The highly cationic nature of sPLA2-IIA allows the enzyme to bind and hydrolyze an anionic phospholipid on the cell membrane, especially when damaged and stressed, causing the release of lysophospholipid and free fatty acid. These products are eventually converted into eicosanoids such as arachidonic acid, leukotrienes, and prostaglandins, leading to inflammation. Recent studies have shown that a high level of sPLA2-IIA is associated with ocular inflammatory diseases, including dry eye disease, chronic blepharitis and allergic conjunctivitis. Therefore, inhibition of sPLA2-IIA may serve as a promising strategy to alleviate ocular inflammation. In this review, we survey the current literature of sPLA2-IIA on inflammatory diseases and aim to provide new insight into the study of sPLA2-IIA inhibition for inflammatory ocular diseases.
All phospholipase A2 (PLA2) enzymes are capable of hydrolyzing the center (sn-2) ester bond of a natural phospholipid substrate, giving lysophospholipid and free fatty acids, including arachidonic acid [1]. However, due to variable functional and structural features including sequence, molecular weight, disulfide bonding patterns, requirement for Ca2+, as well as catalytic mechanisms (His/Asp, Ser/Asp or Ser/His/Asp hydrolase), evolutionary relationships, and localization [1-8], the superfamily of PLA2 is assigned into at least fifteen separate groups and numerous subgroups [1,2,7-9]. While new groups of PLA2 such as adipose-specific PLA2 (AdPLA2) have been identified, most research has focused on the five main groups: secretory PLA2 (sPLA2), pancreatic or cytosolic PLA2 (cPLA2), Ca2+ independent PLA2 (iPLA2), platelet-activating factor (PAF) acetylhydrolase (PAF-AH), and lysosomal PLA2 [5].
The secretory PLA2 (sPLA2) family, originally purified from patients with rheumatoid arthritis, consists of 10 catalytic active enzymes [5,10,11]. Besides the common characteristics of the PLA2 superfamily mentioned above, members of the sPLA2 family share the unique features of low molecular weight, Ca2+ requirement for catalysis, the presence of a His/Asp dyad and six conserved disulfide bonds [2,8]. High-resolution crystal structure models revealed that two water molecules are bonded to the catalytic histidine and a nearby conserved aspartate is used as a ligand for Ca2+ [12]. Together they help to form a positively charged oxyanion hole that stabilizes the negatively charged sPLA2 enzyme [2-4,12].
Currently, more than 11 sPLA2 isoforms have been identified in mammalian secretions [2,5]. A few sPLA2 isoforms are associated with disease, such as sPLA2-IIB with obesity [13] and sPLA2-III with atherosclerosis and colon cancer [14]. The prototypic member of the group II sPLA2 subfamily, sPLA2-IIa, has been shown to be induced by pro-inflammatory stimuli in a wide variety of cells and tissues of animal species as well as been associated with inflammatory, autoimmune, allergic diseases, such as rheumatoid arthritis, asthma, septic shock, Crohn’s disease, acute respiratory distress syndrome, coronary artery disease, atherosclerosis, sepsis and cancer, earning its nickname “the inflammatory sPLA2” [10,11,15-35].
The sPLA2-IIA enzyme is best known as a bactericide involved in the degradation of bacterial membrane thus providing a host defense mechanism against microbial infection [36-40]. The enzyme has a high affinity for anionic phospholipids and prefers binding to phosphatidylethanolamine (PE) than phosphatidylcholine (PC), which are found abundantly on bacterial membranes [37]. Due to its highly cationic nature, sPLA2-IIA binds tightly to the anionic phospholipids on Gram-positive bacteria, allowing the enzyme to penetrate the cell wall and hydrolyze membrane phospholipids, causing bacterial death [41].
Under physiological conditions in mammalian membranes, the inner leaflet contains anionic phosphatidylserine (PS) and PE while the exposed outer leaflet contains neutral phospholipids, such as PC, allowing for relatively low levels of sPLA2-IIA enzymatic activity [42-45]. When the phospholipid distribution is perturbed by stress from the oxidation of phospholipids, increased levels of anionic PS and PE are transported to the outer leaflet, which activates sPLA2-IIA leading to indiscriminate hydrolysis of outer leaflet phospholipids and inflammation [46-50].
The activation of its enzymatic function is triggered by environmental stress or diseased conditions, promoting release of arachidonic acid (AA) and the cyclocoxygenase (COX)-mediated production of prostaglandin (PG) (Figure 1) [51-57]. Once AA is released, biosynthesis of a multitude of eicosanoids, including prostaglandins, such as prostaglandin E2 (PGE2), thromboxanes and leukotrienes is initiated through either the external plasma membrane pathway or heparan sulfate proteoglycan (HSPG)-shuttling pathway with the help of cyclooxygenase and lipoxygenase enzymes [52,58-60]. The newly synthesized eicosanoids then bind to specific G-protein coupled receptors, activating multiple signaling transduction pathways that lead to acute or chronic inflammation or even cancers [61-65]. In addition, the lysophospholipids generated by sPLA2-IIA hydrolysis also serve as precursors for lipid mediators, such as platelet activating factor (PAF), which lead to inflammation [66]. Since spontaneous AA release hardly occurs in unstressed human cells, activating sPLA2-IIA is the critical first step of the inflammatory cascade and therefore inhibiting sPLA2-IIA will ultimately prevent the downward formation of additional inflammatory products [67].
