Introduction: Prenatal counselling for cerebral ventriculomegaly is challenging due to varied factors and prognoses. There is limited data about the natural history of affected pregnancies managed expectantly. We sought to review the prenatal course, obstetric and paediatric outcomes in cases of moderate to severe cerebral ventriculomegaly in an Irish tertiary maternity unit.
Materials and methods: Retrospective review of patients attending the Fetal Assessment Unit from 2006-2014 with lateral cerebral ventricular measurements >12 mm on ultrasound.
Results: During the nine-year period, 93 cases were identified with pregnancy outcome data available for 80 cases and 54 continuing in our institution. Vaginal delivery was achieved in 28.9% of women with 71.1% undergoing caesarean. There were 9 cases of intrauterine demise and an additional six neonatal deaths and three paediatric deaths.
An isolated neural tube defect was present in 12 babies. Of the remaining 33 babies a diagnosis was confirmed in 60.6% of which, 45% were achieved antenatally.
Conclusions: A diagnosis was obtained in 70% of live births. The presence of ventriculomegaly had a significant impact on the mode of delivery and maternal morbidity. The survival rate was 66.6% with high rate of neurodevelopmental delay recognized in survivors particularly in cases without a clear diagnosis.
caesarean, morbidity, neonatology, ultrasound, termination of pregnancy, prenatal diagnosis
Ventriculomegaly is a pathological condition where the fetal cerebral lateral ventricles are enlarged. This can occur due to several aetiologies such as obstruction of the cerebrospinal fluid flow, as a consequence of abnormal cerebral development or secondary to the destruction of brain matter [1]. Sonographic evaluation of the fetal ventricles forms an integral part of the anatomical survey. The measurement of the lateral ventricle width is obtained in the trans-ventricular plane with the callipers positioned perpendicular to the ventricular axis [2].
The incidence of isolated ventriculomegaly is reported 0.5-1.5 per 1000 pregnancies [3]. This is defined as mild (10-12 mm), moderate (12-15 mm) and severe (>15 mm) [1,4]. There is limited data about the antenatal course and obstetric outcome of pregnancies affected by moderate to severe ventriculomegaly, which are managed expectantly. Prenatal counselling for cerebral ventriculomegaly >12 mm is challenging due to the variable factors and prognoses [5-8]. It is known that the presence of additional CNS or extra CNS malformations has been associated with a poorer outcome [9]. Optimal counselling of parents requires accurate knowledge regarding the natural history, need for surgical intervention and expected long-term prognosis [10-13]. Current recommended investigations include ultrasonographic assessment, maternal TORCH screening, Magnetic resonance imaging (MRI) evaluation and specialized paediatric postnatal follow-up [14,15].
We sought to review the prenatal course, obstetric and paediatric outcomes in all cases of moderate to severe ventriculomegaly expectantly managed in an Irish tertiary referral centre. The current study aims to provide an evidence-based rationale to assist in the counselling of parents with a pregnancy complicated by moderate or severe ventriculomegaly.
The Rotunda hospital is a tertiary referral maternity unit with approximately 9000 deliveries per year. As part of routine antenatal care an anatomy ultrasound is performed between 18-22 weeks gestation. Abnormalities identified in peripheral centres are also referred to our unit for review and management by the Fetal Medicine Specialist Team. A retrospective review of our ultrasound software system (Viewpoint; MDI Viewpoint, Jacksonville, FL) was performed between 1st January 2006 and 31st December 2014. Search criterion included a measurement of the lateral cerebral ventricles (Vp) equal or greater to 12 mm.
All cases of ventriculomegaly were referred for Fetal Medicine evaluation when the lateral ventricular width was greater or equal to 10 mm and measured as described by Salomon, et al. [2]. All documented cases of moderate–severe ventriculomegaly were then confirmed by retrospective reassessment by a Fetal Medicine Consultant of images stored on the ultrasound software system (Viewpoint; MDI Viewpoint, Jacksonville, FL) and were included if the lateral cerebral ventricles (Vp) measured ≥ 12 mm. Cases arising in multiple gestations were excluded from the analysis. All singleton pregnancies with confirmed moderate to severe ventriculomegaly (>12 mm) were included to form a retrospective population-based case series. These pregnancies were managed expectantly within the fetal medicine clinic with interval ultrasound assessment.
