Introduction: To investigate the correlation between the positive expression of Bcl-2 and the clinical characteristics of breast cancer patients.
Methods: A total of 124 patients with breast cancer were enrolled in the study. The relationship between the positive expression of Bcl-2 and the clinical characteristics [age, maximum tumor diameter, axillary lymphnode metastasis, clinical stage, histological grade, estrogen receptor (ER), progestogen receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki-67] in breast cancer patients was analyzed. All the patients with 10-year follow-up information were analyzed, and the logistic regression analysis and multivariable COX analyses was regression are utilized to screen for risk factors associated with positive Bcl-2 expression.
Results: (1) The positive expression rate of Bcl-2 differed significantly among breast cancer patients with different clinical stage and histological grade, while breast cancer patients with different maximum tumor diameter had varied Bcl-2 positive expression rate, with statistical significance (P<0.05); (2) the positive expression rate of Bcl-2 among patients with negative axillary lymph node metastasis was significantly higher than that of those with positive axillary lymph node metastasis (P<0.05); (3) the positive expression rate of Bcl-2 among luminal typle breast cancer patients was significantly higher than that in Tripple negative breast cancer and Her-2 positive breast cancer patients. (P<0.05); (4) Frome the 5-year and 10-year follow-up information of all the patients, Bcl-2 positive breast cancer patients have significantly better prognosis than those of Bcl-2 negative breast cancer patients. With the extension of follow-up time, the prognosis of Bcl-2 positive patients is further improved; (5) Multivariable COX analyses were regression indicated that the independent risk factors for 10 years overall survival were axillary lymph node metastasis, Positive expression of Bcl-2 is a good prognostic factor, but there are not enough data to show that Bcl-2 is an independent risk factor for breast cancer.
Conclusion: There was a suggestion of a strong protective effect of Bcl-2 positive expression patients, thus the detection of Bcl-2 is somewhat meaningful value for evaluating the medical conditions and prognosis of breast cancer patients.
Breast cancer, Bcl-2, prognosis
Based on relevant data, about 1.2 million women are diagnosed with breast cancer every year, and the death toll is about 450000. Breast cancer has become one of the most common malignant tumors that threaten the health of women in the world [1]. At present, surgical resection is still the main treatment for breast cancer. Histopathology-based diagnostic results play a unique role not only in understanding the pathological characteristics of patients, but also in accurately determining the prognosis of patients. It has been reported in a large number of literature that due to the high heterogeneity of breast cancer, the changes in molecular genetic content of breast cancer may not be the same even if there are identical histomorphological structures, which may be one of the reasons for the differences in the treatment effect of patients [2]. It is, therefore, of great significance to investigate the molecular changes in proteins associated with infiltration, metastasis and infinite proliferation. B-cell leukemia-2 (Bcl-2) gene is a crucial anti-apoptosis gene for tumor cells, which is an important indicator for the effective evaluation of cell proliferation [3,4]. This study examined the expression of Bcl-2 Ki-67 in breast cancer tissue, analyzed its relationship with the clinicopathological parameters of breast cancer and the prognosis of patients, and explored the value of Bcl-2 in the diagnosis, treatment and prognosis evaluation of breast cancer.
Patients: A total of 124 paraffin-embedded specimens of breast cancer that were surgically removed by the Department of Breast Surgery of the First Affiliated Hospital of Jinan University from February 2008 to February 2011 were collected. All specimens were confirmed by more than two senior pathologists in our hospital by HE staining and routine immunohistochemical staining.
Experiment procedure: The mouse anti-human Bcl-2 polyclonal antibody was purchased from BD company in the United States, with a detection concentration of 1:250; Ready-to-use GTVisionTM immunohistochemical test kit (Mouse/Rabbit) was purchased from Guangzhou Dake Biotechnology Development Co., Ltd., p-dimethylaminoazobenzene (DAB) was purchased from Fuzhou Maixin Biotechnology Co., Ltd.; German Thermo HM325 microtome for rotary microscope was used.
