Abstract
The maternal inheritance of the mitochondrial DNA is one of its amazing characteristics. It is located in the matrix of a mitochondrion. The cellular organelle that is responsible for the generation of ATPs is the mitochondrion. A lot of studies have linked the mitochondria with the generation of cancer cells. There is a crosstalk in the medical research field that whether the cancer cells were originated from a nuclear defect or from a mitochondrial dysfunction. In this review paper, I will highlight the role of mitochondria in tumorigenesis.
Introduction
The mitochondrial genome, which is the DNA of the mitochondria, is strictly inherited maternally, it is a 16569 base pair long circular double stranded DNA (heavy strand from the outside and light strand from the inside), which has a coding region and non-coding region [it is called the control region that consists of Hypervariable Region 1 (HV1) and Hypervariable Region 2 (HV2)], and the coding region codes for 2 ribosomal RNA genes, 22 transfer RNA genes, and 13 protein-coding sequences [2 subunits of ATP synthase, 3 cytochrome oxidase subunits, 7 NADH dehydrogenase subunits and one cytochrome b] that are involved in the electron transport chain [1]. The mitochondria are the organelles of the cytoplasm, they have double membranes [an outer membrane and inner membrane, and in between an inter-membrane space], the ATP synthase and the electron transport chain proteins are located on the inner membrane of the mitochondria [2]. The mitochondria are responsible for the energy production that fuels the entire cells, which is represented by the generation of adenosine triphosphates [ATPs], those are formed by oxidative phosphorylation process, which is known as the citric acid cycle in the mitochondria [1]. Moreover, there are other mitochondrial proteins that are not directed by the mitochondrial genome, the nuclear DNA might encode for proteins to be synthesized in the ribosomes of the mitochondria [2]. In this review paper, I will discuss the role of mitochondria in tumorigenesis by linking the origin and the mechanism of the tumor cells with the function of the mitochondria in leading to those fundamental concepts.
Cancer as a mitochondrial metabolic disease
Dogma roles! Cancer is a genetic disease. The dogma is irrefutable truth, it is a concept that it is no longer challenged or investigated; the dogma is solid. Hanahan & Weinberg have solidified this dogma when they have published a paper titled as “Hallmarks of Cancer: The Next Generation” on 2011. As they have declared, the somatic mutation theory is the foundation of the dogma, which means that cancer cells proliferate in an accelerated rate due to mutations in genes [tumor suppressor genes or proto-oncogenes] that inhibit or stimulate cell division, and they have preserved the dogma when they have claimed that cancer cells have dysregulated energy metabolism that are controlled by proliferation-inducing oncogenes [3]. This means that cancer cell is purely result from a defect in the nuclear DNA of a normal cell. Another example of maintaining the dogma is the work of Stratton, Campbell, & Futreal, which have already mentioned in their published paper “The cancer genome.” on 2009 that cancer cells are raised from series of chance mutations, and they have predict that the minimum accumulation of 4 random mutations in a normal cell will be enough to transform it to a cancer cell [4].
Seyfried, Flores, Poff, & D’Agostino have questioned the dogma, when they have published a paper on 2014 “Cancer as a metabolic disease: implications for novel therapeutics”, they have emphasized that metastasis of the cancer occurs in a stepwise cascade, the cancer cells 1) invade to the local tissues, 2) enter into the circulation, 3) survive the immune system, 4) immunosuppress the immune system, 5) leave the circulation, 6) form secondary tumors, and this is a non-random metastatic cascade, which is hard to imagine that it is caused by random somatic mutations [14]. They have collected the previous experimental studies that have challenged the somatic mutation theory; in order to draw their conclusion.