Following identification, a comprehensive chart review was performed of all available maternal and paediatric records. Maternal data including demographics and antenatal course (details of ultrasound assessments fetal MRI, TORCH screen and results of amniocentesis) were collected. Intrapartum data collected included gestational age at delivery, mode of labour initiation and mode of delivery. Postnatal data collected included both the initial neonatal course and details on longer-term developmental surveillance. In cases of stillbirth or neonatal death histopathology and cytogenetics were reviewed where available. As this was a retrospective review there was no predefined paediatric investigative pathway and this was individualised at the discretion of the managing physician.
This study is largely descriptive in nature. Where applicable statistical analysis was performed via SPSS v22. Categorical data was reported as % (n). Two group comparisons were conducted using the independent t-test and a p value of <0.05 was deemed significant.
In the nine-year period a total of 78776 women delivered at the Rotunda Hospital. In that interval, there were 93 prenatally identified cases of moderate- severe ventriculomegaly attending the Rotunda Fetal Assessment Unit, with outcome data available for 80 cases. The incidence of moderate–severe ventriculomegaly within our unit was 1.18/1000 births. There was a total of 3 cases occurring in a multiple gestation pregnancy and were excluded from the analysis. Regarding pregnancy outcome 11.1% (n=10) were lost to follow-up and outcome data was available in the remaining 80 cases detailed further in Figure 1. Overall, 60.0% (n=54) of identified cases were managed expectantly. Within this cohort 3.7% (n=2) suffered a miscarriage and the incidence of intrauterine death was 13.0% (n=7). The live birth rate was 83.3% (n=45) in expectantly managed pregnancies. There were six (13.3%) neonatal deaths and three (6.7%) paediatric deaths. The overall survival rate of cases managed expectantly was 66.7% (n=36).
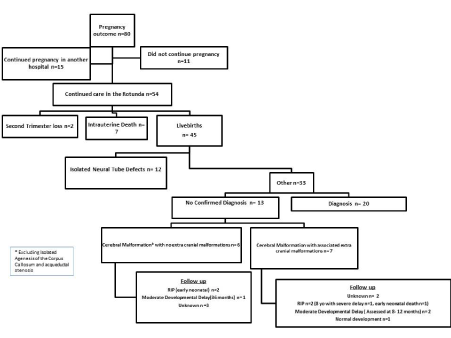
Figure 1. Pregnancy outcome within the study population
Maternal demographics are detailed in Table 1. A diagnosis of moderate ventriculomegaly was apparent in 28.9% (n=13) live births and in 53.8% (n=7) this was an isolated finding. The remaining 71.1% (n=32) had a diagnosis of severe ventriculomegaly of whom only 25% had isolated ventriculomegaly. The mean gestational age at time of diagnosis of moderate–severe ventriculomegaly was 25.5 ± 5.9 weeks with a mean Vp of 16.2 ± 5.7 mm at time of diagnosis. Reasons for diagnosis greater than 23 weeks’ gestation included late first antenatal visit, external referral and progression of a diagnosis of borderline/mild ventriculomegaly at the anatomy ultrasound. Antenatal progression of moderate- severe ventriculomegaly was defined in this cohort as an increase of the Vp by greater or equal to 4 mm. The rate of antenatal progression was 55.6% (n=25) with no cases of antenatal progression in moderate ventriculomegaly. Of cases with severe ventriculomegaly, 78% (n=25) progressed antenatally. The maximal Vp measurement was recorded at a mean gestational age of 32.8 ± 5.4 weeks. The mean of the maximum Vp measurement recorded was 23.6 ± 12.5 mm. The mean degree of antenatal progression was 7.4 ± 10.8 mm (range-3 mm to 43 mm). The maximum head circumference measurement was found at a mean of 36.3 ± 2.8 weeks gestation and a mean measurement of 336.2 ± 43.9 mm. A total of 33.3% of cases had a head circumference >95th centile. A diagnosis of severe ventriculomegaly combined with an additional anomaly conveyed a mortality rate of 25% (n=6).
