Introduction
The special review article “Indications for and adverse effects of red cell transfusion” by J.L. Carson, D.J. Triulzi, and P.M. Ness in NEJM 377:1261-1272, 2017 reports the need to develop non-invasive methods to assess tissue oxygenation and to better identify the indications for red blood cell transfusion [1]. In 1969, the Naval Blood Research Laboratory (NBRL) at the Chelsea Naval Hospital, Chelsea, MA reported the increase in red blood cell 2,3 diphosphoglycerate (DPG) and red blood cell creatine levels in patients with red cell mass deficits or cardiopulmonary insufficiency [2]. The data indicated that the red blood cell 2,3 DPG and creatine levels assessed the state of tissue oxygenation and suggested that these two measurements could be use to evaluate the treatment of patients with red cell mass deficits and patients with cardiopulmonary insufficiency.
We studied 161 male patients, whose ages ranged from 18 to 65, with a mean of 28 years [2]. These patients suffered from a variety of diseases: traumatic injuries (86); cardiopulmonary diseases (16); gastrointestinal bleeding (5); carcinoma (37); and a miscellaneous group that included uremia, hepatitis, cirrhosis, epilepsy, cerebrovascular disease, toxic myositis, and lymphproliferative disorders (17).
The red cell creatine levels were significantly correlated to the red cell mass deficits (r=0.52, p<0.001) in 124 patients with various diagnoses. The correlation between red cell 2,3 DPG level and the red cell mass deficit was also significant (r=0.55, p<0.001) in 123 patients. Likewise, there was a significant correlation between red cell creatine and 2,3 diphosphoglycerate concentration in 161 patients (r=0.60, p<0.001).
Whole blood lactic acid concentrations varied from 0.5 to 3.0 µmoles per mL in 123 patients with varying degrees of red cell mass deficit. However, the correlation was not significant (r=0.12, p>0.05) in 123 patients. The highest lactic acid concentrations were observed in the 28 patients with carcinoma.
Since 2,3 DPG has been shown to facilitate the release of oxygen from hemoglobin, we interpret our data to suggest that the increased 2,3 DPG concentration in the erythrocyte is a compensatory mechanism for delivering more oxygen to tissue. Although the mechanisms for the observed increases in red cell creatine and DPG are not known, the clinical importance of these red cell compounds deserves exploration because of their possible application both as diagnostic aids and for measuring the effectiveness of therapy. We have already shown that the measurement of red cell levels of 2,3 DPG in patients without cardiopulmonary disease is useful in assessing the indications for and effectiveness of red blood cell transfusions. Red cell 2,3 DPG measurements in patients with cardiopulmonary disease may help in the evaluation of the treatment of patients suffering from chronic pulmonary disease and congestive heart failure.
The measurement of red cell 2,3 DPG is affected by changes of acid-base balance, because acidosis decreases the red cell 2,3 DPG level whereas alkalosis increases this pool. Since the measurement of 2,3 DPG is technically difficult and because of the excellent correlation between red cell levels of 2,3 DPG and creatine, the red cell creatine measurement represents a simpler clinical assay for evaluating tissue hypoxia as a manifestation of both cardiopulmonary insufficiency and decreased circulating red cell mass.
Dr. Ernest Beutler in a commentary on the measurement of red cell creatine suggested that red cell creatine is metabolic garbage and has no known function [3,4]. In our study, patients with a decrease in red cell mass and with cardiopulmonary insufficiency produced red cells with increased 2,3 DPG and creatine levels. The release of muscle creatine occurred from the hypoperfused hypoxic muscles in these patients. Two red cell indicators of tissue hypoxia: increases in RBC 2,3 DPG and RBC creatine levels were observed in patients hospitalized at the Chelsea Naval Hospital [2]. Studies performed at the NBRL were unable to demonstrate that creatine could enter the RBC under the various in vitro conditions used in our investigations. However, in vivo studies showed that the increased RBC creatine levels observed in patients with chronic hypovolemic anemia of trauma following RBC transfusions to increase the peripheral red blood cell volume to normal were associated with a decrease in the red blood cell creatine levels. In vivo studies suggested that red blood cell creatine level in these patients with chronic hypovolemic anemia of trauma correlated to the hypoxia in their muscles. The decrease in the peripheral red blood cell volume reduced the perfusion of the muscle of the extremities and increased the RBC DPG and creatine levels. Restoration of the peripheral blood volume by the red blood cell transfusions restored perfusion to the hypoxic muscles and the reduction in red blood cell DPG and creatine levels occurred (Figureure 1 and 2).