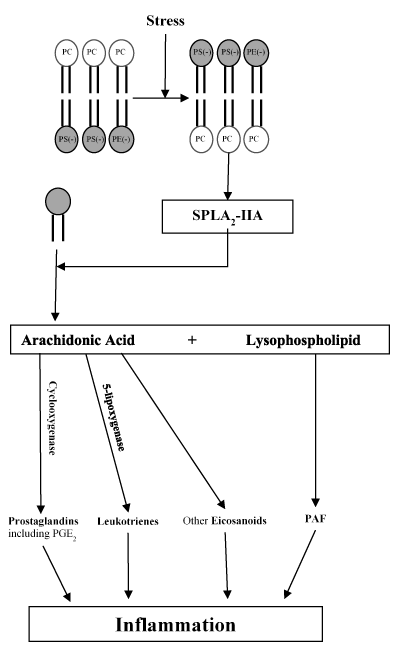
Figure 1. sPLA2-IIA activation promotes inflammation. The oxidation of phospholipids produces stress causing anionic phospholipids (phosphatidylserine (PS) and phosphatidylethanolamine (PE)) to be transported to the outer leaflet, activating cationic sPLA2-IIA. Higher sPLA2-IIA activity promotes the hydrolysis of outer leaflet phospholipids into arachidonic acid and lysophospholipids. Through cyclooxygenase and 5-lipoxygenase enzymes, arachidonic acid is converted into prostaglandins, leukotrienes, and other eicosanoids. Lysophospholipids is converted into platelet-activating factors (PAFs). All four products have been linked to inflammation. Many inflammatory treatments target the arachidonic acid pathway and its enzymes, but targeting sPLA2-IIA may offer direct inhibition to the formation of all downstream inflammatory products
High levels of sPLA2-IIA activity have been found in the progression of non-ocular inflammatory diseases and cancers, such as atherosclerosis [26,27], astrocytoma [28], colorectal cancer [29], lung cancer [30-32], prostate cancer [33], rheumatoid arthritis [34] and acute respiratory distress syndrome (ARDS; [35]) as well as many inflammatory ocular diseases. Since phospholipids serve as surfactants between the aqueous and oil layers of the tear film, higher levels of sPLA2-IIA disrupt the phospholipid layer causing the production of eicosanoids in tears and potentially chronic blepharitis, a condition characterized by the inflammation of the eyelid margins [1,68,69]. In addition, an increase in the number of free fatty acid and sPLA2-IIA have been found on contact lenses of patients with contact lens intolerance, emphasizing sPLA2-IIA’s ability to damage the ocular surface, which leads to discomfort and dryness [70,71].
In allergic conjunctivitis (AC), an eye inflammation caused by an allergic reaction to substances, such as pollen, sPLA2-IIA acts as a chemoattractant for eosinophils, which have been activated by allergens irritating the conjunctiva [72]. Higher levels of sPLA2-IIA create tear film instability, leading to the further progression of allergic conjunctivitis [73, 74]. In patients with dry eye disease (DED) or keratoconjunctivitis sicca, a disease noted by aqueous tear deficiency, high levels of sPLA2-IIA activity and protein concentration stimulate PGE2 production in compromised ocular surface epithelial cells, resulting in the excess production of cytokines in tears and inflammatory cell infiltration [75-78].
The most commonly prescribed drugs for treatment of ocular inflammatory conditions are non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase, thereby suppressing arachidonic acid metabolism and the synthesis of prostaglandins as seen in Figure 1 [79,80]. However, previous studies have shown the possibility of corneal melting and perforation following the use of NSAIDS after ocular surgery in patients with or without systemic diseases, such as rheumatoid arthritis, Sjögren Syndrome, and rosacea [81-83]. Since NSAIDs only inhibit COX-1 and COX-2, having no effect on leukotrienes or PAF [84], inhibiting sPLA2-IIA offers a better solution in blocking the production of additional inflammatory substances.

Figure 1. sPLA2-IIA activation promotes inflammation. The oxidation of phospholipids produces stress causing anionic phospholipids (phosphatidylserine (PS) and phosphatidylethanolamine (PE)) to be transported to the outer leaflet, activating cationic sPLA2-IIA. Higher sPLA2-IIA activity promotes the hydrolysis of outer leaflet phospholipids into arachidonic acid and lysophospholipids. Through cyclooxygenase and 5-lipoxygenase enzymes, arachidonic acid is converted into prostaglandins, leukotrienes, and other eicosanoids. Lysophospholipids is converted into platelet-activating factors (PAFs). All four products have been linked to inflammation. Many inflammatory treatments target the arachidonic acid pathway and its enzymes, but targeting sPLA2-IIA may offer direct inhibition to the formation of all downstream inflammatory products
Furthermore, a natural inhibitor of sPLA2-IIA, ochnaflavone has been shown to strongly inhibit sPLA2-IIA activity by preventing the progression of calcium-tetrachloride (CCl4)-induced PE hydrolysis [85]. Since several ocular inflammatory diseases, such as chronic blepharitis [68] involve the degradation of the tear film, implicated by sPLA2-IIA-initiated PS hydrolysis, inhibiting sPLA2-IIA may be a promising alternative to prevent the formation of inflammatory mediators and the progression of inflammatory ocular diseases.