One of their selections is the work of McKinnell, Deggins, & Labat, they have transplanted the nucleus of a renal tumor cell from a triploid frog into enucleated eggs, those eggs have matured to become a tadpole, but cannot further differentiate to triploid frog, and the tadpole [which was cloned from the nucleus of a frog renal cell tumor] was clear from any tumor [6]. Another work that have been highlighted is the research of Li, Connelly, Wetmore, Curran, & Morgan, they have studied medulloblastoma [which is a brain tumor] by transplanting its nuclei into embryonic stem cells, the results were that those embryonic stem cells stopped differentiation and there was no sign of medulloblastoma [7]. Hochedlinger, et al. published a paper “Reprogramming of a melanoma genome by nuclear transplantation” on 2004, they took the melanoma tumors and characterized genetically the mutations that were in the melanoma genome, then they transplanted the nuclei of the melanoma tumors into embryonic stem cells; to clone the mice, astonishingly they have observed that there were no evidence that the mice were cloned from melanoma tumors as there were no tumor seen, and the mice were aborted [8]. Seyfried, Flores, Poff, & D’Agostino pointed that there was clearly something in the transplanted nucleus of the cancer cell that blocks development in the recipient cell, and did not cause the signature phenotype, which is according to the dogma that cancer cell arise from a defect in the nucleus, and those findings are totally inconsistent with the somatic mutation theory of tumorigenesis [5].
An evolutionary study by Kaipparettu, et al. titled as “Crosstalk from non-cancerous mitochondria can inhibit tumor properties of metastatic cells by suppressing oncogenic pathways.” was published on 2013, they have replaced the mitochondria of highly malignant breast cancer cells with the mitochondria of non-cancerous cells, the results were magnificent, the cancer cells stopped proliferating, then they have decided to reverse the experiment, which means that they have replaced the mitochondria of slow growing cancer cells with the mitochondria of aggressively metastatic breast cancer cells, interestingly they found out that the slow growing cancer cells become aggressively rapid growing cancer cells [9]. Seyfried, Flores, Poff, & D’Agostino summarized the experimental study of Kaipparettu, et al. in figure 1. Therefore, this means that cancer is not resulted from a dysfunction of the nucleus, rather it is resulted from the dysregulation of the mitochondria, and this mechanism of the origin of cancer was explained by the Warburg theory of cancer.
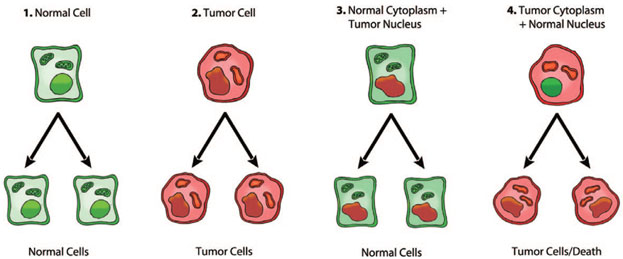
Figure 1. The green nucleus represents normal nucleus and the green cytoplasm represents normal mitochondria, whereas the red nucleus represents tumor nucleus and the red cytoplasm represents tumor mitochondria
1) & 2) as expected, normal nucleus and normal mitochondria result in normal cells, while tumor nucleus and tumor mitochondria result in tumor cells; 3) tumor nucleus with normal mitochondria lead to normal cells; 4) normal nucleus with tumor mitochondria leads to tumor cells. Adapted from “Cancer as a metabolic disease: implications for novel therapeutics” by Seyfried, Flores, Poff, & D’Agostino, 2014, Cancer energy metabolism and therapy, p. 2. Copyright 2013 by Oxford University Press [5]
Warburg theory of cancer
A paper was published by Otto Warburg on 24th of February 1956 titled as “On the Origin of Cancer Cells”, he clearly stated how the cancer cells are working. He declares, the shift from normal cells to cancer cells is due to impaired cell respiration, which takes place in the mitochondria, and increased fermentation, which takes place in the cytoplasm of a cell [10]. The glucose is taken up by the cells through a metabolic pathway called glycolysis, which results in converting glucose to pyruvate [one molecule of glucose will breakdown into two molecules of pyruvates] [10]. The destination of pyruvate molecules will be determined according to the availability of oxygen in the cell. If there is an adequate level of oxygen in the cell, the pyruvate will enter the citric acid cycle in the mitochondria to be processed by oxidative phosphorylation [the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers]; to generate 36 molecules of adenosine triphosphates [ATPs], and this mechanism is known as the cell respiration [10].