Immunohistochemical staining: All paraffin-embedded specimens of breast cancer were continuously sectioned by a microtome with a thickness of 4um, and the immunohistochemical staining was performed by the EnVision two-step method. Paraffin sections were conventionally dewaxed, and then completely immersed in 0.01m citrate buffer solution with pH 6.0. Subsequently, the paraffin sections were hermetically placed in a microwave oven and heated at high temperature for 5min until boiling, then heated at low temperature for 10min. Finally, the paraffin sections were naturally cooled to room temperature for heat-induced antigen retrieval. After washing with PBS, the sections were dripped with mouse anti-human Bcl-2 polyclonal antibody (1:250) and Ki-67 working fluid respectively and incubated at 4°C overnight. The ready-to-use enzyme-labelled II anti-GTVisionTM was added dropwise and incubated in a humidified box at room temperature for 30min. DAB was displayed for 3min, and the color development on the surface of the sections was observed under a microscope for timely blocking. The sections were stained with hematoxylin, dehydrated with conventional gradient alcohol and sealed with xylene. The experimental results were observed under an optical microscope. Positive control sections were known positive expression sections, while negative control sections were performed with PBS solution instead of I antibody. Negative control sections were set for each experiment in each group, and independent sections were observed by more than 2 pathologists with rich clinical experience.
Criterion of results: Criterion of Bcl-2 [5] Cells with brown granules in cytoplasm and nuclear membrane are considered as positive cells. Five high-power visual fields were randomly selected from each section and classified according to the percentage of positive cells in the number of breast cancer cells in the visual field. Cells with almost no positive results are (-), with a score of 0 point; 0-25% of positive cells are weakly positive (+), with a score of 1 point; If 25%-75% cells are positive, they are moderately positive (++), with a score of 2 points; When the proportion of positive cells is more than 75%, it is a strong positive expression (+++), with a score of 3 points; 0 point indicates Bcl-2 negative, 1-3 points indicate Bcl-2 positive.
Postoperative follow-up: All patients were followed up every three months after surgery for the first three years and subsequently every six months in 4-5 years, and then followed up once a year in 6-10years. All the clinical data were obtained from hospital and outpatient medical records. The primary endpoint was disease-free survival (DFS), which was defined as the time interval between the date of surgical resection and the date of first locoregional recurrence, or death because of breast cancer or the last follow-up. The follow-up period was conducted in February 2021.
Statistical analysis: All statistical analyses were performed using the SPSS version 24.0 software. The c2 test was used to compare the correlation between Bcl-2 and clinicopathological parameters of breast cancer. All the patients with 10-year follow-up information were analyzed, include DFS and OS. Kaplan-Meier survival curve analysis was used to study the linear trend of Bcl-2 and patients. multivariate cox regression analysis was performed to estimate the significant impact of Bcl-2 and other factors on the prognosis, and the hazard ratio (HR) was calculated for 95% confidence intervals (95% CI). Statistical analysis was two sided, and a p-value <0.05 was considered statistically significant.
Expression of Bcl-2, Ki-67 in breast cancer tissues: In 124 cases of breast cancer, Bcl-2 positive rate was 68.5% (85/124), Among them Bcl-2(+)20 cases, Bcl-2(++)20 cases, Bcl-2(+++)45 cases.
Relationship between Bcl-2, Ki-67 and various clinical prognostic factors: The expression of Bcl-2 in breast cancer tissues was negatively correlated with the expression of tumor size and clinical stage (P<0.05), while it was low in premenopausal women (P<0.05) and had no significant correlation with lymph node metastasis (P>0.05), (see Table 1 for details).
Table 1. The relationship between Bcl-2, Ki-67 expression and other clinicopathologic factors.
Prognosis factors |
n |
|
|
Bcl-2 |
|
|
- |
+ |
++ |
+++ |
P |
Menstruation |
|
|
|
|
|
0.018 |
Premenopausal |
45 |
15 |
7 |
6 |
29 |
|
Promenopausal |
67 |
24 |
13 |
14 |
16 |
|
Tumor size |
|
|
|
|
|
0.007 |
T≤2cm |
35 |
7 |
3 |
4 |
21 |
|
T>2cm |
89 |
32 |
17 |
16 |
24 |
|
Lymph node |
|
|
|
|
|
0.229 |
(-) |
65 |
22 |
11 |
10 |
16 |
|
(+) |
59 |
17 |
9 |
10 |
29 |
|
TNM stage |
|
|
|
|
|
0.007 |
I |
28 |
2 |
2 |
4 |
20 |
|
II |
63 |
23 |
12 |
12 |
16 |
|
III |
33 |
14 |
6 |
4 |
9 |
|
Relationship between Bcl-2 and various pathological prognostic factors: In breast cancer tissues, the expression of Bcl-2 was positively correlated with the expression of ER and PR (P≤0.05), and negatively correlated with vascular tumor thrombus and histological grade (P≤0.05). However, no significant difference can be observed between the expression of Bcl-2 and Her-2 (P>0.05) (see Table 2 for details).
