of hair follicles, causing a decrease in growth and transformation to vellus hair. Ultimately, the anagen phase is shortened. AGA is a progressive hair loss disorder in which patients experience gradual hair loss without proper treatment. To date, oral administration of finasteride and dutasteride and topical application of minoxidil have been commonly used as treatments. Furthermore, surgical methods, such as scalp skin auto-implantation and follicle autografting, have been implemented, and LLLT is the only FDA-cleared procedure for the treatment of AGA [1-4]. The main side effects of topical minoxidil are hypertrichosis due to excessive local application, which can also lead to irritation and allergic contact dermatitis. The side effects of oral finasteride include loss of libido, erectile dysfunction, and decreased sexual desire [12]. The need for a non-invasive, easy-to-use treatment has recently emerged because of the side effects that can impair the patients’ quality of life.
Several cases of AGA improvement have been reported in the literature with the utilization of GFCs injected using microneedles [5-9,11], and iontophoresis studies [10] have also been conducted to improve the absorption of GFCs. Recently, platelet-rich plasma administered using a 32-G microneedle has been used to treat patients with AGA, which significantly improved the hair count, density, and percentage in anagen hair compared to the control [13].
Dhurat et al. introduced microneedling as an effective treatment for AGA [5]. According to the literature, there are three sets of hair regeneration mechanisms induced by microneedling: The activities of platelets increase the release of platelet-derived growth factors, increase the activity of stem cells in the hair bubble area, and overexpress hair growth-related genes in the skin wound regeneration process [5]. Studies of microneedling techniques by Jeong et al. [14] and Kim et al. [15] demonstrated an overexpression of hair-related genes and stimulation of hair in mice.
This study changed several active ingredients to yield longer-acting effects compared with those of a previous GFC (Cellcurin™) [11]. Previous studies have used GFCs from IGF1, SCF, KGF2, FGF9, Noggin peptide, FGF5s, stable aFGF, protaetide, and NMN; in contrast, our study changed the Noggin peptide to Noggin, protaetide to phloretin, and KGF2 to KGF1. Although KGF1 has an almost the same mechanism as KGF2, it is reported to be more effective in terms of the thickness, strength, and wound healing of keratinocytes [16]. Noggin is an original protein form different from the Noggin peptide, and phloretin has an anti-inflammatory effect similar to that of protaetide [17]. These growth factors are more stable than the wild type and are expected to remain more stable within the formulation, resulting in greater effectiveness. The GFC used in this study consisted of longer-acting GFCs than those used in prior studies, and the study analysis showed significant increases in both the count and thickness of hair in the patients with AGA.
The limitation of this study is the lack of significant numbers of subjects. Additional research is needed in the same setting model as in previous GFCs to prove the effectiveness of the new long-acting GFC; however, because the microneedle used was different, accurate comparison is difficult. Subsequent studies in the future will require a similar group of patients but a significantly larger sample size to evaluate the efficacy by setting a long-term period of more than a few months, rather than only 12 weeks. Further research on the efficacy of GFCs, which varies owing to differences in the depth of the microneedles, is also needed. In some cases, complaints of pain may lead to inconsistencies in the pressure at which the GFC is injected by a doctor. In addition, because the scalp was curved instead of flat, all GFCs to be administered were not injected into the scalp, and some loss occurred. The average NRS score of the patients in our study was 4.04 points, which was higher than normal, with some patients interrupting the procedure and complaining of severe pain at the injection site. Although 1.2-mm microneedling is more accurate and effective than the conventional 0.8-mm microneedling, it is likely to require further supplementation in cases of pain. According to existing literature, patients experiencing hair loss are reported to have thinner scalps than normal individuals [18]; further research using ultrasonography is needed to confirm whether the thickness of the scalp is related to the degree of pain caused by microneedling.
Furthermore, the injection site of the microneedle affected the patients psychologically. Other side effects, such as infections accompanied by simple bleeding or secondary infections caused by bleeding from scalp wounds, were not reported in this study; however, they are also likely to occur in the future, which then requires further long-term research.
In this study, a new long-acting GFC (Cellcurex™) injected via microneedling was evaluated and found to be an effective treatment for hair regrowth and thickness in patients with AGA at a time frame of 12 weeks. Since this treatment has few systemic adverse effects other than pain or bleeding at the injection site, it can be used for patients who are sensitive to drug side effects or those in whom treatment with drugs is difficult owing to underlying diseases. In addition to conventional AGA treatments, this study identified a new and effective long-acting GFC treatment. However, further studies on the long-term efficacy of this new long-acting GFC injected via microneedling and relief of bleeding and pain caused by microneedling are needed.
There is no potential conflict of interest relevant to this article to declare.
Cellcurex™ was provided by PNP Biopharm (Seoul, Korea). This study was supported by the Technology Development Program (S2843228) funded by the Ministry of Small and Medium-Sized Enterprises and Startups (Korea). This study (2020-10-046) was performed as part of the research program for clinical professors of Myongji Hospital.
- Adil A, Godwin M (2017) The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol 77: 136-141.
- Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, et al. (2011) S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol 164: 5-15. [Crossref]
- Tsuboi R, Arano O, Nishikawa T, Yamada H, Katsuoka K (2009) Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese men. J Dermatol 36: 437-446. [Crossref]
- Hoffmann R, Happle R (2000) Current understanding of androgenetic alopecia. Part II: clinical aspects and treatment. Eur J Dermatol 10: 410-417.
- Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, et al. (2013) A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol 5: 6-11.
- Lee YB, Eun YS, Lee JH, Cheon MS, Park YG, et al. (2013) Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol 40: 81-83.
- Ro BI, Lee SY, Kim JB, Shin HC (2017) Therapeutic effects of growth factor cocktail including fibroblast growth Factor 9 in patients with pattern hair loss. Korean J Dermatol 55: 504-511.
- Ro BI, Son HO, Chun SW, Shin HC (2016) Therapeutic effects of growth factor cocktail treatment in patients with androgenetic alopecia according to the depth of microneedle. Korean J Dermatol 54: 184-190.
- Ro BI, Chun SW, Lee SY, Shin HC (2016) Therapeutic effects of growth factor cocktail including fibroblast growth factor 9 in patients with androgenetic alopecia – A split study. Glob Dermatol 3: 404-408.
- RO BI, Kim SM, Kang JW, Shin HC (2020) Effect of iontophoresis with growth factor cocktail containing fibroblast growth factor 5-short applied to the scalp in patients with androgenetic alopecia - A split study. Glob Dermatol 7: 1-4.
- RO BI, Kang JW, Kim SM, Shin HC (2020) Therapeutic effects of growth factor cocktail (Cellcurin™) containing FGF5s (fibroblast growth factor 5 short) and NMN (nicotinamide mononucleotide) in patients with androgenetic alopecia: A split study. Glob Dermatol 7: 1-5.
- Kanti V, Messenger A, Dobos G, Reygagne P, Finner A, et al. (2018) Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men–short version. J Eur Acad Dermatol Venereol 32: 11-22. [Crossref]
- Rodrigues BL, Montalvão SAL, Cancela RBB, Silva FAR, Urban A, et al. (2019) Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol 80: 694-700.
- Jeong K, Lee YJ, Kim JE, Park YM, Kim BJ, et al. (2012) Repeated microneedle stimulation induce the enhanced expression of hair-growth-related genes. Int J Trichol 4: 117.
- Kim BJ, Lim YY, Kim HM, Lee YW, Won CH, et al. (2012) Hair follicle regeneration in mice after wounding by microneedle roller. Int J Trichol 4: 117.
- Peng Y, Wu S, Tang Q, Li S, Peng C (2019) KGF-1 accelerates wound contraction through the TGF-β1/Smad signaling pathway in a double-paracrine manner. J Biol Chem 294: 8361-8370.
- Anunciato Casarini TP, Frank LA, Pohlmann AR, Guterres SS (2020) Dermatological applications of the flavonoid phloretin. Eur J Pharmacol 889: 173593.
- Hori H, Moretti G, Rebora A, Crovato F (1972) The thickness of human scalp: normal and bald. J Invest Dermatol 58: 396-399.
Editorial Information
Editor-in-Chief
Dr. Dennis Mans
Article Type
Research Article
Publication history
Received date: July 17, 2021
Accepted date: July 29, 2021
Published date: August 03, 2021
Copyright
©2021 Byung In Ro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Ro BI, Kim WJ, Kang JW, Jo YS, Shin HC (2021) Therapeutic effects of a new long-acting growth factor cocktail (Cellcurex™) injected using a microneedle on the scalp in patients with androgenetic alopecia: A split study. Glob Dermatol 8: DOI: 10.15761/GOD.1000231