One of the first small-molecule inhibitors specific for human sPLA2-IIA, LY311727, was designed to fit the active site of human non-pancreatic sPLA2 enzyme through tight binding interactions, in contrast to the weak or lack of inhibition of previously used snake venom and pancreatic enzyme derivatives [86-88]. While LY311727 has a high affinity for group II sPLA2 at an enzyme concentration that is 20,000 times less than the phospholipid substrate concentration, studies have found that the inhibitor can also inhibit group V sPLA2 with an IC50 of 36 nanomolar (nM) [79,89], stressing one of the main obstacles in sPLA2-IIA inhibition research, sufficient sPLA2-IIA selectivity.
The most heavily researched sPLA2 inhibitor, Varespladib sodium (also known as LY315920 and S-5920) was optimized to be five to ten-folds less active against sPLA2 group V and 40-fold less active against group IB, with negligible activity against groups IV, VI and cytosolic phospholipases [90-92]. The small-molecule indole-based sPLA2 inhibitor has IC50 as low as 9-14 nM for human sPLA2-IIA [93] and was found to mechanistically inhibit surfactant degradation [94]. Having wide existing pharmacological data, Varespladib has already undertaken phase II and III clinical studies in adults (both orally and intravenously) for a variety of clinical symptoms, such as sepsis, acute coronary syndrome, and sickle cell disease-induced acute chest syndrome (IMPACTS trial, NCT00434473) [9,95-100]. However, the oral pro-drug of Varespladib sodium, Varespladib-methyl (LY333013) has been found to have varying efficacies in the treatment of coronary artery disease (CAD) [96-98] as well as been ineffective in treatment of rheumatoid arthritis, leading researchers to believe that Varespladib has limited efficacy in clinical studies [101].
One reason for this failure may be due to the incomplete inactivation of sPLA2 influenced by an inadequate inhibitor concentration in the trial, which the authors fail to report [102]. In some diseases, Varespladib was found to bind non-specifically to high levels of proteins before reaching the sPLA2 catalytic site for effective inhibition [91,100]. Increasing Varespladib dosage has also been noted as a solution to overcoming incomplete inactivation of sPLA2 [100]. Furthermore, an alternative small molecule sPLA2 inhibitor (PIP-18) was proposed to have a stronger suppressive effect on sPLA2 transcription and translation than Varespladib which offers another solution to the limited efficacy found in Varespladib clinical studies [103,104]. This 18-residue peptide contains two presumed pharmacophores. Each pharmacophore binds to more than one molecule of sPLA2 through the hydrophobic binding pocket near the N-terminal helix of sPLA2, inhibiting more than 70% of sPLA2 secreted and lowering PGE2 production [103-106].
On the other hand, a naturally produced triterpene, Celastrol, has shown multiple inhibitory effects on sPLA2-IIA and other enzymes along the AA pathway including COX-2 and 5-LOX, in an apparent concentration-dependent manner [107]. The inhibitor demonstrates positive pharmacological effects and use as a therapeutic molecule in inflammatory diseases, such as arthritis and cancer [108], suggesting that targeting both sPLA2-IIA and inflammatory mediators may serve as a useful and potentially powerful strategy.
Human integrins αvβ3 and α4β1 on leukocytes have also recently been implicated as two sPLA2-IIA human receptors and targets for chronic inflammatory diseases [109-111]. Using docking simulation, Takada et al. designed a peptide, named compound 21, which was able to inhibit nearly 93% of sPLA2-IIA-integrin αvβ3 interactions in water soluble conditions thus suppressing αvβ3-mediated cell adhesion, migration and inflammation [112]. In addition, other inhibitors with heterocycle-peptide conjugate structures have been identified, such as compound 4, compound 8 and compound 16, which binds to integrins and blocks the ligand function of sPLA2-IIA [110]. This type of inhibitor may provide a different and effective strategy to suppressing sPLA2-IIA-induced inflammation.
There is an increasing body of evidence demonstrating the beneficial use of sPLA2-IIA inhibitors in ocular inflammatory diseases. Oleanolic acid was found to effectively reduce production of sPLA2-IIA and other pro-inflammatory mediators by eosinophils and mast cells in the conjunctiva of allergic mice [74]. Varespladib and a recently studied sPLA2-IIA inhibitor, S-3319, have also shown to decrease symptoms of corneal superficial punctate keratitis (SPK), a disorder noted by the death of small group of cells in DED mice [113]. S-3319 was shown to selectively bind to sPLA2-IIA in vivo in mice with an IC50 as high as 29 nM [114-116] as well as in mouse conjunctiva [77,117]. S-3319 also has a significant inhibitory role on PGE2 production in the conjunctiva of BALB/c mouse models, used for their functional sPLA2-IIA gene [77,118]. Like Varespladib, increasing dosage (0.08μM to 10μM) reduced PGE2 production by 29% [77], indicating that current sPLA2-IIA inhibition may have low potency. All three inhibitors decrease symptoms in conjunctival or corneal epithelia, suggesting their involvement with sPLA2-IIA activity and/or production. However, the current basic research on sPLA2-IIA in ocular inflammatory diseases is limited to only a few inhibitors (Table 1) and future research will need to experiment with inhibitors used in other inflammatory diseases to possibly combat issues of potency and high IC50.
Table 1. List of inhibitors of sPLA2-IIA used in inflammation studies.