Table 2. The relationship between Bcl-2, Ki-67 expression and other pathology factors.
Prognosis factors |
n |
Bcl-2 |
- |
+ |
++ |
+++ |
P |
ER |
|
|
|
|
|
0.000 |
(-) |
39 |
22 |
11 |
4 |
2 |
|
(+) |
85 |
17 |
9 |
16 |
43 |
|
PR |
|
|
|
|
|
0.000 |
(-) |
48 |
24 |
10 |
5 |
8 |
|
(+) |
76 |
15 |
10 |
15 |
37 |
|
Her-2 |
|
|
|
|
|
0.28 |
(-) |
106 |
31 |
17 |
16 |
42 |
|
(+) |
18 |
8 |
3 |
4 |
3 |
|
Vasculat invasion |
|
|
|
|
|
0000 |
(-) |
68 |
12 |
9 |
12 |
35 |
|
(+) |
56 |
27 |
11 |
8 |
10 |
|
Histological tumor grade |
|
|
|
|
|
0.042 |
I grade |
16 |
1 |
1 |
3 |
11 |
|
II grade |
49 |
19 |
7 |
6 |
17 |
|
III grade |
59 |
19 |
12 |
11 |
17 |
|
Relationship between bcl-2, Ki-67 and molecular typing of breast cancer: According to the expression of ER, PR, Her-2 and Ki-67 in breast cancer tissues, breast cancer was divided into three types: Luminal A (36 cases), Luminal B (60 cases), Her-2 overexpression (11 cases) and triple negative breast cancer (27 cases). The comparative analysis of sample composition ratio showed that Bcl-2 was highly expressed in luminal type breast cancer (P≤0.05). Ki-67 was highly expressed in triple-negative breast cancer by the test of the four-fold table data (P≤0.05).
Relationship between Bcl-2 and postoperative 5 and 10-year disease-free survival: According to the expression of Bcl-2, all breast cancers were divided into two groups: the positive group and the negative group. About 85 cases (68.5%) in the positive group and 39 cases (31.5%) in the negative group. Kaplan-Meier survival analysis showed that the 5-year DFS of Bcl-2 positive expression was 90.8%, and the 5-year DFS of Bcl-2 negative expression was 62.2%, and the absolute benefit was 28.6%, (P≤0.05), see Figure 1A; To further extend the follow-up time, the Kaplan-Meier survival analysis showed that the 10-year DFS of Bcl-2 positive expression was 80.5%, the 10-year DFS of Bcl-2 negative expression was 48.6%, and the absolute benefit was 31.9% (P≤0.05), see Figure 1B. The results suggest that Bcl-2 positive expression has a better prognosis, and with the extension of follow-up time, the DFS of Bcl-2 positive patients was further improved.
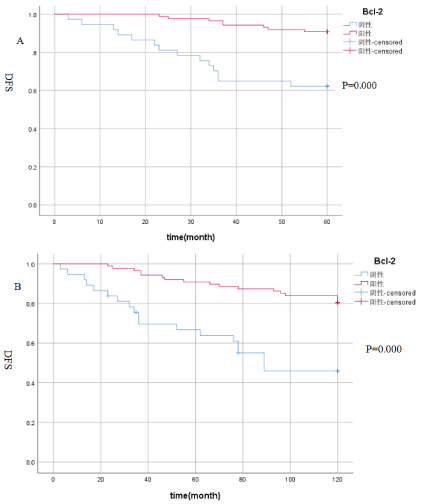
Figure 1A. The relationship between Bcl-2 expression and 5-year disease-free survival: the 5-year DFS of Bcl-2 positive expression was 90.8%, and the 5-year DFS of Bcl-2 negative expression was 62.2%(P≤0.05); Figure 1B. The relationship between Bcl-2 expression and 10-year disease-free survival: the 10-year DFS of Bcl-2 positive expression was 80.5%, the 10-year DFS of Bcl-2 negative expression was 48.6% (P≤0.05).