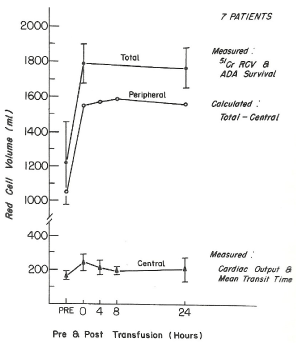
Figure 1. The total, central, and peripheral red blood cell volumes measured prior to and following transfusion of three to five units of preserved red blood cells with low 2,3 DPG and with increased affinity for oxygen in seven patients. The central red blood cell volume was measured from the cardiac output, the pulmonary circulation mean transit time, and the hematocrit of the blood circulating through the lungs. Before transfusion, the red blood cell volume was measured using 51Cr-labeled autologous red blood cells, and after transfusion it was estimated from the red blood cell volume measured before transfusion and the increase in red blood cell volume due to the circulating donor red blood cells calculated from the volume and survival percentage of the transfused donor red blood cells. The automated differential agglutination procedure was used to measure the donor red blood cell survival. The peripheral red blood cell volume was calculated from the total red blood cell volume minus the central red blood cell volume [6].
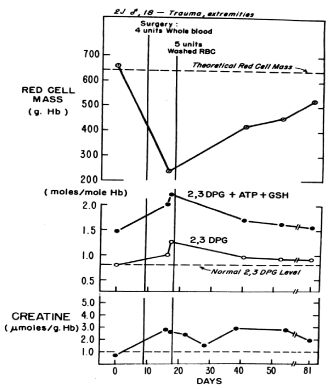
Figure 2. Red blood cell mass (g Hb), red blood cell 2,3 DPG and creatine levels, and the sum of the red blood cell 2,3 DPG, ATP, and glutathione (GSH) levels in an 18-year-old white man with traumatic injuries (2J). Measurements were made prior to operation and again following the surgical procedure. The patient received four units of whole blood intraoperatively and five units of washed red blood cells during the postoperative period. The patient had a normal red blood cell mass, and normal red blood cell levels of 2,3 DPG, creatine, ATP, and GSH prior to operation. Although four units of whole blood were transfused intraoperatively, 7 days postoperatively the red blood cell mass was decreased to 35% of normal and the levels of red blood cell 2, 3 DPG, creatine, and the sum of the 2,3 DPG, ATP, and GSH levels were increased significantly. With restoration of the red blood cell mass towards normal, the red blood cell levels of 2,3 DPG and the sum of 2,3 DPG, ATP, and GSH returned towards normal [6].
The book The Hypovolemic Anemia of Trauma: The Missing Blood Syndrome published by CRC Press, Boca Raton, FL in 1981 was written to report on our clinical experience during the years 1968 to 1974 when over 300 patients who sustained war injuries in South Vietnam were transferred to Chelsea Naval Hospital, Chelsea, MA. The patients were called to the attention of our research facility when the use of general anesthesia for routine debridement of wounds precipitated a life threatening state of hypotension. A series of tests established the presence of chronic hypovolemia [5,6].
When red blood cells were administered to the patients with this clinical disorder before their surgical procedure, hypotension during operations and post-operatively was eliminated. Determinations of blood volumes were made using 51 Cr labeled autologous red blood cells to measure red blood cell volume and 125I iodinated cold agglutinin to measure the plasma volume. Cardiac output and the distribution of blood flow was measured in these patients to understand how the vital organs such as the brain, heart, and kidneys were protected in spite of 30-40% reduction in total blood volume.
To gain some understanding of the mechanism of the reduce blood volume, we studied the patient’s red blood cell production as well as the destruction of the patient’s own red blood cells and the donor red blood cells. Red blood cell production was impaired and both the patient and the donor RBC were being destroyed through an alternative pathway producing porphyrin-like substances instead of the usual urobilinogen breakdown product of the hemoglobin with the release of carbon monoxide. The degradation of red blood cell hemoglobin through the alternative pathway was thought to be responsible for the so-called missing blood syndrome [6].
The experience of Stinner and associates reported in Military Medicine, 175:1027-1029, 2010 is very different from our experience treating patients at the Chelsea Naval Hospital during the Vietnam War. They report that of 348 service members who sustained combat injuries in Afghanistan and Iraq, fifty-three (15.2%) required amputations 12 weeks to 5.5 years following their initial extremity injuries [7].
In our patients at the Chelsea Naval Hospital although these orthopedic patients had chronic hypovolemic anemia of trauma with reduction in both the RBC and plasma volume, normal hemoglobin and hematocrit values, and normal central blood volumes, they suffered from severely contracted peripheral red blood cell volumes to their extremities (Figure 1). The aggressive transfusion of washed liquid preserved RBC and washed previously frozen deglycerolized RBC restored their peripheral red blood cell volume and total blood volume. We studied 300 servicemen for at least 2 years during their hospitalization at Chelsea Naval Hospital. Chronic hypovolemic patients with traumatic injuries to their extremities were transfused RBC in order to restore their peripheral red blood cell volume and to repair the injured extremities (Figure 3-9).