On the other hand, in the absence of oxygen level in a cell, the pyruvate molecules are converted by fermentation into lactate [deprotonated form of lactic acid], which results in generating 2 molecules of adenosine triphosphates [ATPs], this shift from the oxidative phosphorylation to glycolysis [which represents the fermentation only] is described as anaerobic glycolysis, which is known as the Pasteur effect [10].
In contrast, Warburg observed that cancer cells behave differently. Warburg mentioned that cancer cells have an adequate level of oxygen, rather than processing the pyruvates by oxidative phosphorylation, they intend to go for fermentation, and he have described this as aerobic glycolysis, which is named as the Warburg effect [10]. Therefore, reduced or destruction of cell respiration as a consequence of malfunctioned mitochondria leads to significantly increased of cell fermentation, which are the fundamental characteristics of cancer cells, and this is the definition of Warburg theory of cancer.
Another paper was unleashed on 2009 by Pavlides, et al. that have introduced a new role of mitochondria in tumorigenesis, which was titled as “The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma”. The communication between cancer cells and the cells in the surrounding environment [known as the stromal cells] helped to drive tumor progression [11]. Pavlides, et al. explained that cancer cells secrete reactive oxygen species [ROS] to induce oxidative stress in the stromal cells, which will result in inevitable damage of the mitochondria of the stromal cells, then aerobic glycolysis will dominate in the stromal cells [Warburg effect] generating lactate as their byproduct, this lactate will be transferred to the cancer cells, the lactate will be converted to pyruvate by the act of lactate dehydrogenase, and it will enter the citric acid cycle in the mitochondria of the cancer cells where it will get processed by oxidative phosphorylation in order to make elevated amount of adenosine triphosphates [ATPs], and this phenomenon is termed as the reverse Warburg effect [11]. This new understanding of Warburg effect is become one of the hallmarks of cancer.
Conclusion
To sum up, tumor cells are not merely the consequence of nuclear genomic defects. In fact, it is obviously that the dysregulation of cell respiration is the origin of tumorigenesis. It is clearly approved that normal mitochondria can suppress tumorigenesis in a cancerous cell, whereas dysfunctional mitochondria can stimulate tumorigenesis in a non-cancerous cell, and that is the critical role of mitochondria in tumorigenesis.
References
- Scheffler IE (1999) Mitochondria. New York, United States of America: Wiley-Liss, Inc.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al. (2008) Energy conversion: Mitochondria and chloroplasts. in molecular biology of the cell (pp. 813-878). New York: Garland Science, Taylor & Francis Group, LLC.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674. [Crossref]
- Stratton MR1, Campbell PJ, Futreal PA (2009) The cancer genome. Nature 458: 719-724. [Crossref]
- Seyfried TN, Flores RE, Poff AM, D’Agostino DP (2014) Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 35: 515–527. [Crossref]
- McKinnell RG, Deggins BA, Labat DD (1969) Transplantation of pluripotential nuclei from triploid frog tumors. Science 165: 394-396. [Crossref]
- Li L, Connelly MC, Wetmore C, Curran T, Morgan JI (2003) Mouse embryos cloned from brain tumors. Cancer Res 63: 2733-2736. [Crossref]
- Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, et al. (2004) Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev 18: 1875-1885. [Crossref]
- Kaipparettu BA, Ma Y, Park JH, Lee TL, Zhang Y, et al. (2013) Crosstalk from non-cancerous mitochondria can inhibit tumor properties of metastatic cells by suppressing oncogenic pathways. PLOS ONE 1-9.
- Warburg O (1956) On the origin of cancer cells. Science 123: 309-314. [Crossref]
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, et al. (2009) The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8: 3984-4001. [Crossref]
2021 Copyright OAT. All rights reserv