Relationship between Bcl-2 and postoperative overall survival: According to the expression of Bcl-2, all breast cancers were divided into two groups: the positive group and the negative group. Kaplan-Meier survival analysis showed that the 5-year OS of Bcl-2 positive expression was 94.3 %, and the 5-year OS of Bcl-2 negative expression was 64.9%, and the absolute benefit was 29.4%, (P≤0.05), see Figure 2A; To further extend the follow-up time, the Kaplan-Meier survival analysis showed that the 10-year OS of Bcl-2 positive expression was 87.4%, the 10-year OS of Bcl-2 negative expression was 54.1%, and the absolute benefit was 33.3% (P≤0.05), see Figure 2B. The results suggest that Bcl-2 positive expression has a better prognosis, and with the extension of follow-up time, the OS of Bcl-2 positive patients was further improved.
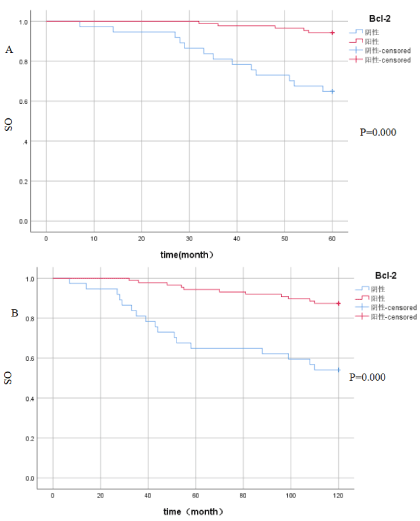
Figure 2A. The relationship between Bcl-2 expression and 5-year overall survival: the 5-year OS of Bcl-2 positive expression was 94.3 %, and the 5-year OS of Bcl-2 negative expression was 64.9% (P≤0.05); Figure 2B. The 10-year OS of Bcl-2 positive expression was 87.4%, the 10-year OS of Bcl-2 negative expression was 54.1%(P≤0.05).
Multivariate COX regression analysis of independent prognostic factors for breast cancer: The multivariate COX stepwise regression model was used to analyze the independent prognostic factors of patients with 10-year OS. The results suggest that lymph node metastasis is an independent prognostic factor of 10-year OS, and positive expression of Bcl-2 is a good prognostic factor, but there are not enough data to explain it, Bcl-2 is an independent risk factor for breast cancer (see Table 3 for details).
Table 3. Multivariate analysis indicated the independent risk factors of breast cancer.
|
|
|
sig |
Exp(B) |
95.0% CI for Exp(B) |
ER |
|
|
.624 |
|
lower |
upper |
LN |
|
|
.001 |
|
|
|
Tumor size |
.351 |
.545 |
.519 |
1.421 |
.488 |
4.136 |
Ki-67 |
.017 |
.505 |
.974 |
.378 |
.378 |
2.736 |
Bcl-2 |
-.267 |
.540 |
.621 |
.265 |
.265 |
2.207 |
Apoptosis refers to the process of cell death under the control of programmed factors. When apoptosis is inhibited, the survival time of some DNA-damaged cells can be moderately prolonged [6,7]. If the body fails to remove harmful mutant cells in time, favourable conditions will be created for the formation of tumor cells, supplemented by continuous proliferation and division of malignant tumor cells to promote the rapid growth of tumors [8,9]. The inhibition of apoptosis and the rapid division of tumor cells have a close bearing on the formation, occurrence and development of tumor cells. In this study, the value of the anti-apoptotic protein Bcl-2 in the diagnosis, treatment and prognosis of breast cancer was analyzed by detecting their expression in breast cancer tissues.