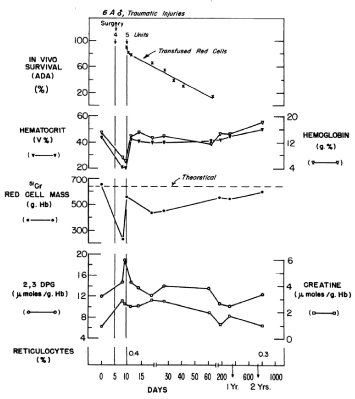
Figure 3. Hematocrit and hemoglobin concentrations, red blood cell mass, and red blood cell 2,3 DPG and creatine levels, and reticulocyte counts in Patient 6A, a 20-year old male with traumatic injuries who was treated and evaluated over a 2-year period. The red blood cell survival of five units of liquid-stored washed red blood cells was measured by the automated differential agglutination procedure [6].
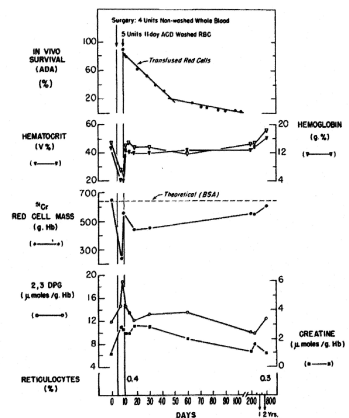
Figure 4. Serial measurements of red-cell mass (grams of hemoglobin), peripheral venous hematocrit and hemoglobin values, red-cell 2,3 diphosphoglycerate (DPG) and creatine levels, and reticulocyte counts in patient S.S. Time when blood was transfused, and survival of transfused red cells are shown. The theoretical red-cell mass in grams of hemoglobin was calculated from the body surface area (BSA). The in vivo survival was determined by the automated differential agglutination (ADA) method [5].
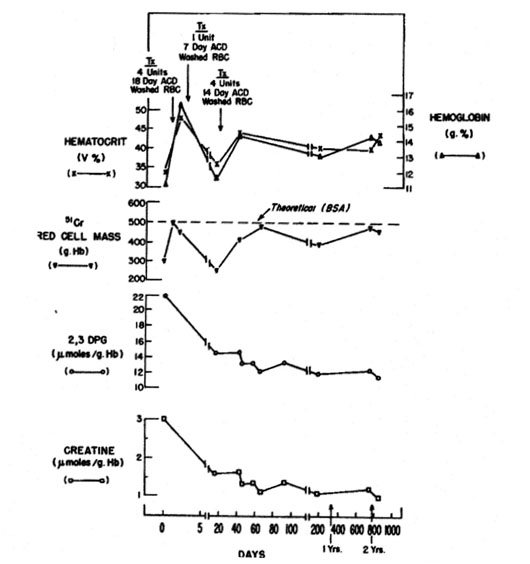
Figure 5. Serial hematologic and biochemical measurements in patient S.D. The time when blood was transfused is shown [5].
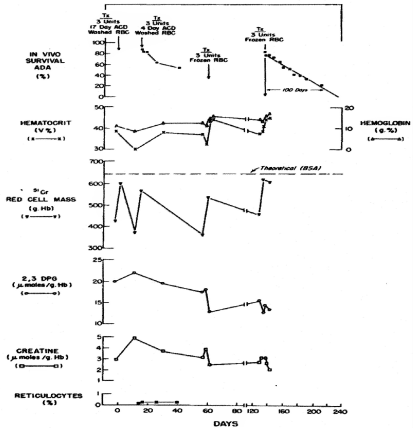
Figure 6. Serial hematologic and biochemical measurements in patient J.B. Time when blood was transfused and survival of transfused red cells are shown [5].
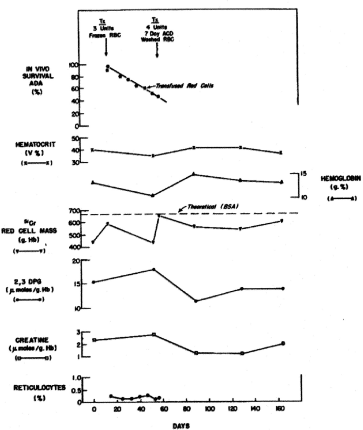
Figure 7. Serial hematologic and biochemical measurements in patient E.S. Time when blood was transfused and survival of the transfused red cells are shown [5].

Figure 8. Hematocrit and hemoglobin concentrations, red blood cell mass, and red blood cell 2,3 DPG and creatine levels in Patient 6B, a 21-year old male with traumatic injuries who was treated and evaluated for a 10-month period [6].