Number |
Inhibitor |
IC50 |
Used in ocular inflammatory studies |
Reference |
1 |
Oleanolic Acid |
- |
Yes |
[74] |
2 |
Ochnaflavone |
3.45 μM |
No |
[85] |
3 |
LY311727 |
36 nM |
No |
[79,89] |
4 |
LY315920/LY333013 |
9 – 14 nM |
Yes |
[93,113] |
5 |
PIP-18 |
1.19 μM |
No |
[103-106] |
6 |
Celastrol |
- |
No |
[107] |
7 |
Compound 4 |
85 μM |
No |
[110] |
8 |
Compound 8 |
20 μM |
No |
[110] |
9 |
Compound 16 |
- |
No |
[110] |
10 |
Compound 21 |
71 μM |
No |
[112] |
11 |
S-3319 |
29 nM |
Yes |
[114-116] |
12 |
Fucoidan |
- |
No |
[119] |
13 |
KH064 |
29 nM |
No |
[120] |
14 |
PX-18 |
- |
No |
[121] |
15 |
Ursolic Acid |
12 – 18 μM |
No |
[122] |
sPLA2-IIA is confirmed to be higher in concentration and activity in ocular inflammatory diseases, such as DED, allergic conjunctivitis, and chronic blepharitis, which demonstrates a need for sPLA2-IIA inhibition in treatment of inflammation on the human ocular surface. However, due to the multiple mechanisms of action of sPLA2-IIA enzymes as well as the limited efficacy of inhibitors in clinical trials, the usefulness of inhibiting sPLA2-IIA has not yet been realized. Basic research in sPLA2-IIA inhibition with tissue culture and the ocular surface of animal models will continue to be effective tools leading to potential effective inhibitors and clinical studies in human diseases. Future research will need to focus on identifying novel or existing sPLA2-IIA inhibitors with higher affinity for sPLA2-IIA, as well as determining the mechanism of sPLA2-IIA in ocular inflammatory diseases. Only then will designing new inhibitors, with methods such as docking simulation, as well as specific humanized and sPLA2-IIA neutralizing antibodies for the study of inhibition be effective. Consideration must also focus on sPLA2-IIA inhibitors with high water solubility to allow for dissolution on the ocular surface with high potency. Identifying effective and selective sPLA2-IIA inhibitors will provide the key to future approaches in the treatment of ocular surface inflammation.
- Burke JE, Dennis EA (2009) Phospholipase A2 biochemistry. Cardiovasc Drugs Ther 23: 49-59. [Crossref]
- Schaloske RH, Dennis EA (2006) The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761: 1246-1259. [Crossref]
- Hiraoka M, Abe A, Shayman JA (2002) Cloning and characterization of a lysosomal phospholipase A2, 1-O-acylceramide synthase. J Biol Chem 277: 10090-10099. [Crossref]
- Hiraoka M1, Abe A, Shayman JA (2005) Structure and function of lysosomal phospholipase A2: identification of the catalytic triad and the role of cysteine residues. J Lipid Res 46: 2441-2447. [Crossref]
- Murakami M1, Taketomi Y, Miki Y, Sato H, Hirabayashi T, et al. (2011) Recent progress in phospholipase A2 research: from cells to animals to humans. Prog Lipid Res 50: 152-192. [Crossref]
- Abe A, Shayman JA (1998) Purification and characterization of 1-O-acylceramide synthase, a novel phospholipase A2 with transacylase activity. J Biol Chem 273: 8467-8474. [Crossref]
- Dennis EA1 (1994) Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 269: 13057-13060. [Crossref]
- Six DA, Dennis EA (2000) The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta 1488: 1-19. [Crossref]
- Abraham E, Naum C, Bandi V, Gervich D, Lowry SF, et al. Efficacy and safety of LY315920Na/S-5920, a selective inhibitor of 14-kDa group IIA secretory phospholipase A2, in patients with suspected sepsis and organ failure. Crit Care Med 31: 718-728. [Crossref]
- Kramer RM, Hession C, Johansen B, Hayes G, McGray P, et al. (1989) Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem 264: 5768-5775. [Crossref]
- Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, et al. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem 264: 5335-5338. [Crossref]
- Arni RK1, Ward RJ (1996) Phospholipase A2--a structural review. Toxicon 34: 827-841. [Crossref]
- Hui D, Cope M, Labonté E, Chang H-T, Shao J, et al. (2009) The phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br J Pharmacol 157: 1263-1269. [Crossref]
- Mounier CM, Wendum D, Greenspan E, Fléjou JF, Rosenberg DW, et al. (2008) Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br J Cancer 98: 587-595. [Crossref]
- Nakano T, Ohara O, Teraoka H, Arita H (1990) Group II phospholipase A2 mRNA synthesis is stimulated by two distinct mechanisms in rat vascular smooth muscle cells. FEBS Lett 261: 171-174. [Crossref]
- Crowl RM, Stoller TJ, Conroy RR, Stoner CR (1991) Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J Biol Chem 266: 2647-2651. [Crossref]
- Oka S, Arita H (1991) Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. Two distinct pathways of the gene expression. J Biol Chem 266: 9956-9960. [Crossref]
- Pfeilschifter J, Mühl H, Pignat W, Märki F, van den Bosch H (1993) Cytokine regulation of Group II phospholipase A2 expression in glomerular mesangial cells. Eur J Clin Pharmacol 44: S7-S9. [Crossref]