Bcl-2 gene, which falls into the proto-oncogene and is located in 18q21, is currently recognized as an anti-apoptotic gene. When it transposes with the IgH site of 14q32 immunoglobulin, the expression of Bcl-2 protein increases [10,11]. Bcl-2, theoretically, is beneficial in inhibiting cell apoptosis. Therefore, the stronger the expression of Bcl-2, the higher the malignant degree of the tumor and the worse the biological behavior of the tumor. Bcl-2, however, has been shown in more and more clinical studies to be associated with some favorable outcomes in breast cancer [12,13]. It was found by Melella et al. [14], that estrogen can stimulate the expression of Bcl-2 in MCF-7 cancer cell line, confirming that the expression of Bcl-2 is regulated by estrogen, and the expression of this protein is mostly limited to ER-positive breast cancer cells. The expression of ER/PR in breast cancer often indicates a high degree of differentiation, low malignancy, and sensitivity to endocrine therapy of tumor cells. Dawswon discovered that Bcl-2 was relatively highly expressed in highly differentiated breast cancer, and the higher the histological grade, the worse the tissue differentiation and the worse the prognosis [15]. In this experiment, the expression of Bcl-2 was strongly positively correlated with ER and PR and was highly expressed in Luminal type breast cancer. However, the expression of Bcl-2 was negatively correlated with poor prognostic factors such as tumor size, clinical stage, vascular tumor emboli and histological grade, and was often low in premenopausal women. The experimental results are roughly the same as those of current domestic and foreign studies, confirming that breast cancer patients with high Bcl-2 expression tend to have a favourable prognosis, which is specifically reflected in their sensitivity to endocrine therapy and low malignant degree of tumor. In this experiment, all patients were followed up and it was found that those with low expression of Bcl-2 were more likely to relapse after surgery than those with high expression of Bcl-2 (P≤0.05), which supported that those with high Bcl-2 expression had a preferable prognosis. High expression of Bcl-2 may lead to better prognosis, which may be attributed to the following four factors:① The presence of Bcl-2 in some well-differentiated breast tumor tissues not only inhibits cell apoptosis, but also prolongs cell cycle, thus delaying the proliferation of tumor cells [16,17]; ② Bcl-2 is highly expressed in estrogen/progesterone receptor-positive breast cancer, which is touted to have a better effect on endocrine therapy [18]; ③ Bcl-2 is highly expressed in breast cancer with low histological grade, with low malignant degree of tumor [19,20]; ④ Low expression of Bcl-2 is associated with poor prognosis, which may be attributable to the fact that some genes replace the function of Bcl-2 at a certain stage of tumor progression [21].
It was found in this experiment that the expression of Bcl-2 was negatively correlated with the expression of Ki-67 (c2=5.14, P=0.032), confirming that patients with high expression of Bcl-2 and low expression of Ki-67 had preferable prognosis. The reasons for this situation are speculated as follows in combination with the analysis of its pathological characteristics: ① Low expression of Ki-67 and high expression of Bcl-2 are more common in patients with estrogen/progesterone receptor-positive breast cancer, who boast superior endocrine therapy efficacy [22-24]; ② Patients with early pathological stage, no lymphatic metastasis or small tumor have superior surgical efficacy. It has been found in some studies that in neoadjuvant chemotherapy, the expression of Bcl-2 and Ki-67 is markedly correlated with the grade of chemotherapy efficacy. With the improvement of chemotherapy efficacy, the expression of Bcl-2 protein significantly increased while the expression of Ki-67 significantly decreased compared with the previous level [25,26].
Most studies have confirmed that Bcl-2 is positively correlated with the expression of ER in breast cancer, and patients with high Bcl-2 expression have a better prognosis [27-30]. However, there are few studies that directly study Bcl-2 and patients with DFS and OS for 5 years or even 10 years. This study found that the 5-year DFS of Bcl-2 positive expression was 90.8%, and the 5-year DFS of Bcl-2 negative expression was 62.2%, the absolute benefit was 28.6%, (P≤0.05). To further extend the follow-up time, the Kaplan-Meier survival analysis showed that the 10-year DFS of Bcl-2 positive expression was 86.2%, the 10-year DFS of Bcl-2 negative expression was 45.9%, and the absolute benefit was 40.3% (P≤0.05). The results suggest that Bcl-2 positive expression has a better prognosis, and with the extension of follow-up time, the DFS of Bcl-2 positive patients was further improved. In addition, Kaplan-Meier survival analysis showed that the 5-year OS of Bcl-2 positive expression was 94.3 %, and the 5-year OS of Bcl-2 negative expression was 64.9% (P≤0.05), and the absolute benefit was 29.4%. To further extend the follow-up time, the Kaplan-Meier survival analysis showed that the 10-year OS of Bcl-2 positive expression was 87.4%, the 10-year OS of Bcl-2 negative expression was 45.9% (P≤0.05), and the absolute benefit was 41.5%. The results suggest that Bcl-2 positive expression has a better prognosis, and with the extension of follow-up time, the OS of Bcl-2 positive patients was further improved. But in this study, Multivariate COX regression analysis model was used to analyze the independent prognostic factors of patients with 10-year OS. The results suggest that lymph node metastasis is an independent prognostic factor of 10-year OS, and positive expression of Bcl-2 is a good prognostic factor, but there are not enough data to explain it, Bcl-2 is an independent risk factor for breast cancer.