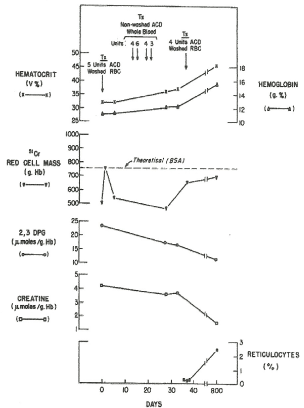
Figure 9. Serial hematologic and biochemical measurements in patient J.D. The time when blood was transfused is shown [5].
The recent paper by Stinner DJ and associates in Military Medicine reported the incidence of late amputation of 15% occurred 12 weeks to 5.5 years following combat related injuries sustained by U.S. military personnel in Afghanistan and Iraq [7]. During the Vietnam War from 1968 to 1974 our laboratory studied and treated over 300 wounded servicemen who returned to the Chelsea Naval Hospital from South Vietnam with severe traumatic injuries to their extremities who were treated by the orthopedic service (Figure 3-9). These patients with traumatic injuries to their extremities had chronic hypovolemic anemia of trauma with reduction in total blood volume of 30 to 40%, normal hemoglobin and hematocrit values, normal central blood volume but severely reduced peripheral blood volume to their extremities (Figure 1). Aggressive transfusion of washed liquid preserved red blood cells and previously frozen deglycerolized red blood cells were administered to restore the peripheral blood volume to their extremities. The repeated red blood cell transfusion treatment to restore to normal the peripheral blood volume to their extremities was associated with repair of the wounded extremities with the rare need for an amputation in the 300 servicemen who were studied for one to two years during their hospitalization at the Chelsea Naval Hospital. Chronic hypovolemic patients with traumatic injuries to their extremities need to be transfused red blood cells to restore the peripheral blood volume to repair the injured extremities of these patients. Our studies demonstrate the limitation of the hematocrit and hemoglobin concentration measurements; the maintenance of the central blood volume and the 30-40% reduction in the peripheral blood volume in patients with chronic hypovolemia with traumatic injuries to their extremities. The repeated transfusions of viable and identifiable compatible red blood cells restored the peripheral red blood cell volume and permitted the healing of their injured extremities without the need for amputations. In these hypovolemic anemic patients both the RBC 2,3 DPG and RBC creatine levels were increased due to the muscle hypoxia associated with the reduction in the red blood cell volume. Red blood cell transfusions restored the RBC volume and reduced the increased levels of RBC 2,3 DPG and creatine in these patients with traumatic injuries (Figure 3-9).
The aggressive transfusion of patients with traumatic injuries and chronic hypovolemia who had multiple fractures of the upper and lower extremities restored the blood volume to these areas. In the study of 300 wounded servicemen from Vietnam who were transferred to Chelsea Naval Hospital from 1968 to 1974, amputation of their extremities rarely occurred. The transfusion of these patients with washed liquid preserved red blood cells and washed previously frozen deglycerolized RBC increased the red blood cell volume and the plasma volume towards their normal values. The multiple fractures of the upper and lower extremities healed without the need to amputate these extremities because of poor perfusion.
Studies in wounded servicemen that returned from Vietnam between 1968 to 1974 provided data to document the maintenance of the central blood volume with the reduction in the peripheral blood volume in hypovolemic anemic patients who were subjected to traumatic wounds. Transfusion of compatible but identifiable allogeneic red blood cells increased the peripheral blood volume to the gastrointestinal tract and muscle, bone and skin of the extremities. The increase in peripheral blood volume occurred following the transfusion of compatible and identifiable red blood cells. The studies performed in the chronic hypovolemic anemic patients demonstrated that transfusion of washed RBC not only increased the peripheral red blood cell volume but also increased the plasma volume. The red blood cell volume, the plasma volume and total blood volume in these chronic hypovolemic anemic patients were reduced. The normal hematocrit and hemoglobin concentration could not be used to assess the hypovolemic state in these patients with “stress anemia” [5]. The red blood cell volume was measured with 51Cr labeled autologous RBC and the plasma volume was measured with radiolabeled 125I cold agglutinin 19S macroglobulin with a molecular weight of 1,000,000. Radiolabeled human albumin could not be used to measure the plasma volume in patients because of the rapid loss of the radiolabeled albumin with molecular weight of 68,000 into the extravascular volume which overestimates the plasma volume [8]. The normal hematocrit in these patients reduced the non-surgical blood loss from open wounds of the extremities which required surgical debridement [9]. The chronic hypovolemic anemic patients subjected to general anesthesia for the debridement of their wounds of the extremities became hypotensive and a few of these wounded servicemen had cardiac arrests associated with the administration of general anesthesia. This observation confirmed that general anesthesia permitted redistribution of the blood volume from the central blood volume in the brain, heart, lungs, and kidneys into the hypoperfused peripheral blood volume which consists of the blood volume in the gastrointestinal tract, muscle, bones and skin of the extremities. In these patients with normal hematocrit and hemoglobin concentration values the debridement of wounds of the extremities was associated with minimal non-surgical blood loss from the open wounds of the extremities [9]. Another clinical observation was that following the transfusion of RBC which increased the peripheral red blood cell volume to the extremities, the edema in the tissues of the extremities disappeared. The reduction in the tissue edema in the extremities was associated with an increase in the plasma volume [10].