Table 1. Maternal demographics
Maternal/Neonatal characteristic |
N (%) / mean ± SD |
Age, years |
28.3 ± 6.2 |
Ethnicity (white European) |
39 (86.7%) |
Nulliparous |
14 (31.1%) |
Spontaneous conception |
42 (93.3%) |
Smoking |
9 (20%) |
Severe ventriculomegaly |
32 (71.1%) |
Bilateral |
35 (77.8%) |
Additional CNS abnormality |
22 (48.9%) |
Extra CNS abnormality |
14 (31.1%) |
Isolated ventriculomegaly |
15 (33.3%) |
HC >95th centile |
15 (33.3%) |
CNS: Central nervous system; HC: Head circumference
A diagnosis of ventriculomegaly conferred an increased risk of caesarean section delivery with only 28.9% (n=13) of women achieving a vaginal delivery. Table 2 further details delivery details and peripartum neonatal findings. The Elective caesarean section delivery rate was 48.9% (n=22) and was similar among both a diagnosis of moderate or severe ventriculomegaly 53.8% (n=7) or 46.9% (n=15) respectively. There were 14 cases of severe ventriculomegaly associated with macrocephaly of which 71.4% (n=10) underwent an elective caesarean section.
Table 2. Delivery details
Characteristic |
N (%) / mean ± SD |
Trial of Labour |
23 (51.1%) |
Successful Trial of Labour |
13 (28.9%) |
Overall LSCS rate |
32 (71.1%)* |
Elective LSCS rate |
22 (48.9%)* |
GA at delivery, weeks |
37.1 ± 5.4 |
Significant resuscitation |
3 (6.7%) |
Apgar’s <7 at 5 minutes |
5 (11.1%) |
Birthweight, grams |
2840 ± 1118 |
Birth OFC, cm |
36.0 ± 5.0 |
Normal neuro exam in first 1/52 |
15 (33.3%) |
NICU admission |
23 (51.1%) |
Average Length of Stay, days |
11 ± 15.2 |
LSCS: lower segment cesarean section; HC: Head circumference; GA: gestational age; OFC: Occipitofrontal circumference; NICU: Neonatal Intensive Care. *p<0.001 in comparison to hospital CS rate in study period
Highest diagnostic yield was from postnatal genetic studies 15.6% (n=5) and MR imaging 18.8% (n=6). MRI was not performed for all infants during this cohort as the MRI service became available to our unit in 2011. Antenatal MRI was performed in 26.7% (n=12) and in a further 17.8% (n=18) postpartum. There were two cases diagnosed since MRI facilities became available where imaging was deferred; one case had a diagnosis of neural tube defect and the second case was a moderate ventriculomegaly with a normal postnatal cranial ultrasound. Of the 64.4% (n=29) who underwent TORCH bloods there was only one equivocal result for toxoplasmosis with the remainder being negative. Uptake of amniocentesis was high at 64.4% (n=29). However, despite all seven cases where a genetic diagnosis was achieved having undergone invasive testing only 28.6% (n=2) of these had an abnormal antenatal result. Of these antenatal diagnoses the first case was an antenatal diagnosis of Goldenhar, which continued to term. The second case was an infant with a Chromosome 13 deletion with significant associated abnormalities and intrauterine growth restriction who was delivered at term, managed palliatively and died within the first few hours of life.