- Bingham CO, Murakami M, Fujishima H, Hunt JE, Austen KF, et al. (1996) A heparin-sensitive phospholipase A2 and prostaglandin endoperoxide synthase-2 are functionally linked in the delayed phase of prostaglandin D2 generation in mouse bone marrow-derived mast cells. J Biol Chem 271: 25936-25944. [Crossref]
- Couturier C, Brouillet A, Couriaud C, Koumanov K, Béréziat G, et al. (1999) Interleukin 1beta induces type II-secreted phospholipase A(2) gene in vascular smooth muscle cells by a nuclear factor kappaB and peroxisome proliferator-activated receptor-mediated process. J Biol Chem 274: 23085-23093. [Crossref]
- Kuwata H, Yamamoto S, Miyazaki Y, Shimbara S, Nakatani Y, et al. (2000) Studies on a mechanism by which cytosolic phospholipase A2 regulates the expression and function of type IIA secretory phospholipase A2. J Immunol 165: 4024-4031. [Crossref]
- Peilot H, Rosengren B, Bondjers G, Hurt-Camejo E (2000) Interferon-gamma induces secretory group IIA phospholipase A2 in human arterial smooth muscle cells. Involvement of cell differentiation, STAT-3 activation, and modulation by other cytokines. J Biol Chem 275: 22895-22904. [Crossref]
- Massaad C, Paradon M, Jacques C, Salvat C, Bereziat G, et al. (2000) Induction of secreted type IIA phospholipase A2 gene transcription by interleukin-1beta. Role of C/EBP factors. J Biol Chem 275: 22686-22694. [Crossref]
- Akiba S, Hatazawa R, Ono K, Kitatani K, Hayama M, et al. (2001) Secretory phospholipase A2 mediates cooperative prostaglandin generation by growth factor and cytokine independently of preceding cytosolic phospholipase A2 expression in rat gastric epithelial cells. J Biol Chem 276: 21854-21862. [Crossref]
- Pruzanski W, Vadas P (1991) Phospholipase A2- a mediator between proximal and distal effectors of inflammation. Immunol Today 12: 143-146. [Crossref]
- Anthonsen M, Stengel D, Hourton D, Ninio E, Johansen B (2000) Mildly oxidized LDL induces expression of Group IIa secretory phospholipase A2 in human monocyte–derived macrophages. Arterioscler Thromb Vasc Biol 20: 1276-1282.
- Ghesquiere SAI, Gijbels MJJ, Anthonsen M, van Gorp PJJ, van der Made I, et al. (2005) Macrophage-specific overexpression of group IIa sPLA2 increases atherosclerosis and enhances collagen deposition. J Lipid Res 46: 201-210. [Crossref]
- Martín R, Cordova C, Gutiérrez B, Hernández M, Nieto ML (2017) A dangerous liaison: Leptin and sPLA2-IIA join forces to induce proliferation and migration of astrocytoma cells. PLoS One 12: e0170675. [Crossref]
- Tribler L, Jensen LT, Jørgensen K, Brünner N, Gelb MH, et al. (2007) Increased Expression and Activity of Group IIA and X Secretory Phospholipase A2 in Peritumoral versus Central Colon Carcinoma Tissue. Anticancer Res 27: 3179-3185. [Crossref]
- Wang M, Hao FY, Wang JG, Xiao W (2014) Group IIa secretory phospholipase A2 (spla2IIa) and progression in patients with lung cancer. Eur Rev Med Pharmacol Sci 18: 2648-2654. [Crossref]
- Kupert E, Anderson M, Liu Y, Succop P, Levin L, et al. (2011) Plasma secretory phospholipase A2-IIa as a potential biomarker for lung cancer in patients with solitary pulmonary nodules. BMC Cancer 11: 513. [Crossref]
- Yu JA, Mauchley D, Li H, Meng X, Nemenoff RA, et al. (2012) Knockdown of secretory phospholipase A2 IIa reduces lung cancer growth in vitro and in vivo. J Thorac Cardiovasc Surg 144: 1185-1191. [Crossref]
- Oleksowicz L, Liu Y, Bracken RB, Gaitonde K, Burke B, et al. (2012) Secretory phospholipase A2-IIa is a target gene of the HER/HER2-elicited pathway and a potential plasma biomarker for poor prognosis of prostate cancer. Prostate 72: 1140-1149. [Crossref]
- Masuda S, Murakami M, Komiyama K, Ishihara M, Ishikawa Y, et al. (2005) Various secretory phospholipase A2 enzymes are expressed in rheumatoid arthritis and augment prostaglandin production in cultured synovial cells. FEBS J 272: 655-672. [Crossref]
- Kitsiouli E, Nakos G, Lekka ME (2009) Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim Biophys Acta1792: 941-953. [Crossref]
- Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J (1998) Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest 102: 633-638. [Crossref]
- Nevalainen TJ1, Graham GG, Scott KF (2008) Antibacterial actions of secreted phospholipases A2. Review. Biochim Biophys Acta 1781: 1-9. [Crossref]
- Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, et al. (2002) The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem 277: 1788-1793. [Crossref]
- Girgis DO, Dajcs JJ, O’Callaghan RJ (2003) Phospholipase A2 activity in normal and staphylococcus aureus-infected rabbit eyes. Invest Ophthalmol Vis Sci 44: 197-202. [Crossref]
- Qu XD, Lehrer RI (1998) Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun 66: 2791-2797. [Crossref]
- Koprivnjak T, Peschel A, Gelb MH, Liang NS, Weiss JP (2002) Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against staphylococcus aureus. J Biol Chem 277: 47636-47644. [Crossref]