The transfusion of washed liquid preserved red blood cells and washed previously frozen deglycerolized red blood cells to chronic hypovolemic anemic patients increased both the red blood cell volume and the plasma volume. At the same time studies were done to assess whether washed autologous dog red blood cell transfusions would increase the plasma volume in dogs following acute hypovolemic anemia produced by removal of whole blood from the dogs. Dogs subjected to acute hypovolemia with reduced RBC volume and plasma volume were transfused with washed autologous dog red blood cells. Following transfusion, an increase in red blood cell volume and plasma volume occurred. The study demonstrated that plasma volume increased following the transfusion of washed autologous dog red blood cells to acute hypovolemic dogs. These studies indicated that the red blood cell volume regulated the plasma volume and the total blood volume in chronic hypovolemic patients and in acute hypovolemic dogs [10].
The hematocrit and the hemoglobin concentration of the chronic hypovolemic anemic patients did not increase following the transfusion of several units of compatible identifiable viable RBC because the plasma volume of the patients increased [10]. Both the red blood cell 2,3 DPG and the RBC creatine levels decreased following the restoration of the red blood cell volume in these patients. The chronic hypovolemic anemic patients had normal hematocrit values and hemoglobin concentrations but they had significantly elevated RBC 2,3 DPG levels and RBC creatine levels prior to the RBC transfusions. The aggressive transfusions of the chronic hypovolemic anemic patients with extremity injuries to restore the peripheral blood volume prevented the need to amputate extremities in the 300 wounded servicemen hospitalized at the Chelsea Naval Hospital between 1968 to 1974 [6].
The increase in RBC 2,3 DPG level was a compensatory mechanism to permit the reduced volume of red blood cells to improve oxygen delivery to tissue to maintain high p02 tensions in the brain, heart and kidneys without the need to increase blood flow to the brain, heart, and kidneys. The hypoperfusion of the muscle permitted leakage of creatine from the muscle which accumulated in the circulating RBC to increase the RBC creatine level. In patients with cardiopulmonary insufficiency increases in RBC 2,3 DPG and creatine levels were observed. Again, the tissue hypoxia produced in patients with cardiopulmonary insufficiency stimulated the RBC to increase the 2,3 DPG level to improve oxygen release from the RBC. The tissue hypoxia that was present in patients with cardiopulmonary insufficiency permitted loss of creatine from the hypoxic muscle which accumulated in the red blood cells. Both the red blood cell creatine and 2,3 DPG levels were elevated in patients with cardiopulmonary insufficiency reflected tissue hypoxia in the non-vital tissues like the muscle of the extremities.
The aggressive transfusion of the patients with traumatic injuries who had multiple fractures of the upper and lower extremities restored the blood volume to these areas. In the study of 300 wounded servicemen from Vietnam who were transferred to Chelsea Naval Hospital from 1968 to 1974, amputation of their extremities rarely occurred. The transfusion of these patients to restore the red blood cell volume, especially the peripheral red blood cell volume to extremities with washed liquid preserved red blood cells and washed previously frozen deglycerolized RBC increased the red blood cell volume, the plasma volume and total blood volume towards their normal values. The multiple fractures of the upper and lower extremities healed without the need to amputate these extremities because of poor perfusion (Figure 3-8).
Following transfusion in these chronic hypovolemic and anemic patients improvement in appetite occurred together with improvement in their libido and general state of health in these wounded servicemen. Studies were done to investigate the mechanisms of the red blood cell volume deficits observed in these wounded servicemen with the chronic hypovolemic anemia of trauma. The red blood cell survival of autologous and allogeneic compatible but identifiable red blood cells, the rate of red blood cell production, and the red blood cell destruction were measured [6].
Biphasic survival of allogeneic compatible but identifiable red blood cells was observed suggesting the presence and subsequent removal of an extrinsic factor that accelerated the destruction of the donor red blood cells and patient red blood cells. Treatment of the patient’s blood with adrenochrome, a metabolite of epinephrine, showed increase hemolysis which decreased following the RBC transfusions. The extrinsic factor in the patient’s blood correlated to the increased hemolysis in their blood following the in vitro treatment with adrenochrome [11,12]. Red blood cell production was reduced and destruction of preserved compatible nonviable red blood cells was not associated with increases of carbon monoxide, bilirubin, and urinary and fecal urobilinogen excretion. The destruction of preserved allogeneic compatible nonviable red blood cells was associated with porphyrin accumulation in the wounds and increase in urinary excretion of dipyrroles and oxyporphyrin. The data suggested that the degradation of preserved compatible nonviable RBC was not through the carbon monoxide – bilirubin – urobilinogen pathway but by degradation into heme and porphyrin derivatives without release of carbon monoxide. In vitro testing to assess the susceptibility of the red blood cells to adrenochrome, a degradation product of epinephrine, suggested a potential mechanism for the degradation of hemoglobin into heme and porphyrin. In vitro testing of blood from the chronic hypovolemic anemic patients showed an increase in hemolysis when treated with adrenochrome [11,12].