Postnatal diagnoses are detailed in Figure 2. Despite extensive investigation a clear diagnosis was not found in 39.4% (n=13) of live born babies (excluding isolated neural tube defect). Outcome in this group appeared worse in the presence of associated extracranial malformations (Figure 1). Likewise, outcome was very poor in babies with a known genetic, chromosomal or metabolic syndrome (Figure 2).
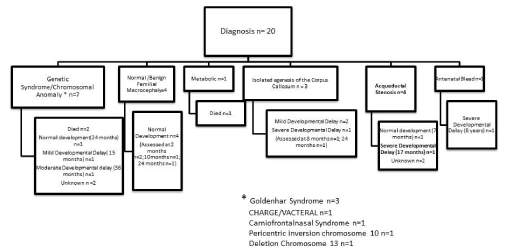
Figure 2. Diagnosis excluding isolated neural tube defects
The incidence of moderate- severe ventriculomegaly was 1.18/1000 births and is similar to that experienced internationally [16]. In Ireland the majority of women with a ventriculomegaly affected pregnancy opt to continue with their pregnancy, as prior to 2019 the Irish Constitution did not permit termination except in circumstances where there was an imminent threat to the life of the woman [17]. As a result, the majority of cases with ventriculomegaly >12mm were managed expectantly with a survival rate of 66.7%. However, the presence of ventriculomegaly had a significant impact on maternal morbidity, as 71.1% of women required a caesarean section delivery (p<0.001). A trial of labour was attempted in 23 women and it was successful in 56.5% (n=13).
The range of diagnoses and neurological outcome was diverse with a poorer neurological outcome demonstrated when there was no clear diagnosis and additional extracranial anomalies were present. Genetic studies had the highest diagnostic yield especially postpartum. There was poor diagnostic yield from TORCH screening which is similar to that found in previous studies and again brings into question its role as a routine investigation for ventriculomegaly [18].
All cases in this cohort with a confirmed chromosomal abnormality had undergone invasive prenatal testing but despite this only 28.6% of these cases achieved an antenatal diagnosis. The improved diagnostic yield postnatally can be attributed to the use of microarray and molecular studies and as a result presents further evidence in favour of performing a microarray panel as part of routine antenatal testing in cases of ventriculomegaly.
Where a diagnosis was obtained, ventriculomegaly was associated with a genetic abnormality in almost 40%. The most common demonstrated genetic disorder within this cohort was Goldenhar (Oculo-Auriculo-Vertebral) Spectrum. There have been a variety of genetic abnormalities linked to this syndrome including trisomy 7 mosaic [19], trisomy 9 mosaic [20], del(18q), del(22q) [21], dup(22q) [22], trisomy 22 mosaic[23] and unbalanced translocation(5p:8p) [24]. An antenatal diagnosis was possible in one case of Goldenhar and similar to other studies there was a variable neurological outcome with this diagnosis [25].
A paper by Kallen, et al. [26] suggested that the similarities between Goldenhar syndrome and CHARGE can be attributed to disturbed neural crest development but its genetic component is primarily caused by a mutation in the CHD7 gene [27]. In this analysis, comparison was not possible, as the infant affected by CHARGE/VACTERYL in this population did not survive the neonatal period. Similar to Goldenhar it also has a variable neurological outcome with up to 70% reported to have developmental delay, which in part may be secondary to sensory deficits [28].
The other antenatally detected genetic abnormality was a chromosome 13 deletion and the infant died on day 1 of life. Again, this is similar to findings by Walsh, et al. where the infant in their review did not survive infancy. In their review they also detailed ventriculomegaly to be a prevalent feature of the Chromosome 13 deletion phenotype [29]. Craniofrontonasal syndrome is an X-linked condition again characterized by diverse phenotypes and in 87% is a result of mutations in the EFNB1 gene [30].
Despite efforts to document follow up on all babies a large proportion 27.3% (n=9) were lost to follow up. Follow up data is also limited due to the retrospective nature of the study, with information being retrieved only from chart review. A significant strength of this study is the ability to characterize the diverse spread of diagnoses and neurological outcome in this cohort given constitutional limitations to termination in this jurisdiction and the resultant high proportion of cases following expectant management.