- Bretscher MS (1972) Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol 236: 11-12. [Crossref]
- Devaux PF (1991) Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30: 1163-1173. [Crossref]
- Williamson P, Schlegel RA (1994) Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol 11: 199-216. [Crossref]
- Kay JG, Grinstein S (2011) Sensing phosphatidylserine in cellular membranes. Sensors (Basel) 11: 1744-1755. [Crossref]
- Zwaal RF1, Comfurius P, Bevers EM (2005) Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci 62: 971-988. [Crossref]
- Fijneman RJ1, Cormier RT (2008) The roles of sPLA2-IIA (Pla2g2a) in cancer of the small and large intestine. Front Biosci 13: 4144-4174. [Crossref]
- Fadeel B, Xue D (2009) The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol 44: 264-277. [Crossref]
- Boon JM, Smith BD (2002) Chemical control of phospholipid distribution across bilayer membranes. Med Res Rev 22: 251-281. [Crossref]
- Volinsky R, Kinnunen PKJ (2013) Oxidized phosphatidylcholines in membrane-level cellular signaling: from biophysics to physiology and molecular pathology. FEBS J 280: 2806-2816. [Crossref]
- Murakami M, Kambe T, Shimbara S, Yamamoto S, Kuwata H, et al. (1999) Functional association of type IIA secretory phospholipase A(2) with the glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan in the cyclooxygenase-2-mediated delayed prostanoid-biosynthetic pathway. J Biol Chem 274: 29927-29936. [Crossref]
- Murakami M, Koduri RS, Enomoto A, Shimbara S, Seki M, et al. (2001) Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J Biol Chem 276: 10083-10096. [Crossref]
- Kuwata H, Nakatani Y, Murakami M, Kudo I (1998) Cytosolic phospholipase A2 is required for cytokine-induced expression of type IIA secretory phospholipase A2 that mediates optimal cyclooxygenase-2-dependent delayed prostaglandin E2 generation in rat 3Y1 fibroblasts. J Biol Chem 273:1733-1740. [Crossref]
- Koduri RS, Baker SF, Snitko Y, Han SK, Cho W, et al. (1998) Action of human group IIa secreted phospholipase A2 on cell membranes. Vesicle but not heparinoid binding determines rate of fatty acid release by exogenously added enzyme. J Biol Chem 273: 32142-32153. [Crossref]
- Ricciotti E, FitzGerald GA (2011) Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol 31: 986-1000. [Crossref]
- Bidgood MJ, Jamal OS, Cunningham AM, Brooks PM, Scott KF (2000) Type IIA secretory phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J Immunol 165: 2790-2797. [Crossref]
- Masuda S, Murakami M, Matsumoto S, Eguchi N, Urade Y, et al. (2004) Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim Biophys Acta 1686: 61-76. [Crossref]
- Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111: 6130-6185. [Crossref]
- Simmons DL, Botting RM, Hla T (2004) Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387-437. [Crossref]
- Rådmark O, Werz O, Steinhilber D, Samuelsson B (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta 1851: 331-339. [Crossref]
- Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871-1875. [Crossref]
- Tsuboi K, Sugimoto Y, Ichikawa A (2002) Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat 68-69: 535-556. [Crossref]
- Bingham CO 3rd, Austen KF (1999) Phospholipase A2 enzymes in eicosanoid generation. Proc Assoc Am Physicians 111: 516-524. [Crossref]
- Devillier P, Baccard N, Advenier C (1999) Leukotrienes, leukotriene receptor antagonists and leukotriene synthesis inhibitors in asthma: an update. Part I: synthesis, receptors and role of leukotrienes in asthma. Pharmacol Res 40: 3-13. [Crossref]
- Gomes RN, Felipe da Costa S, Colquhoun A (2018) Eicosanoids and cancer. Clinics (Sao Paulo) 73: e530s. [Crossref]
- Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM (2000) Platelet-Activating Factor and Related Lipid Mediators. Annu Rev Biochem 69: 419-445. [Crossref]
- Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, et al. (1998) The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem 273: 14411-14423. [Crossref]
- Song CH, Choi JS, Kim DK, Kim JC (1999) Enhanced secretory group II PLA2 activity in the tears of chronic blepharitis patients. Invest Ophthalmol Vis Sci 40: 2744-2748. [Crossref]
- Eberhardt M, Rammohan G (2017) Blepharitis. StatPearls [Internet]: StatPearls Publishing, Treasure Island (FL), USA. [Crossref]
- Glasson MJ, Stapleton F, Willcox MDP (2002) Lipid, lipase and lipocalin differences between tolerant and intolerant contact lens wearers. Curr Eye Res 25: 227-235. [Crossref]
- Mochizuki H, Yamada M, Hatou S, Kawashima M, Hata S (2008) Deposition of lipid, protein, and secretory phospholipase A2 on hydrophilic contact lenses. Eye Contact Lens 34: 46-49. [Crossref]
- Patel DS, Arunakirinathan M, Stuart A, Angunawela R (2017) Allergic eye disease. BMJ 359: j4706. [Crossref]