The subsequent studies of non-surgical blood loss in patients with anemia and thrombocytopenia following cardiopulmonary bypass surgery provided an explanation for the observed reduced non-surgical blood loss from open wounds of the extremities observed in the chronic hypovolemic anemic patients with traumatic injuries. The patients with chronic hypovolemia of trauma had normal or slightly increased hematocrit values. The effect of the normal or slightly increased hematocrit values on the bleeding time and non-surgical blood loss was associated with the decrease in non-surgical blood loss in the open extremity wounds of these patients [9]. The other important observation was that RBC transfusions increased the peripheral red blood cell volume and reduced the edema in the extremities to increase the plasma volume and the total blood volume [10].
The experience of Stinner and associates reported in Military Medicine, 175:1027-1029, 2010 is very different from our experience treating patients at the Chelsea Naval Hospital during the Vietnam War. They report that of 348 service members who sustained combat injuries in Afghanistan and Iraq, fifty-three (15.2%) required amputations 12 weeks to 5.5 years following their initial extremity injuries [7].
In our patients at the Chelsea Naval Hospital although these orthopedic patients had chronic hypovolemic anemia of trauma with reduction in both the RBC and plasma volume, normal hemoglobin and hematocrit values, and normal central blood volumes, they suffered from severely contracted peripheral blood volumes to their extremities. The aggressive transfusion of washed liquid preserved RBC and washed previously frozen deglycerolized RBC restored their peripheral blood volume and total blood volume. We studied 300 servicemen for at least 2 years during their hospitalization at Chelsea Naval Hospital. Chronic hypovolemic patients with traumatic injuries to their extremities were transfused RBC in order to restore their peripheral blood volume and to repair the injured extremities (Figure 3-9).
The observation of biphasic survival of compatible identifiable allogeneic RBC transfused to patients with traumatic injuries suggested the presence and removal of a toxic substance from the blood. The observation of biphasic survival curves suggested the removal from the patient’s circulation of a toxic substance which had adversely affected the lifespan of donor RBC populations and of the patient’s own RBC as well. Out hypothesis was that the presumed toxic substance had two adverse effects: it decreased the survival of the autologous and donor compatible and identifiable RBC and impaired erythropoiesis. The disappearance of the presumed toxic substance was accompanied by improvement in the survival of donor RBC populations as shown by the biphasic survival curves, and by improvement in the patient’s RBC production as manifested by an increase in the reticulocyte count in peripheral blood. In patients with biphasic linear donor RBC survival curves, the lifespan in the initial phase ranged from 38 to 60 days, and in the second phase ranged from 78 to 113 days. This finding suggested that it took 30 to 40 days after transfusion for the toxic substance to be removed from the circulation and for the lifespan to increase [6].
Studies were done at the NBRL between 1968 to 1974 to document that patients with chronic hypovolemic anemia following traumatic injuries and patients with cardiopulmonary insufficiency had red blood cells with increased levels of RBC 2,3 DPG and RBC creatine. Two significant serendipitous observations were made at the NBRL. Tissue hypoxia in patients with decrease in red blood cell volume and in patients with cardiopulmonary insufficiency produced red blood cell with increased 2,3 DPG and creatine levels. The release of muscle creatine occurred from the hypoperfused hypoxic muscles in these patients. In 1968, two red blood cell indicators of tissue hypoxia; increases in RBC 2,3 DPG and RBC creatine levels were observed in patients hospitalized at the Chelsea Naval Hospital [2]. Studies performed at the NBRL were unable to demonstrate that creatine could enter the RBC under the various in vitro conditions used in our investigations. However, in vivo studies showed that the increased RBC creatine levels observed in patients with chronic hypovolemic anemia of trauma following RBC transfusions to increase the red blood cell volume and the peripheral red blood cell volume to normal were associated with a decrease in the red blood cell creatine levels. In vivo studies suggested that the red blood cell creatine level in these patients with chronic hypovolemic anemia of trauma correlated to the hypoxia in their muscles. The decrease in the peripheral red blood cell volume reduced the perfusion of the muscle of the extremities. Restoration of the peripheral blood volume by the red blood cell transfusions restored perfusion to the hypoxic muscles and the reduction in red blood cell creatine level occurred [2,6].