Bcl-2 is closely related to the occurrence and development of breast cancer by inhibiting cell apoptosis. In breast cancer, Bcl-2 is highly expressed in Luminal breast cancer and is positively correlated with ER/PR. Its high expression indicates that patients have a better prognosis; Bcl-2 positive breast cancer patients have better DFS and OS than Bcl -2 negative patients, and with the extension of follow-up time, the benefit is more significant. Therefore, clinically, by detecting the expression of Bcl-2 in breast cancer tissues, the biological characteristics of the tumor can be further clarified and the risk of postoperative recurrence can be evaluated to guide the choice of clinical treatment options.
We would like to thank Dr Wang Ning-Xia for his valuable suggestions on this manuscript. This work was supported by Flagship specialty construction project-General surgery (Funding No.:711003).
The authors confirm that they have no conflict of interest.
Flagship specialty construction project-General surgery (Funding No.: 711003) Natural Science Foundation of Guangdong Province(Funding No.: 2019A1515011847)
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. (2013) Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24: 2206-2223. [Crossref]
- Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, et al. (2013) Breast cancer follow-up and management after primary treatment: american society of clinical oncology clinical practice guideline update. J Clin Oncol 31: 961-965. [Crossref]
- Gross A (2016) BCL-2 family proteins as regulators of mitochondria metabolism. Biochim Biophys Acta 1857: 1243-1246. [Crossref]
- Mansouri H, Mnango LF , Magorosa EP, Sauli E, Mpolya EA (2019) Ki-67, p53 and BCL-2 Expressions and their Association with Clinical Histopathology of Breast Cancer among Women in Tanzania. Open Scientific Reports 9: 11.
- Dawson S-J, Makretsov N, Blows FM, Driver KE, Provenzano E, et al. (2010) Bcl-2 in breast cancer:a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer103: 668-675. [Crossref]
- Ayadi EZ, Cherif B, Ben Hamed Y, Mokni M, Rebai A, et al. (2018) Prognostic Value of BCL2 in Women Patients with Invasive Breast Cancer. Asian Pac J Cancer Prev Dec 19: 3557-3564. [Crossref]
- Min KW, Kim DH, Do SI, Pyo JS, Chae SW, et al. (2016) High Ki67/BCL2 index is associated with worse outcome in early-stage breast cancer. Postgrad Med J 92: 707-714. [Crossref]
- Akram M, Iqbal M, Daniyal M, Khan AU (2017) Awareness and current knowledge of breast cancer. Biol Res 50: 33. [Crossref]
- Fabi A, Mottolese M, Benedetto AD, Sperati F, Ercolani C, et al. (2020) p53 and BCL2 immunohistochemical expression across molecular subtypes in 1099 early breast cancer with long-term follow-up: an observational study. Clin Breast Cancer 6: e761-e770.
- Llambi F, Geen DR (2011) Apoptosis and Oncogenesis: Give and Take in the Bcl-2 Family. Curr Opin Genet Dev 21: 12-20. [Crossref]
- Um HD (2016) Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget 7: 5193-5203. [Crossref]
- J Yang, Li Q, Zhou R, Zhou M, Lin X, et al. (2021) Combination of Mitochondrial Targeting Doxorubicin with Bcl-2 Function Converting Peptide NuBCP-9 for Synergistic Breast Cancer Metastasis Inhibition. J Mater Chem B 9: 1336-1350.