- Li K, Liu X, Chen Z, Huang Q, Wu K (2010) Quantification of tear proteins and sPLA2-IIa alteration in patients with allergic conjunctivitis. Mol Vis 16: 2084-2091. [Crossref]
- Córdova C, Gutiérrez B, Martínez-García C, Martín R, Gallego-Muñoz P, et al. (2014) Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS One 9: e91282. [Crossref]
- Chen D, Wei Y, Li X, Epstein S, Wolosin JM, et al. (2009) sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res 88: 880-888. [Crossref]
- Aho VV, Nevalainen TJ, Saari MK (2002) Group IIA phospholipase A2 content of tears in patients with keratoconjunctivitis sicca. Graefes Arch Clin Exp Ophthalmol 240: 521-523. [Crossref]
- Wei Y, Epstein SP, Fukuoka S, Birmingham NP, Li XM, et al. sPLA2-IIa Amplifies Ocular Surface Inflammation in the Experimental Dry Eye (DE) BALB/c Mouse Model. Invest Ophthalmol Vis Sci 52: 4780-4788. [Crossref]
- Touqui L, Alaoui-El-Azher M (2001) Mammalian Secreted Phospholipases A2 and Their Pathophysiolo-gical Significance in Inflammatory Diseases. Curr Mol Med 1: 739-754. [Crossref]
- Vane JR, Botting RM (1998) Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med 104: 2S-8S. [Crossref]
- Ahuja M, Dhake AS, Sharma SK, Majumdar DK (2008) Topical ocular delivery of NSAIDs. AAPS J 10: 229-241. [Crossref]
- Flach AJ (2001) Corneal melts associated with topically applied nonsteroidal anti-inflammatory drugs. Trans Am Ophthalmol Soc 99: 205-212. [Crossref]
- Guidera AC, Luchs JI, Udell IJ (2001) Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology 108: 936-944. [Crossref]
- Lin JC, Rapuano CJ, Laibson PR, Eagle RC Jr, Cohen EJ (2000) Corneal melting associated with use of topical nonsteroidal anti-inflammatory drugs after ocular surgery. Arch Ophthalmol 118: 1129-1132. [Crossref]
- Reid RC (2005) Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem 12: 3011-3026. [Crossref]
- Moon TC, Hwang HS, Quan Z, Son KH, Kim C-H, et al. (2006) Ochnaflavone, naturally occurring biflavonoid, inhibits phospholipase A2 dependent phosphatidylethanolamine degradation in a CCl4-induced rat liver microsome. Biol Pharm Bull 29: 2359-2361. [Crossref]
- Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, et al. (1995) Structure-based design of the first potent and selective inhibitor of human non-pancreatic secretory phospholipase A2. Nat Struct Biol 2: 458. [Crossref]
- Pisabarro MT, Ortiz AR, Palomer A, Cabre F, Garcia L, et al. (1994) Rational modification of human synovial fluid phospholipase A2 inhibitors. J Med Chem 37: 337-341. [Crossref]
- Beaton HG, Bennion C, Connolly S, Cook AR, Gensmantel NP, et al. (1994) Discovery of new non-phospholipid inhibitors of the secretory phospholipases A2. J Med Chem 37: 557-559. [Crossref]
- Balsinde J, Shinohara H, Lefkowitz LJ, Johnson CA, Balboa MaA, et al. (1999) Group V phospholipase A2-dependent Induction of cyclooxygenase-2 in macrophages. J Biol Chem 274: 25967-25970. [Crossref]
- Springer DM1 (2001) An update on inhibitors of human 14 kDa Type II s-PLA2 in development. Curr Pharm Des 7: 181-198. [Crossref]
- Snyder DW, Bach NJ, Dillard RD, Draheim SE, Carlson DG, et al. (1999) Pharmacology of LY315920/S-5920, [[3-(aminooxoacetyl)-2-ethyl-1- (phenylmethyl)-1H-indol-4-yl]oxy] acetate, a potent and selective secretory phospholipase A2 inhibitor: A new class of anti-inflammatory drugs, SPI. J Pharmacol Exp Ther 288: 1117-1124. [Crossref]
- Smart BP, Oslund RC, Walsh LA, Gelb MH (2006) The first potent inhibitor of mammalian group X secreted phospholipase A2: elucidation of sites for enhanced binding. J Med Chem 49: 2858-2860. [Crossref]
- Rosenson RS, Hurt-Camejo E (2012) Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J 33: 2899-2909. [Crossref]
- Furue S, Kuwabara K, Mikawa K, Nishina K, Shiga M, et al. (1999) Crucial role of group IIA phospholipase A(2) in oleic acid-induced acute lung injury in rabbits. Am J Respir Crit Care Med 160: 1292-1302. [Crossref]
- Tomillero A, Moral M (2008) Gateway to Clinical Trials. Methods Find Exp Clin Pharmacol 30: 543-588. [Crossref]
- Rosenson RS, Hislop C, Elliott M, Stasiv Y, Goulder M, et al. (2010) Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J Am Coll Cardiol 56: 1079-1088. [Crossref]
- Rosenson RS, Hislop C, McConnell D, Elliott M, Stasiv Y, et al. (2009) Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet 373: 649-658. [Crossref]
- Dzavik V, Lavi S, Thorpe K, Yip PM, Plante S, et al. (2010) The sPLA2 Inhibition to decrease enzyme release after percutaneous coronary intervention (SPIDER-PCI) trial. Circulation 122: 2411-2418. [Crossref]
- Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, et al. (2005) LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit Care Med 33: 1741-1748. [Crossref]
- De Luca D, Minucci A, Piastra M, Cogo PE, Vendittelli F, et al. (2012) Ex vivo effect of varespladib on secretory phospholipase A2 alveolar activity in infants with ARDS. PLoS One 7: e47066. [Crossref]
- Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, et al. (2005) A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J Rheumatol 32: 417-423. [Crossref]
- Pruzanski W (2005) Phospholipase A2: quo vadis? J Rheumatol 32: 400-402. [Crossref]
- Thwin MM, Douni E, Arjunan P, Kollias G, Kumar PV, et al. (2009) Suppressive effect of secretory phospholipase A(2 )inhibitory peptide on interleukin-1ß-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts, and its antiarthritic activity in hTNFtg mice. Arthritis Res Ther 11: R138. [Crossref]
- Thwin MM, Satyanarayanajois SD, Nagarajarao LM, Sato K, Arjunan P, et al. (2007) Novel peptide inhibitors of human secretory phospholipase A2 with antiinflammatory activity: solution structure and molecular modeling. J Med Chem 50: 5938-5950. [Crossref]
- Thwin MM, Gopalakrishnakone P, Kini RM, Armugam A, Jeyaseelan K (2000) Recombinant antitoxic and antiinflammatory factor from the nonvenomous snake Python reticulatus: phospholipase A2 inhibition and venom neutralizing potential. Biochemistry 39: 9604-9611. [Crossref]
- Thwin MM, Ong WY, Fong CW, Sato K, Kodama K, et al. (2003) Secretory phospholipase A2 activity in the normal and kainate injected rat brain, and inhibition by a peptide derived from python serum. Exp Brain Res 150: 427-433. [Crossref]
- Joshi V, Venkatesha SH, Ramakrishnan C, Nanjaraj Urs AN, Hiremath V, et al. (2016) Celastrol modulates inflammation through inhibition of the catalytic activity of mediators of arachidonic acid pathway: Secretory phospholipase A2 group IIA, 5-lipoxygenase and cyclooxygenase-2. Pharmacol Res 113: 265-275. [Crossref]
- Kannaiyan R, Shanmugam MK, Sethi G (2011) Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303: 9-20. [Crossref]
- Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, et al. (2008) Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. J Biol Chem 283: 26107-26115. [Crossref]
- Ye L, Dickerson T, Kaur H, Takada YK, Fujita M, et al. (2013) Identification of inhibitors against interaction between pro-inflammatory sPLA2-IIA protein and integrin avß3. Bioorg Med Chem Lett 23: 340-345. [Crossref]
- Fujita M, Takada YK, Takada Y (2014) The chemokine fractalkine can activate integrins without CX3CR1 through direct binding to a ligand-binding site distinct from the classical RGD-binding site. PLoS One 9: e96372. [Crossref]
- Takada Y, Fujita M (2017) Secreted phospholipase A2 Type IIA (sPLA2-IIA) activates integrins in an allosteric manner. Adv Exp Med Biol 925: 103-115 [Crossref]
- Wei Y, Li P, Zou J, Epstein S, Gadaria-Rathod N, et al. (2013) Secretory group two a phospholipase (spla2) inhibition decreases corneal superficial punctate keratitis (SPK) in dry eye mice. Invest Ophthalmol Vis Sci 54: 4326-4330.
- Arumugam TV, Arnold N, Proctor LM, Newman M, Reid RC, et al. (2003) Comparative protection against rat intestinal reperfusion injury by a new inhibitor of sPLA(2), COX-1 and COX-2 selective inhibitors, and an LTC(4) receptor antagonist. Br J Pharmacol 140: 71-80. [Crossref]
- Hansford KA, Reid RC, Clark CI, Tyndall JDA, Whitehouse MW, et al. (2003) D-Tyrosine as a chiral precusor to potent inhibitors of human nonpancreatic secretory phospholipase A2 (IIa) with antiinflammatory activity. Chembiochem 4: 181-185. [Crossref]
- Titsworth WL, Cheng X, Ke Y, Deng L, Burckardt KA, et al. (2009) Differential expression of sPLA2 following spinal cord injury and a functional role for sPLA2-IIA in mediating oligodendrocyte death. Glia 57: 1521-1537. [Crossref]
- Wei Y, Pinhas A, Liu Y, Epstein S, Wang J, et al. (2012) Isoforms of secretory group two phospholipase A (sPLA2) in mouse ocular surface epithelia and lacrimal glands. Invest Ophthalmol Vis Sci 53: 2845-2855. [Crossref]
- Tischfield JA, Xia YR, Shih DM, Klisak I, Chen J, et al. (1996) Low-molecular-weight, calcium-dependent phospholipase a2genes are linked and map to homologous chromosome regions in mouse and human. Genomics 32: 328-333. [Crossref]
- Maruyama H, Suzuki K, Miyai S, Ohtsuki K (2008) Characterization of meFucoidan as a selective inhibitor for secretory phospholipase A2-IIA and the phosphorylation of meFucoidan-binding proteins by A-kinase in vitro. Biol Pharm Bull 31: 714-718. [Crossref]
- Levick S, Loch D, Rolfe B, Reid RC, Fairlie DP, et al. Antifibrotic activity of an inhibitor of group iia secretory phospholipase A2 in young spontaneously hypertensive rats. J Immunol 176: 7000-7007. [Crossref]
- Kupreishvili K, Stooker W, Emmens RW, Vonk ABA, Sipkens JA, et al. (20170 PX-18 protects human saphenous vein endothelial cells under arterial blood pressure. Ann Vasc Surg 42: 293-298. [Crossref]
- Nataraj A, Gowda CDR, Rajesh R, Vishwanath BS (2007) Group IIA secretory PLA2 inhibition by ursolic acid: a potent anti-inflammatory molecule. Curr Top Med Chem 7: 801-809. [Crossref]