Figure 1. The microneedle device (Crystal MESO™; Nobamedi Co., Ltd., Yongin, Korea) consists of five multi-pins, which are injected at a 1.2-mm depth in 32 G

Figure 2. Schematic diagram of the division of the scalp into the right and left sides for the split test. GFC, growth factor cocktail

Figure 3. Evaluation of the phototrichogram images after treatment for 12 weeks in patient no. 8, who was categorized under male pattern hair loss type III. (A: baseline, right side of the scalp; B: baseline, left side of the scalp; C: after 12 weeks, right side of the scalp; D: after 12 weeks, left side of the scalp)

Figure 4. Change from baseline to 12 weeks after treatment. The graph shows that the increase in the right-side parameters (GFC) is greater than that in the left-side parameters (normal saline). GFC, growth factor cocktail
Table 1. Component ratio of the new long-acting growth factor cocktail (Cellcurex™)
No. |
Ingredient |
PPM |
1 |
Insulin-like growth factor-1 |
2.5 |
2 |
Stem cell factor |
5 |
3 |
Keratinocyte growth factor-1 |
5 |
4 |
Fibroblast growth factor 9 |
5 |
5 |
Noggin |
10 |
6 |
Fibroblast growth factor 5-short |
10 |
7 |
Stable acidic fibroblast growth factor |
5 |
8 |
Phloretin |
80 |
9 |
Nicotinamide mononucleotide |
500 |
Table 2. Baseline characteristics of the study patients.
|
|
Total |
Men |
Women |
Patients |
|
22 |
12 |
10 |
Mean age (year) |
|
34.9±7.5 |
38.4±6.7 |
30.7±6.5 |
Pattern hair loss |
|
|
|
|
Hamilton-Norwood types |
MPHL III |
|
6 |
|
MPHL IV |
|
4 |
|
MPHL V |
|
2 |
|
Ludwig types |
FPHL I |
|
|
5 |
FPHL II |
|
|
5 |
MPHL, male pattern hair loss.
Table 3. Statistical analysis of the changes in the hair density and diameter between baseline and 2 weeks after treatment
| |
|
Baseline |
12 weeks |
| |
|
Mean ± SD |
Mean ± SD |
Δ |
p-value |
Hair density |
GFC |
165.9±27.1 |
178.3±27.8 |
12.4 |
0.0024 |
(number/cm2) |
Normal saline |
176.7±30.6 |
176.3±31.3 |
-0.4 |
0.9352 |
Hair diameter |
GFC |
61.27±10.50 |
62.18±10.61 |
0.91 |
0.0485 |
(mm) |
Normal saline |
62.18±9.67 |
59.55±10.00 |
2.63 |
0.1156 |
GFC, growth factor cocktail; SD, standard deviation.