In the hypovolemic anemic patients the decrease in red blood cell volume stimulated an increase in red blood cell 2,3 DPG level. Our subsequent studies were done to ensure that the transfused RBC not only circulated but functioned to provide oxygen to the brain, heart and kidneys which require high tissue oxygen tension. In addition, RBC transfusions need to increase the peripheral red blood cell volume to perfuse the muscle of the extremities and prevent the loss of muscle creatine which increased the RBC creatine level. Previous clinical studies have reported that patients with congestive heart failure had increased level of creatine in their urine. Reduction in urinary creatine was associated with treatment of the congestive heart failure. Studies between 1968 to 1974 at the NBRL documented that both red blood cell. 2,3 DPG level and RBC creatine level were useful measurements to assess the presence of tissue hypoxia. The level of RBC 2,3 DPG level was affected by the patient’s blood pH and the level of plasma inorganic phosphorus.
Since 1968 the NBRL has studied not only the in vivo survival of preserved RBC but their function to provide oxygen at high tensions to the brain, heart and kidneys. Studies performed at the NBRL located at Chelsea Naval Hospital demonstrated that wounded servicemen who returned from South Vietnam with wounds of the extremities had chronic hypovolemic anemia of trauma. The hematocrit values and hemoglobin concentrations did not reflect the red blood cell volume, plasma volume, and total blood volume deficits in these patients. The direct measurement of the red blood cell volume with the radioisotope 51Cr was routinely done to measure the red blood cell volume. In addition, the plasma volume was measured using 125I human albumin. The use of the radiolabeled albumin demonstrated rapid loss of the 125I labeled albumin into the extravascular volume and overestimation of the plasma volume was observed in these patients. The simultaneous measurement of the plasma volume using 125I radiolabeled human 7S albumin with a molecular weight of 68,000 and 13II radiolabeled human 19S cold agglutinin with a molecular weight of 1,000,000 demonstrated that human albumin cannot be used to measure the plasma volume in patients and should not be used as a resuscitation solution to increase the plasma volume and blood volume in patients subjected to hemorrhagic shock [8]. The data demonstrated that iodinated human albumin and Evans blue dye which binds to human albumin should not be used to measure the plasma volume in patients. However, in normal volunteers 125I human albumin can estimate the plasma volume and with the total body hematocrit (peripheral venous hematocrit multiplied by 0.89) to estimate the red blood cell volume and the total blood volume but not in patients [8].
Studies in wounded servicemen who returned from Vietnam with primarily orthopedic injuries of the extremities had chronic hypovolemia of trauma with significant decreases in the peripheral blood volume and total blood volume [6]. The peripheral venous hematocrit and hemoglobin concentration did not reflect the hypovolemic state and the significant reductions in the red blood cell volume and plasma volume. The central blood volume assessed by arterial blood pressure, heart rate, arterial blood gases, cardiac output, pulmonary artery wedge pressure, and renal function was normal. The deficit in red blood cell volume was documented by the measured 51Cr red blood cell volume, the deficit in the plasma volume by its measurement using 125I labeled cold agglutinin. The deficit in peripheral blood volume was demonstrated by the transfusion of multiple units of compatible but identifiable red blood cells that were washed liquid preserved RBC and washed previously frozen deglycerolized red blood cells. The aggressive transfusion of multiple units of washed compatible identifiable liquid preserved and previously frozen deglycerolized red blood cells increased the red blood cell volume and the plasma volume with only a slight increase in the hematocrit values and hemoglobin concentrations in the peripheral venous blood. The central red blood cell volume increased slightly following the transfusion but within 4 hours returned to the baseline level [6].
The effect of the transfusion of washed red blood cells to restore the red blood cell volume and the plasma volume was associated with improvement in blood flow to the gastrointestinal tract and increase in appetite, improvement in libido, improvement in general health and decrease in the edema which was present in extremity wounds. The increase in red blood cell volume restored perfusion to the extremities with only rare amputation of the extremities by the aggressive transfusion to increase the peripheral red blood cell volume. The increase in the plasma volume associated with the transfusion of washed red blood cells was associated with decrease in the edema in the wounds of the extremities [6,10].
The therapeutic effectiveness of the transfusion of compatible, identifiable, viable washed red blood cells produced a significant reduction in both the elevated RBC 2,3 DPG and creatine levels. The elevated red blood cell creatine level could not be explained by an increased in red blood cell production which was reduced in these patients. The increased red blood cell creatine in these patients with chronic hypovolemia of trauma was not due to an increase in erythropoiesis. RBC creatine level correlated to the red blood cell volume deficit and not to the mean red blood cell age [6].
The study of the patients wounded in Vietnam revealed that their wounds required debridement under anesthesia but bleeding of these wounds was minimal. The reduction in nonsurgical blood loss observed with debridement of these wounds of the extremities was related to the normal or slightly increased hematocrit of these patients. This serendipitous clinical observation stimulated the NBRL to study the effect of hematocrit on the bleeding time and nonsurgical blood loss. Viable and functional RBC reduce the bleeding time and nonsurgical blood [9].
