Abstract
Background: Pulmonary embolism (PE) is a life-threatening condition with overall mortality of up to 20 % when left untreated. CTPA is the preferred investigation for diagnosis of PE for patients with normal kidney function. Several studies showed this test is being over employed without using available predictive tools i.e. Wells score, Modified Geneva score & D-Dimer
Methodology: A retrospective review of patients’ records who had CTPA for suspected pulmonary embolism from the period of 1st March 2015 to 28th Feb 2016. Patient’s demographics and components of different scoring system were obtained by manual review of patient’s electronic records.
Result: A total of 249 CTPAs were done during this study period. In term of patient demographics, median age was 63 years and 48.6 % of patients were females. PE was diagnosed in 34 (13.7%) patients. Only 196 (76.6%) patients had a CXR as an initial investigation. Acute kidney injury was noted in 23 (14.0%) patients post CTPA.
Revised Geneva score was calculated for all patients who had CTP for PE, only 10 (4 %) patients had a high probability on Revised Geneva score (score >11). Wells score could not be accurately calculated due to the retrospective nature of the study.
Conclusion: The diagnostic yield of CTPA with a positive result for pulmonary embolism was 13.7%, which is below the recommended standards by the Royal College of Radiology (UK) (15.4% to 37.4%). Also, these findings were noted to be inferior compared with a similar study done in an Australian hospital. These findings may be attributed to the poor utilization of risk assessment tools (Geneva, Wells and D-Dimer) and not performing simple chest X-ray prior to CTPA. A protocol to request CTPAs is needed which is suited to the regional settings to avoid unnecessary CTPAs and its complications.
Introduction
Pulmonary embolism (PE) is a potentially fatal condition if left untreated. The annual crude incidence rate of PE in Australian was estimated to be 0.31 per 1000 in a community setting [1]. The 1-year case-fatality rate for PE is approximately 23% [2]. Efficient clinical evaluation and diagnostic testing is necessary to avoid delays in initiating therapy, which in turn reduces morbidity and mortality from PE [3]. Pulmonary angiography is the historical criterion standard for the diagnosis of PE, which has now been largely replaced by less invasive alternatives. CTPA is the investigation of choice for diagnosis of PE but requires the use of potentially nephrotoxic contrast agents and radiation [4,5]. Alternatively, as a result V/Q scans are commonly considered in patients with renal impairment, pregnant women and in young patients due to the lower dose of radiation [6]. Over the last decade, there has been a significant increase in CTPA use for diagnosis of PE [7]. Inappropriate CTPA use is associated with increases in health care expenditure and risks causing unnecessary harm for patient such as contrast-induced nephropathy and hypersensitivity reactions [8].
The positive rate of CTPA for PE can be as low as 7% but this rate can be increased to more than 30% with use of pre-test risk assessment tools [9-11]. Commonly applied stratifying tools are the Wells score and the revised Geneva score [12]. Depending on the risk factors for pulmonary embolism, patients are categorized into three groups: low (Geneva score 0–3, Wells score 0–1), intermediate (Geneva score 4–10, Wells score 2–6) or high (Geneva score ≥11, Wells score ≥7) [13].
The D-Dimer value is a very useful marker in low/intermediate probability groups and its use has been validated in several studies [12]. In the general population, the combination of D-Dimer testing and clinical assessment tools miss less than 2% of PEs [14].
Objectives of the study
The objectives of this audit are:
1)To evaluate the appropriate use of CTPA (overuse/underuse) and the rate of positive results of CTPA for PE in a regional health setting.
2)To check the degree of adherence to an existing algorithm for using CTPA for PE.
3)To estimate the rate of contrast induced nephropathy.
Setting
Goulburn Valley Health is a rural hospital located 200km northeast of Melbourne that pro- vides care for a catchment population of approximately 120,000 people from the City of Greater Shepparton extending to southern New South Wales. The Medical imaging provides variety of services including CT scans and nuclear medicine.
Methodology
We performed a retrospective review of patients’ records who had CTPA for suspected pulmonary embolism from the 1st March 2015 to the 28th Feb 2016. Patients demographics and components of different scoring system were obtained by manual review of patient’s electronic records. Patients’ individual baseline creatinine values were identified along with their serum creatinine within 72 hours after the CTPA. We defined contrast-induced nephropathy as renal function impairment within 48-72 hours of contrast administration and was measured as a 25% increase in serum creatinine from baseline. Our study was reviewed and approved by the human research ethics committee at Goulburn Valley Health.
Result
We have a total of 249 CTPAs the study period. a 48.6% of patients were females with median age was 63 (IQR =25) years. Only 34 (13.7%) CTPAs were positive for PE. The most common risk factor for PE in our cohort was immobilization or surgery within 4 weeks (19.2%). In term of symptoms, the most common symptom was shortness of breath (70.7%) followed by chest pain (52.2 %) (Table 1).