- Gross A (2016) BCL-2 family proteins as regulators of mitochondria metabolism. Biochim Biophys Acta 1857: 1243-1246. [Crossref]
- Milella M, Trisciuoglio D, Bruno T, Ciuffreda L, Mottolese M, et al. (2004) Trastuzumab down regulates Bcl-2 expression and potentiates apoptosis induction by Bcl-2/Bax-XL bispecific antisense oligonucleotides in HER-2 gene-amplified breast cells. Clin Cancer Res 10: 7747-7756. [Crossref]
- Dawson S-J, Makretsov N, Blows FM, Driver KE, Provenzano E, et al. (2010) BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 103: 668-675. [Crossref]
- Bing Yu, Xin Sun, Hong-yan Shen, Gao F, Fan Y-M, et al. (2010) Expression of the apoptosis-relates genes BCL-2 and BAD in human breast carcinoma and their associated relationship with chemosensitivity. J Exp Clin Cancer Res 29: 107-114. [Crossref]
- Pavlenko IA, Povilatite PE, Gorelik MZ, Petrov AV (2012) Bcl-2 as a prognostic factor in different molecular genetic subtypes of breast cancer. Arkh Patol 74: 36-40. [Crossref]
- Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, et al. (2006) Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res 12: 2468-2475. [Crossref]
- Kerkhofs M, Rovere RL, Welkenhuysen K, Janssens A, Vandenberghe P, et al. (2021) BIRD-2, a BH4-domain-targeting peptide of Bcl-2, provokes Bax/Bak-independent cell death in B-cell cancers through mitochondrial Ca2+-dependent mPTP opening. Cell Calcium 94: 102333. [Crossref]
- Hong W, Bei X, Shi J (2020) N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene 722: 144076. [Crossref]
- Paul R, Das T, Debnath M, Chauhan A, Dash J (2020) G-quadruplex Binding Small Molecule Induces Synthetic Lethality in Breast Cancer Cells by Inhibiting c-MYC and BCL2Expression. ChemBioChem 21: 963-970. [Crossref]
- Léon P, Cancel-Tassin G, Drouin S, Audouin M, Varinot J, et al. (2018) Comparison of cell cycle progression score with two immunohistochemical markers (PTEN and Ki-67) for predicting outcome in prostate cancer after radical prostatectomy. World J Urol 36: 1495-1500. [Crossref]
- Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) ki67 in breastcancer:prognostic and predictive potential. The Lancet Oncol 18: 287-292. [Crossref]
- Léon P, Cancel-Tassin G, Drouin S, Audouin M, Varinot J, et al. (2018) Comparison of cell cycle progression score with two immunohistochemical markers (PTEN and Ki-67) for predicting outcome in prostate cancer after radical prostatectomy. World J Urol 36: 1495-1500. [Crossref]
- Kapoor NS, Curcio LD, Patrick M (2016) Abstract PD7-05: Multigene panel testing, and the cancers identified in patients at risk for hereditary breast cancer. Cancer Res 76: 874-876.
- Valkonen M, Isola J, Ylinen O, Muhonen V, Saxlin A, et al. (2020) Cytokeratin-Supervised Deep Learning for Automatic Recognition of Epithelial Cells in Breast Cancers Stained for ER, PR, and Ki-67. IEEE Trans Med Imaging 39: 534-542. [Crossref]
- Emi M, Kim R, Tanabe K, Uchida Y, Toge T (2019) Targeted therapy against Bcl-2-related proteins in breast cancer cells. Breast Cancer Res 7: R940–R952. [Crossref]
- Paul R, Das T, Debnath M, Chauhan A, Dash J (2020) G-quadruplex Binding Small Molecule Induces Synthetic Lethality in Breast Cancer Cells by Inhibiting c-MYC and BCL2Expression. ChemBioChem 21: 963-970. [Crossref]
- Kerkhofs M, Rovere RL, Welkenhuysen K, Janssens A, Vandenberghe P (2021) BIRD-2, a BH4-domain-targeting peptide of Bcl-2, provokes Bax/Bak-independent cell death in B-cell cancers through mitochondrial Ca2+-dependent mPTP opening. Cell Calcium 94: 102333. [Crossref]
- Kønig SM, Rissler V, Terkelsen T, Lambrughi M, Papaleo E (2019) Alterations of the interactome of Bcl-2 proteins in breast cancer at the transcriptional, mutational and structural level. PLoS Comput Biol 15: e1007485. [Crossref]
Editorial Information
Editor-in-Chief
Tomoko Hasunuma
Kitasato University School of Medicine
Article Type
Research Article
Publication history
Received: April 18, 2021
Accepted: April 26, 2021
Published: May 05, 2021
Copyright
©2021 Huang X. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Huang X, Zhang Q, Wang N (2021) The significance of Bcl-2 expression in breast cancer: a 10-year follow-up study. J Clin Invest Stud. 4. DOI: 10.15761/JCIS.1000127