In conclusion, this study suggests that when ventriculomegaly is detected in the antenatal setting a microarray panel may be useful as part of the routine investigations. Our experience further outlines the range of differential diagnoses that must be considered and the overall poor prognosis in this cohort.
This study was approved by the local Rotunda Hospital Research Ethics Committee reference RAG- 2015-015.
- Gaglioti P, Oberto M, Todros T (2009) The significance of fetal ventriculomegaly: etiology, short- and long-term outcomes. Prenat Diagn 29: 381-388. [Crossref]
- Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, et al. (2011) Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 37: 116-126. [Crossref]
- Sethna F, Tennant PWG, Rankin J, Robson SC (2011) Prevalence, natural history, and clinical outcome of mild to moderate ventriculomegaly. Obstet Gynecol 117: 867-876. [Crossref]
- Signorelli M, Tiberti A, Valseriati D, Molin E, Cerri V, et al. (2004) Width of the fetal lateral ventricular atrium between 10 and 12 mm: a simple variation of the norm? Ultrasound Obstet Gynecol 23: 14-18. [Crossref]
- Nicolaides KH, Berry S, Snijders RJ, Thorpe-Beeston JG, Gosden C (1990) Fetal lateral cerebral ventriculomegaly: associated malformations and chromosomal defects. Fetal Diagn Ther 5: 5-14. [Crossref]
- Wilhelm C, Keck C, Hess S, Korinthenberg R, Breckwoldt M (1998) Ventriculomegaly diagnosed by prenatal ultrasound and mental development of the children. Fetal Diagn Ther 13: 162-166. [Crossref]
- Lee CS, Hong SH, Wang K-C, Kim S-K, Park JS, et al. (2006) Fetal ventriculomegaly: prognosis in cases in which prenatal neurosurgical consultation was sought. J Neurosurg 105: 265-270. [Crossref]
- Rankin J, Robson S, Wariyar U (1998) Outcome of prenatally detected mild/moderate cerebral ventriculomegaly. Eur J Pediatr Surg 8: 72. [Crossref]
- Gaglioti P, Danelon D, Bontempo S, Mombrò M, Cardaropoli S, et al. (2005) Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol 25: 372-377. [Crossref]
- Beeghly M, Ware J, Soul J, Plessis AD, Khwaja O, et al. (2010) Neurodevelopmental outcome of fetuses referred for ventriculomegaly. Ultrasound Obstet Gynecol 35: 405-416. [Crossref]
- Gaglioti P, Oberto M, Todros T (2009) The significance of fetal ventriculomegaly: etiology, short- and long-term outcomes. Prenat Diagn 29: 381-388. [Crossref]
- den Hollander NS, Vinkesteijn A, Schmitz-van Splunder P, Catsman-Berrevoets CE, Wladimiroff JW (1998) Prenatally diagnosed fetal ventriculomegaly; Prognosis and outcome. Prenat Diagn 18: 557-566. [Crossref]
- Breeze ACG, Alexander PMA, Murdoch EM, Missfelder-Lobos HH, Hackett GA, et al. (2007) Obstetric and neonatal outcomes in severe fetal ventriculomegaly. Prenat Diagn 27: 124-129. [Crossref]
- Irwin K, Henry A, Gopikrishna S, Taylor J, Welsh AW (2016) Utility of fetal MRI for workup of fetal central nervous system anomalies in an Australian maternal-fetal medicine cohort. Aust N Z J Obstet Gynaecol 56: 267-273. [Crossref]
- Emery SP, Hogge WA, Hill LM (2015) Accuracy of prenatal diagnosis of isolated aqueductal stenosis. Prenat Diagn 35: 319-324. [Crossref]