The paper by Stinner DJ and associates in Military Medicine reported the incidence of late amputation of 15% occurred 12 weeks to 5.5 years following combat related injuries sustained by U.S. military personnel in Afghanistan and Iraq [7]. During the Vietnam War from 1968 to 1974 our laboratory studied and treated over 300 wounded servicemen who returned to the Chelsea Naval Hospital from South Vietnam with severe traumatic injuries to their extremities who were treated by the orthopedic service (Figure 3-9). These patients with traumatic injuries to their extremities had chronic hypovolemic anemia of trauma with reduction in total blood volume of 30 to 40%, normal hemoglobin and hematocrit values, normal central blood volume but severely reduced peripheral blood volume to their extremities (Figure 1). Aggressive transfusion of washed liquid preserved red blood cells and previously frozen deglycerolized red blood cells were administered to restore the peripheral blood volume to their extremities. The repeated red blood cell transfusion treatment to restore to normal the peripheral blood volume to their extremities was associated with repair of the wounded extremities with the rare need for an amputation in the 300 servicemen who were studied for one to two years during their hospitalization at the Chelsea Naval Hospital. The book the Hypovolemic Anemia of Trauma: The Missing Blood Syndrome by Valeri CR and Altschule MD, CRC Press, Boca Raton, FL, 1981, was written to report that chronic hypovolemic patients with traumatic injuries to their extremities need to be transfused red blood cells to restore the peripheral blood volume to repair the injured extremities of these patients (Figure 1). The studies reported in the book demonstrate the limitation of the hematocrit and hemoglobin concentration measurements; the maintenance of the central blood volume and the 30-40% reduction in the peripheral blood volume in patients with traumatic injuries to their extremities. The repeated transfusions of viable and identifiable compatible red blood cells restored the peripheral blood volume and permitted the healing of their injured extremities without the need for amputations.
2021 Copyright OAT. All rights reserv
Summary
Red blood cell transfusions are indicated for patients with hypovolemic anemia of trauma with normal hematocrit and hemoglobin levels to restore the peripheral red blood cell volume to normal to prevent the need for amputations. The red blood cell creatine level is not metabolic garbage but a measurement of muscle hypoxia. An increase in red blood cell creatine measures the reduced oxygen tension in muscle and red blood cell transfusions are indicated to restore the peripheral red blood cell volume to normal and to reduce the red blood cell creatine to normal in hypovolemic patients with traumatic injuries to prevent the need for amputations.
References
- Carson JL, Triulzi DJ, Ness PM (2017) Indications for and Adverse Effects of Red-Cell Transfusion. N Engl J Med 377 1261-1272. [Crossref]
- Valeri CR, Fortier NL (1969) Red-cell 2,3-diphosphoglycerate and creatine levels in patients with red-cell mass deficits or with cardiopulmonary insufficiency. N Engl J Med 281: 1452-1455. [Crossref]
- Beutler E (1970) Is red-cell creatine metabolic garbage? N Engl J Med 282: 979-980. [Crossref]
- Valeri CR, Fortier NL (1970) Is red-cell creatine metabolic garbage? N Engl J Med 282: 980. [Crossref]
- Biron PE, Howard J, Valeri CR (1972) Chronic deficits in red-cell mass in patients with orthopaedic injuries (stress anemia). J Bone Joint Surg Am 54: 1001-1014. [Crossref]
- Valeri CR and Altschule MD (1981) Hypovolemic Anemia of Trauma: The Missing Blood Syndrome. Chemical Rubber Company, Boca Raton, Florida.
- Stinner DJ, Burns TC, Kirk KL, Scoville CR, Ficke JR, et al. (2010) Prevalence of late amputations during the current conflicts in Afghanistan and Iraq. Mil Med 175:1027-1029.
- Valeri CR, Cooper AG, Pivacek LE (1973) Limitations of measuring blood volume with iodinated I 125 serum albumin. Arch Int Med 132:534-538.
- Valeri CR, Cassidy G, Pivacek LE, Ragno G, Lieberthal W, et al. (2001) Anemia-induced increase in the bleeding time: indications for treatment of nonsurgical blood loss. Transfusion 41:977-983.
- Valeri CR, Donahue K, Feingold HM, Cassidy GP, Altschule MD (1986) Increase in plasma volume after the transfusion of washed erythrocytes. Surg Gynecol Obstet 162: 30-36. [Crossref]
- Valeri CR, Altschule MD, Pivacek LE (1972) The hemolytic action of adrenochrome, an epinephrine metabolite. J Med 3: 20-40. [Crossref]
- Valeri CR, Altschule MD (1973) Hemolysis in vitro of blood obtained from patients with traumatic injuries. J Trauma 13: 678-686. [Crossref]