Table 1. Patients characteristics and common risk factors, signs and symptoms.
Characteristics: n=249 |
Age (yr): mean (IQR) |
63 (25) |
Sex: female (%) |
48.6 |
Risk Factors |
Active Malignancy (%) |
17.3 |
Immobilization or surgery in the previous 4 weeks (%) |
19.2 |
Previously diagnosed with DVT or PE (%) |
13.2 |
Signs and Symptoms |
Hemoptysis (%) |
2.8 |
Shortness of breath (%) |
70.7 |
Chest pain (%) |
52.2 |
Signs and symptoms of DVT (%) |
11.2 |
Tachycardia (%) |
31.3 |
In regard to the use of the PE risk assessment tools, only 79 (31.7%) patients had D-Dimer and 191 (76.7 %) chest x-ray as the primary imaging. 56 out of 191 patients (29.3%) had findings in their chest x-ray adequate to explain their symptoms but treating doctors opted to proceed with CTPA .We used the modified Geneva score to stratify patients according to PE risk , 76 (30.5%) patients had low risk ,163 (65.5% ) patients had medium risk and 10 (4.0%) patients had high risk for PE .The rate of positive CTPA for PE was linearly proportional to the modified Geneva score (Figure 1). 247 (99.2 %) patients had renal function test pre-CTPA and 164 (65.9%) had follow up renal function test .23 out of these 164 (14.0%) patients had acute kidney injury.
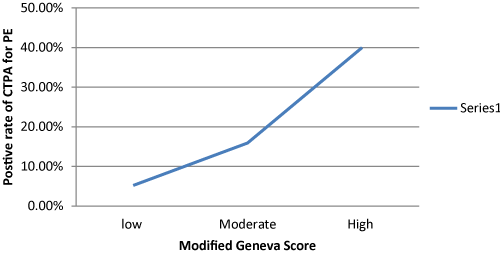
Figure 1. Relationship between Modified Geneva Score and positive rate of CTPA.
Discussion
CTPA is the investigation of choice for diagnosis of PE. The diagnostic yield of CTPA with positive results is only 13.7 % which is lower compared to the recommended standard by the Royal College of Radiology UK (15.4 -37.4%).
The role of clinical assessment tools and D-Dimer for excluding PE in well validated in medical literature and helps in minimizing unnecessary use of CTPA [15]. Furthermore, the introduction of the age adjusted d-dimer has been shown to be more accurate than standard testing and has the potential to reduce the number of unnecessary CTPAs [16]. However, this test is poorly utilized in our institution with less than 32% of patients had D- Dimer.
Only 76.6% had chest x-rays and around 30% of this group had findings in their chest x-ray adequate to explain patients’ symptoms. However, in all occasions, treating doctors opted to proceed with CTPA. We could not calculate Well score due to the retrospective nature of this study, however, when we calculated the modified Geneva score, we found less than 5% of patients -who had CTPA -had high probability score which indicates that majority of these scans were unnecessary.
Easy accessibility to CT is a well-known factor associated with overuse of CTPA [17] and we believe that this is the case in our institution. Another factor that might be associated with overuse of CTPA in regional health settings is the lack of senior decision makers especially after business hours. Also, using CTPA to increase chance of finding alternative diagnosis which is unacceptable stander medical practice and should be avoided [18].
Patients undergoing CTPA are at risk of contrast-induced nephropathy and radiation exposure. In our cohort, only 164 (65.9%) patients had follow up renal function tests and we found 23 (14%) of them had a degree of acute kidney injury. The rate of contrast-induced nephropathy in our study is almost similar to positive rate of CTPA for PE which is similar to previous studies findings [19-22].
2021 Copyright OAT. All rights reserv
In summary, this audit had proven that poor utilization of pre-test risk assessment tool d resulted unnecessary use of CTPA that caused harm for some patients. To minimize this problem, we strongly recommend introducing educational interventions such as seminars as well as devising algorithms targeting clinicians who request imaging without compromising patient safety [23,24]. Guidelines such as these are beneficial to clinicians as they aid in overcoming defensive practice and fear of missing the diagnosis [19].
Conclusion
We have proven that CTPA has been overused at GVH with the low positive rate of PE. Implementation of algorithm-based clinical guidelines and validated pre-test assessment tools will guide the rational use of CTPA. We would recommend carrying out educational interventions directed to medical staff involved with requesting CTPAs. Finally, follow-up audit to monitor the compliance with these recommendations is essential.
Declaration
- 1.This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
- 2.The authors declare no conflict of interest in preparing this article.