- Leitner Y, Stolar O, Rotstein M, Toledano H, Harel S, et al. (2009) The neurocognitive outcome of mild isolated fetal ventriculomegaly verified by prenatal magnetic resonance imaging. Am J Obstet Gynecol 201: E1-E6. [Crossref]
- https://www.oireachtas.ie/en/bills/bill/2013/66/
- Pasquini L, Masini G, Gaini C, Franchi C, Trotta M, et al. (2014) The utility of infection screening in isolated mild ventriculomegaly: an observational retrospective study on 141 fetuses. Prenat Diagn 34: 1295-1300. [Crossref]
- Hodes ME, Gleiser S, DeRosa GP, Yune HY, Girod DA, et al. (1981) Trisomy 7 mosaicism and manifestations of goldenhar syndrome with unilateral radial hypoplasia. J Craniofac Genet Dev Biol 1: 49-55. [Crossref]
- Wilson GN, Barr Jr M (1983) Trisomy 9 mosaicism: another etiology for the manifestations of Goldenhar syndrome. J Craniofac Genet Dev Biol 3: 313-316. [Crossref]
- Herman GE, Greenberg F, Ledbetter DH (1988) Multiple congenital anomaly/mental retardation (MCA/MR) syndrome with Goldenhar complex due to a terminal del(22q). Am J Med Genet 29: 909-915. [Crossref]
- Hathout EH, Elmendorf E, Bartley J (1998) Hemifacial microsomia and abnormal chromosome 22. Am J Med Genet 76: 71-73. [Crossref]
- Pridjian G, Gill WL, Shapira E (1995) Goldenhar sequence and mosaic trisomy 22. Am J Med Genet 59: 411-413. [Crossref]
- Josifova DJ, Patton MA, Marks K (2004) Oculoauriculovertebral spectrum phenotype caused by an unbalanced t(5;8)(p15.31;p23.1) rearrangement. Clin Dysmorphol 13: 151-153. [Crossref]
- Castori M, Brancati F, Rinaldi R, Adami L, Mingarelli R, et al. (2006) Antenatal presentation of the oculo-auriculo-vertebral spectrum (OAVS). Am J Med Genet A 140: 1573-1579. [Crossref]
- Källén K, Robert E, Castilla EE, Mastroiacovo P, Källén B (2004) Relation between oculo-auriculo-vertebral (OAV) dysplasia and three other non-random associations of malformations (VATER, CHARGE, and OEIS). Am J Med Genet A 127: 26-34. [Crossref]
- van Ravenswaaij-Arts CMA, Blake K, Hoefsloot L, Verloes A (2015) Clinical utility gene card for: CHARGE syndrome - update 2015. Eur J Hum Genet 23: ejhg.2015.15. [Crossref]
- Hartshorne N, Hudson A, MacCuspie J, Kennert B, Nacarato T, et al. (2016) Quality of life in adolescents and adults with CHARGE syndrome. Am J Med Genet A 170: 2012-2021. [Crossref]
- Walsh LE, Vance GH, Weaver DD (2001) Distal 13q Deletion Syndrome and the VACTERL association: case report, literature review, and possible implications. Am J Med Genet 98: 137-144. [Crossref]
- Twigg SRF, Kan R, Babbs C, Bochukova EG, Robertson SP, et al. (2004) Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci U S A 101: 8652-8657. [Crossref]
Editorial Information
Editor-in-Chief
Alessandro Buda
University of Milano Bicocca
Italy
Article Type
Research Article
Publication history
Received: January 16, 2021
Accepted: February 02, 2021
Published: February 05, 2021
Copyright
©2021 Monteith C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Monteith C (2021) The natural history of moderate-severe ventriculomegaly: A review of cases managed expectantly in an Irish tertiary Centre. Obstet Gnecol Rep 5(1): DOI: 10.15761/OGR.1000154.
Corresponding author
Cathy Monteith
Department of Obstetrics & Gynaecology, Royal College of Surgeons in Ireland, Rotunda Hospital, Ireland.
E-mail : bhuvaneswari.bibleraaj@uhsm.nhs.uk