References
- Ho WK, Hankey GJ, Eikelboom JW (2008) The incidence of venous thromboembolism: a prospective, community-based study in Perth, Western Australia. Med J Aust 189: 144-7. [Crossref]
- Tagalakis V, Patenaude V, Kahn SR, Suissa S (2013) Incidence of and mortality from venous thromboembolism in a real-world population: The Q-VTE Study Cohort. Am J Med 126: 832 e13-21. [Crossref]
- Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, et al. (2010) Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest 137: 1382-1390. [Crossref]
- Santos RO, Malvar B, Silva R, Ramalho V, Pessegueiro P, et al. (2011) [Contrast-induced nephropathy]. Acta Med Port 24: 809-820. [Crossref]
- Cigarroa RG, Lange RA, Williams RH, Hillis LD (1989) Dosing of contrast material to prevent contrast nephropathy inpatients with renal disease. Am J Med 86(6 Pt 1): 649-52. [Crossref]
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, et al. (2008) Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 29: 2276-2315. [Crossref]
- Molaee S, Ghanaati H, Safavi E, Foroumandi M, Peiman S (2015) Computed Tomography Pulmonary Angiography for Evaluation of Patients with Suspected Pulmonary Embolism: Use or Overuse. Iran J Radiol 12: e22383. [Crossref]
- Morzycki A, Bhatia A, Murphy KJ (2017) Adverse Reactions to Contrast Material: A Canadian Update. Can Assoc Radiol J 68: 187-193. [Crossref]
- Donohoo JH, Mayo-Smith WW, Pezzullo JA, Egglin TK (2008) Utilization patterns and diagnostic yield of 3421 consecutive multidetector row computed tomography pulmonary angiograms in a busy emergency department. J Comput Assist Tomogr 32: 421-425. [Crossref]
- Reagle Z, Tringali S, Gill N, Peterson MW (2012) Diagnostic yield and renal compli- cations after computed tomography pulmonary angiograms performed in a community-based academic hospital. J Community Hosp Intern Med Perspect 2. [Crossref]
- Walen S, de Boer E, Edens MA, van der Worp CA, Boomsma MF, et al. (2016) Mandatory adherence to diagnostic protocol increases the yield of CTPA for pulmonary embolism. Insights Imaging 7: 727-34. [Crossref]
- Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, et al. (2015) Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 36: 2642. [Crossref]
- Nendaz M, Spirk D, Kucher N, Aujesky D, Hayoz D, Beer JH, et al. (2014) Multicentre validation of the Geneva Risk Score for hospitalised medical patients at risk of venous thromboembolism. Explicit Assessment of Thromboembolic Risk and Prophylaxis for Medical PATients in Switzerland (ESTIMATE). Thromb Haemost 111: 531-538. [Crossref]
- Geersing GJ, Erkens PM, Lucassen WA, Buller HR, Cate HT, et al. (2012) Safe exclusion of pulmonary embolism using the Wells rule and qualitative D-dimer testing in primary care: prospective cohort study. BMJ 345: e6564.
- Lucassen W, Geersing GJ, Erkens PM, Reitsma JB, Moons KG, et al. (2011) Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med 155: 448-60. [Crossref]
- Sharp AL, Vinson DR, Alamshaw F, Handler J, Gould MK (2016) An Age-Adjusted D- dimer Threshold for Emergency Department Patients with Suspected Pulmonary Embolus: Accuracy and Clinical Implications. Ann Emerg Med 67: 249-57. [Crossref]
- David S, Beddy P, Babar J, Devaraj A (2012) Evolution of CT pulmonary angiography: referral patterns and diagnostic yield in 2009 compared with 2006. Acta Radiol 53: 39-43. [Crossref]
- Chandra S, Sarkar PK, Chandra D, Ginsberg NE, Cohen RI (2013) Finding an alternative diagnosis does not justify increased use of CT-pulmonary angiography. BMC Pulm Med 13:19. [Crossref]
- Rohacek M, Buatsi J, Szucs-Farkas Z, Kleim B, Zimmermann H, et al. (2012) Ordering CT pulmonary angiography to exclude pulmonary embolism: defense versus evidence in the emergency room. Intensive Care Med 38: 1345-1351. [Crossref]
- Rich MW, Crecelius CA (1990) Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med 150: 1237-1242. [Crossref]
- Huda W (2007) Radiation doses and risks in chest computed tomography examinations. Proc Am Thorac Soc 4: 316-20. [Crossref]
- Mitchell AM, Jones AE, Tumlin JA, Kline JA (2012) Prospective study of the incidence of contrast-induced nephropathy among patients evaluated for pulmonary embolism by contrast-enhanced computed tomography. Acad Emerg Med 19: 618-25. [Crossref]
- Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, et al. (2010) Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. AJR Am J Roentgenol 194: 392-397. [Crossref]
- Sud R, Langfield J, Chu G (2013) Heightened clinical suspicion of pulmonary embolism and disregard of the D-dimer assay: a contemporary trend in an era of in- creased access to computed tomography pulmonary angiogram? Intern Med J 43: 1231-1236. [Crossref]

